Chlorpheniramine Intranasal Spray to Accelerate COVID-19 Clinical Recovery in an Outpatient Setting: The ACCROS Trials
et al., Research Square, doi:10.21203/rs.3.rs-2167465/v1, ACCROS-II, NCT05520944, Oct 2022
47th treatment shown to reduce risk in
December 2022, now with p < 0.00000000001 from 3 studies.
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
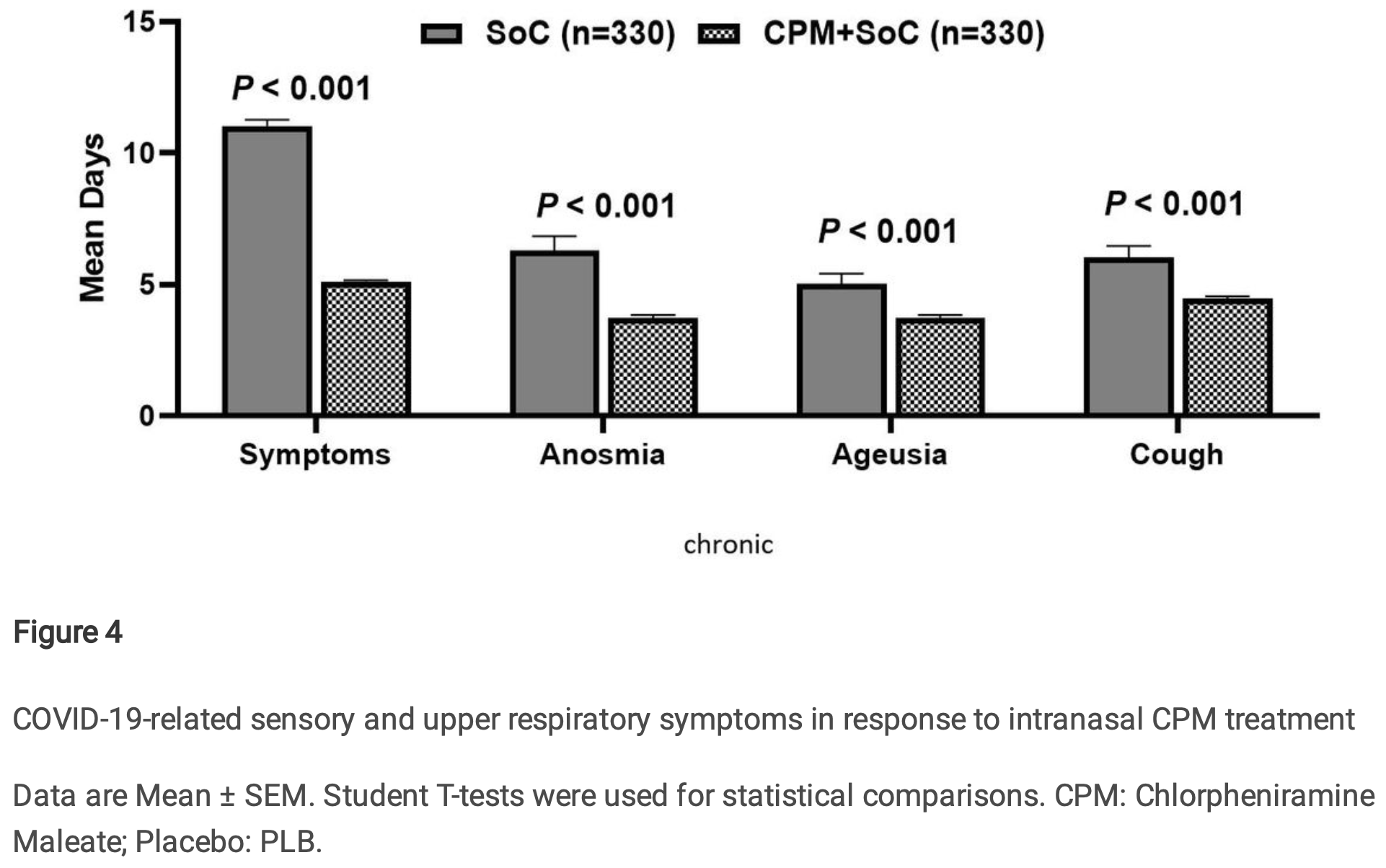
RCT and retrospective study of chlorpheniramine nasal spray for COVID-19. The retrospective study included 660 outpatients showing fewer days with general COVID-19 symptoms, cough, anosmia, and ageusia compared to standard of care alone. The RCT results are listed separately1.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
Study covers antihistamine H1RAs and chlorpheniramine.
|
recovery time, 54.3% lower, relative time 0.46, p < 0.001, treatment mean 4.97 (±3.32) n=330, control mean 10.88 (±6.64) n=330.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Valerio-Pascua et al., Chlorpheniramine Intranasal Spray to Accelerate COVID-19 Clinical Recovery in an Outpatient Setting: The ACCROS Trials, Research Square, doi:10.21203/rs.3.rs-2167465/v1.
2.
Valerio-Pascua (B) et al., Mitigating the risks of post-acute sequelae of SARS-CoV-2 infection (PASC) with intranasal chlorpheniramine: perspectives from the ACCROS studies, BMC Infectious Diseases, doi:10.1186/s12879-024-10211-8.
Valerio-Pascua et al., 18 Oct 2022, retrospective, Honduras, preprint, 16 authors, study period June 2021 - July 2022, trial NCT05520944 (history) (ACCROS-II).
Contact: rahaghf@ccf.org.
DOI record:
{
"DOI": "10.21203/rs.3.rs-2167465/v1",
"URL": "http://dx.doi.org/10.21203/rs.3.rs-2167465/v1",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p><jats:bold>Purpose: </jats:bold>Our group demonstrated the safety, efficacy, and antiviral effect of intranasally administered Chlorpheniramine Maleate (CPM) for treating coronavirus disease 2019 (COVID-19). Since the nasal cavity is the portal of entry for COVID pathogens, sensory and upper respiratory symptoms (URS) (e.g., cough, ageusia, anosmia, nasal congestion, etc.) are significant symptoms in the course of the disease. Intranasal therapies could alleviate the disease-induced URS faster. This study evaluated the effectiveness and safety of intranasal CPM for treating mild to moderate COVID-19-induced URS in the outpatient setting.\n<jats:bold>Methods: </jats:bold>The two-part <jats:bold>A</jats:bold>ccelerating <jats:bold>C</jats:bold>OVID-19 <jats:bold>C</jats:bold>linical <jats:bold>R</jats:bold>ecovery in an <jats:bold>O</jats:bold>utpatient <jats:bold>S</jats:bold>etting (ACCROS) research study was conducted to collect evidence from a randomized, double-blinded placebo-controlled trial (ACCROS-I). Both parts enrolled patients with mild to moderate COVID-19 confirmed by reverse transcription-polymerase chain reaction. The primary endpoint in ACCROS-I was time to clinical recovery, defined as the change from baseline to day 7 in COVID-19 symptoms reported as the percent change (Δ%) in the daily symptoms score (DSS) and the severity of the disease symptoms using a visual analog scale (VAS), on a scale of 1-10 (10=worst symptoms). COVID-19 patients (n = 101) were recruited and assigned to either a 10-day CPM treatment (n=61) or placebo (PLB) (n=40) in addition to standard of care (SoC). Secondary endpoints included the incidence of hospitalization and the proportion of patients with URS on day 7. ACCROS-II data were collected from medical records of COVID-positive subjects using a standardized form. Cohorts of patients treated with CPM and SoC (CPM+Soc) were compared for the duration of general symptoms and URS. Patient information was collected as part of routine visits and telehealth consultations.\n<jats:bold>Results </jats:bold><jats:italic>ACCROS-I:</jats:italic> There was a statistically significant difference in the rate of clinical recovery (P<0.05) in Δ%DSS (M -18.8±SEM 7.9%) and Δ%VAS (-8.6±5.1%), such that the CPM group reported fewer symptoms than PLB. The proportion of patients who reported sensory deficits and URS at day 7 was significantly lower (P<0.05) in CPM vs. PLB for ageusia (1.7% vs. 15.0%), cough (16.4% vs. 35.0%) and nasal congestion (8.1%vs.20%). None of the patients required hospitalization.\n<jats:italic>ACCROS-II</jats:italic>: There was a statistically significant reduction (P<0.05) in total days reporting URS for general symptoms of COVID-19 in CPM+SoC (5.1 ± 0.1) compared to SoC (11.0 ± 0.2). CPM+SoC users also showed fewer days with cough, anosmia, and ageusia. Persistent anosmia (over 29 days) was found in 3% of the patients on SoC, whereas no persistent anosmia was reported in the CPM+SoC cohort (X<jats:sup>2</jats:sup> = 10.18; P<0.001).\n<jats:bold>Conclusion:</jats:bold> The result of this two-part study supports the conclusion that intranasal CPM is an antiviral agent that can be administered intranasally to treat COVID-19-induced symptoms effectively. Intranasal CPM accelerates clinical recovery and reduces URS in patients with mild to moderate COVID-19. This study's important implications include individuals returning to daily life faster, reducing community and individual economic burden, and decreasing healthcare utilization.\n<jats:bold>Trial registration:</jats:bold> ClinicalTrials.gov.; ID: NCT05449405 ACCROS-I retrospectively registered on 7/13/2022, NCT05520944 ACCROS-R retrospectively registered on 08/27/2022.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
10,
14
]
]
},
"author": [
{
"affiliation": [
{
"name": "Hospital CEMESA Cortés"
}
],
"family": "Valerio-Pascua",
"given": "Fernando",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Hospital CEMESA Cortés"
}
],
"family": "Mejia",
"given": "Estela Jackeline Pineda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Aventura Hospital Pulmonary and Critical Care Fellowship"
}
],
"family": "Tesch",
"given": "Mari L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Leonardo Martínez Valenzuela"
}
],
"family": "Godoy",
"given": "Jancy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Universidad Católica de Honduras"
}
],
"family": "Fuentes",
"given": "Carlos López",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Universidad Católica de Honduras"
}
],
"family": "Erazo",
"given": "Gloria B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Universidad Católica de Honduras"
}
],
"family": "Bermúdez",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Saint Barnabas Hospital"
}
],
"family": "Pineda",
"given": "Miguel Fernando Vargas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hampton University School of Pharmacy"
}
],
"family": "Rivzi",
"given": "Syed A.A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Aventura Hospital Pulmonary and Critical Care Fellowship"
}
],
"family": "Cabrera",
"given": "Armando",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Aventura Hospital Pulmonary and Critical Care Fellowship"
}
],
"family": "Chauhan",
"given": "Zeeshan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinica Universitaria Union Medica"
}
],
"family": "Grullón-Franco",
"given": "Scarlet",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinica Universitaria Union Medica"
}
],
"family": "Paulino-Then",
"given": "Jorge L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinica Universitaria Union Medica"
}
],
"family": "Garcia",
"given": "Natalia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The George Washington University"
}
],
"family": "Williams",
"given": "Jeffrey D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cleveland Clinic"
}
],
"family": "Rahaghi",
"given": "Franck F.",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
10,
18
]
],
"date-time": "2022-10-18T18:11:18Z",
"timestamp": 1666116678000
},
"deposited": {
"date-parts": [
[
2022,
10,
24
]
],
"date-time": "2022-10-24T04:15:00Z",
"timestamp": 1666584900000
},
"group-title": "In Review",
"indexed": {
"date-parts": [
[
2022,
10,
25
]
],
"date-time": "2022-10-25T05:06:49Z",
"timestamp": 1666674409162
},
"institution": [
{
"name": "Research Square"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
10,
18
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
18
]
],
"date-time": "2022-10-18T00:00:00Z",
"timestamp": 1666051200000
}
}
],
"link": [
{
"URL": "https://www.researchsquare.com/article/rs-2167465/v1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.researchsquare.com/article/rs-2167465/v1.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8761",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
10,
18
]
]
},
"prefix": "10.21203",
"published": {
"date-parts": [
[
2022,
10,
18
]
]
},
"publisher": "Research Square Platform LLC",
"reference": [
{
"DOI": "10.1093/ofid/ofac138",
"article-title": "A Systematic Review of Coronavirus Disease 2019 Vaccine Efficacy and Effectiveness Against Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Disease",
"author": "Higdon MM",
"doi-asserted-by": "crossref",
"first-page": "ofac138",
"issue": "6",
"journal-title": "Open Forum Infect Dis",
"key": "ref1",
"unstructured": "Higdon, M.M., et al., A Systematic Review of Coronavirus Disease 2019 Vaccine Efficacy and Effectiveness Against Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Disease. Open Forum Infect Dis, 2022. 9(6): p. ofac138.",
"volume": "9",
"year": "2022"
},
{
"author": "Sanchez-Gonzalez MA",
"key": "ref2",
"unstructured": "Sanchez-Gonzalez, M.A., et al., A Pathophysiological Perspective on COVID-19's Lethal Complication: From Viremia to Hypersensitivity Pneumonitis-like Immune Dysregulation. Infect Chemother, 2020.",
"volume-title": "A Pathophysiological Perspective on COVID-19's Lethal Complication: From Viremia to Hypersensitivity Pneumonitis-like Immune Dysregulation",
"year": "2020"
},
{
"DOI": "10.1038/s41392-021-00849-0",
"article-title": "SARS-CoV-2-triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury",
"author": "Wu M-L",
"doi-asserted-by": "crossref",
"first-page": "428",
"issue": "1",
"journal-title": "Signal Transduction and Targeted Therapy",
"key": "ref3",
"unstructured": "Wu, M.-L., et al., SARS-CoV-2-triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury. Signal Transduction and Targeted Therapy, 2021. 6(1): p. 428.",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.633680",
"article-title": "COVID-19: Famotidine, Histamine, Mast Cells, and Mechanisms",
"author": "Malone RW",
"doi-asserted-by": "crossref",
"first-page": "633680",
"journal-title": "Front Pharmacol",
"key": "ref4",
"unstructured": "Malone, R.W., et al., COVID-19: Famotidine, Histamine, Mast Cells, and Mechanisms. Front Pharmacol, 2021. 12: p. 633680.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3390/jcm10030459",
"article-title": "The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases",
"author": "Sarkar A",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "J Clin Med",
"key": "ref5",
"unstructured": "Sarkar, A., et al., The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J Clin Med, 2021. 10(3).",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/j.mehy.2021.110622",
"article-title": "Early multidrug treatment of SARS-CoV-2 infection (COVID-19) and reduced mortality among nursing home (or outpatient/ambulatory) residents",
"author": "Alexander PE",
"doi-asserted-by": "crossref",
"first-page": "110622",
"journal-title": "Med Hypotheses",
"key": "ref6",
"unstructured": "Alexander, P.E., et al., Early multidrug treatment of SARS-CoV-2 infection (COVID-19) and reduced mortality among nursing home (or outpatient/ambulatory) residents. Med Hypotheses, 2021. 153: p. 110622.",
"volume": "153",
"year": "2021"
},
{
"author": "Ontai S",
"key": "ref7",
"unstructured": "Ontai, S., et al., Early multidrug treatment of SARS-CoV-2 (COVID-19) and decreased case fatality rates in Honduras. medRxiv, 2021: p. 2021.07.21.21260223.",
"year": "2021"
},
{
"article-title": "Repurposing Drugs for Covid-19 by a Developing Country",
"author": "Valerio Pascua F",
"issue": "2",
"journal-title": "Epidemol Int J",
"key": "ref8",
"unstructured": "Valerio Pascua, F., et al., Repurposing Drugs for Covid-19 by a Developing Country. Epidemol Int J, 2022. 6(2).",
"volume": "6",
"year": "2022"
},
{
"article-title": "COVID-19: Azelastine nasal spray Reduces Virus-load In Nasal swabs (CARVIN). Early intervention with azelastine nasal sprays reduces viral load in SARS-CoV-2 infected patients. First report on a double-blind placebo-controlled phase II clinical trial",
"author": "Klussmann JP",
"key": "ref9",
"unstructured": "Klussmann, J.P., et al., COVID-19: Azelastine nasal spray Reduces Virus-load In Nasal swabs (CARVIN). Early intervention with azelastine nasal sprays reduces viral load in SARS-CoV-2 infected patients. First report on a double-blind placebo-controlled phase II clinical trial. COVID-19, 2021.",
"volume": "COVID-19",
"year": "2021"
},
{
"DOI": "10.1016/j.pupt.2021.101989",
"article-title": "Antihistamines and azithromycin as a treatment for COVID-19 on primary health care - A retrospective observational study in elderly patients",
"author": "Morán Blanco JI",
"doi-asserted-by": "crossref",
"first-page": "101989",
"journal-title": "Pulm Pharmacol Ther",
"key": "ref10",
"unstructured": "Morán Blanco, J.I., et al., Antihistamines and azithromycin as a treatment for COVID-19 on primary health care - A retrospective observational study in elderly patients. Pulm Pharmacol Ther, 2021. 67: p. 101989.",
"volume": "67",
"year": "2021"
},
{
"DOI": "10.18103/mra.v10i3.2752",
"article-title": "Intranasal Chlorpheniramine Maleate for the treatment of COVID-19: Translational and Clinical Evidence",
"author": "Sanchez-Gonzalez MA",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "Medical Research Archives",
"key": "ref11",
"unstructured": "Sanchez-Gonzalez, M.A., et al., Intranasal Chlorpheniramine Maleate for the treatment of COVID-19: Translational and Clinical Evidence. Medical Research Archives, 2022. 10(3).",
"volume": "10",
"year": "2022"
},
{
"article-title": "Treatment Protocol for COVID-19 Based on T2R Phenotype",
"author": "Taha MA",
"issue": "3",
"journal-title": "Viruses",
"key": "ref12",
"unstructured": "Taha, M.A., et al., Treatment Protocol for COVID-19 Based on T2R Phenotype. Viruses, 2021. 13(3).",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.7189/jogh.11.03084",
"article-title": "Impact of COVID-2019 on school attendance problems",
"author": "Nathwani G",
"doi-asserted-by": "crossref",
"first-page": "03084",
"journal-title": "J Glob Health",
"key": "ref13",
"unstructured": "Nathwani, G., et al., Impact of COVID-2019 on school attendance problems. J Glob Health, 2021. 11: p. 03084.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1183/13993003.01341-2021",
"article-title": "Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: a prospective cohort study",
"author": "Osmanov IM",
"doi-asserted-by": "crossref",
"first-page": "2101341",
"issue": "2",
"journal-title": "European Respiratory Journal",
"key": "ref14",
"unstructured": "Osmanov, I.M., et al., Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: a prospective cohort study. European Respiratory Journal, 2022. 59(2): p. 2101341.",
"volume": "59",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm6927a1",
"article-title": "Increases in Health-Related Workplace Absenteeism Among Workers in Essential Critical Infrastructure Occupations During the COVID-19 Pandemic - United States, March-April 2020",
"author": "Groenewold MR",
"doi-asserted-by": "crossref",
"first-page": "853",
"issue": "27",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "ref15",
"unstructured": "Groenewold, M.R., et al., Increases in Health-Related Workplace Absenteeism Among Workers in Essential Critical Infrastructure Occupations During the COVID-19 Pandemic - United States, March-April 2020. MMWR Morb Mortal Wkly Rep, 2020. 69(27): p. 853–858.",
"volume": "69",
"year": "2020"
},
{
"article-title": "Chlorpheniramine, an Old Drug with New Potential Clinical Applications: A Comprehensive Review of the Literature",
"author": "Rizvi AAS",
"first-page": "1",
"journal-title": "Current Reviews in Clinical and Experimental Pharmacology",
"key": "ref16",
"unstructured": "Rizvi, A.A.S., et al., Chlorpheniramine, an Old Drug with New Potential Clinical Applications: A Comprehensive Review of the Literature. Current Reviews in Clinical and Experimental Pharmacology, 2022. 17: p. 1–1.",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1172/JCI72094",
"article-title": "Bitter and sweet taste receptors regulate human upper respiratory innate immunity",
"author": "Lee RJ",
"doi-asserted-by": "crossref",
"first-page": "1393",
"issue": "3",
"journal-title": "J Clin Invest",
"key": "ref17",
"unstructured": "Lee, R.J., et al., Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest, 2014. 124(3): p. 1393–405.",
"volume": "124",
"year": "2014"
},
{
"article-title": "Molecular Modeling and Preliminary Clinical Data Suggesting Antiviral Activity for Chlorpheniramine (Chlorphenamine) Against COVID-19",
"author": "Black S",
"first-page": "e20980",
"issue": "1",
"journal-title": "Cureus",
"key": "ref18",
"unstructured": "Black, S., Molecular Modeling and Preliminary Clinical Data Suggesting Antiviral Activity for Chlorpheniramine (Chlorphenamine) Against COVID-19. Cureus, 2022. 14(1): p. e20980.",
"volume": "14",
"year": "2022"
},
{
"author": "Mostafa A",
"key": "ref19",
"unstructured": "Mostafa, A., et al., FDA-Approved Drugs with Potent In Vitro Antiviral Activity against Severe Acute Respiratory Syndrome Coronavirus 2. Pharmaceuticals (Basel), 2020. 13(12).",
"year": "2020"
},
{
"article-title": "In Vitro Virucidal Effect of Intranasally Delivered Chlorpheniramine Maleate Compound Against Severe Acute Respiratory Syndrome Coronavirus 2",
"author": "Westover JB",
"first-page": "e10501",
"issue": "9",
"journal-title": "Cureus",
"key": "ref20",
"unstructured": "Westover, J.B., et al., In Vitro Virucidal Effect of Intranasally Delivered Chlorpheniramine Maleate Compound Against Severe Acute Respiratory Syndrome Coronavirus 2. Cureus, 2020. 12(9): p. e10501.",
"volume": "12",
"year": "2020"
},
{
"article-title": "A Randomized Controlled Pilot Trial to Test the Efficacy of Intranasal Chlorpheniramine Maleate With Xylitol for the Treatment of Allergic Rhinitis",
"author": "Sanchez-Gonzalez M",
"first-page": "e14206",
"issue": "3",
"journal-title": "Cureus",
"key": "ref21",
"unstructured": "Sanchez-Gonzalez, M., et al., A Randomized Controlled Pilot Trial to Test the Efficacy of Intranasal Chlorpheniramine Maleate With Xylitol for the Treatment of Allergic Rhinitis. Cureus, 2021. 13(3): p. e14206.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-19145-6",
"article-title": "ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs",
"author": "Lee IT",
"doi-asserted-by": "crossref",
"first-page": "5453",
"issue": "1",
"journal-title": "Nature Communications",
"key": "ref22",
"unstructured": "Lee, I.T., et al., ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs. Nature Communications, 2020. 11(1): p. 5453.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.2307/2529712",
"article-title": "Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial",
"author": "Pocock SJ",
"doi-asserted-by": "crossref",
"first-page": "103",
"issue": "1",
"journal-title": "Biometrics",
"key": "ref23",
"unstructured": "Pocock, S.J. and R. Simon, Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics, 1975. 31(1): p. 103–15.",
"volume": "31",
"year": "1975"
},
{
"author": "FDA US",
"key": "ref24",
"unstructured": "FDA, U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER). Assessing COVID-19-Related Symptoms in Outpatient Adult and Adolescent Subjects in Clinical Trials of Drugs and Biological Products for COVID-19 Prevention or Treatment Guidance for Industry. 2020. https://www.fda.gov/media/142143/download. Accessed 12 May 2021. 2020.",
"year": "2020"
},
{
"author": "Basu S",
"key": "ref25",
"unstructured": "Basu, S., et al., A model-based approach to improve intranasal sprays for respiratory viral infections. medRxiv, 2022: p. 2022.01.26.22269854.",
"year": "2022"
},
{
"DOI": "10.1038/s41598-021-85765-7",
"article-title": "Computational characterization of inhaled droplet transport to the nasopharynx",
"author": "Basu S",
"doi-asserted-by": "crossref",
"first-page": "6652",
"issue": "1",
"journal-title": "Sci Rep",
"key": "ref26",
"unstructured": "Basu, S., Computational characterization of inhaled droplet transport to the nasopharynx. Sci Rep, 2021. 11(1): p. 6652.",
"volume": "11",
"year": "2021"
},
{
"key": "ref27",
"unstructured": "U.S. Department of Health & Human Services, U.S.F.a.D.A., Guidance for industry. Allergic rhinitis: clinical development programs for drug products., U.S.F.a.D.A. U.S. Department of Health & Human Services, Editor. 2018.",
"volume-title": "Guidance for industry. Allergic rhinitis: clinical development programs for drug products.",
"year": "2018"
},
{
"DOI": "10.1111/j.1398-9995.2006.01276.x",
"article-title": "Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines",
"author": "Bousquet PJ",
"doi-asserted-by": "crossref",
"first-page": "367",
"issue": "4",
"journal-title": "Allergy",
"key": "ref28",
"unstructured": "Bousquet, P.J., et al., Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy, 2007. 62(4): p. 367–72.",
"volume": "62",
"year": "2007"
},
{
"article-title": "Chlorpheniramine Maleate Nasal Spray In COVID-19 Patients: Case Series",
"author": "Torres J",
"first-page": "3",
"issue": "2",
"journal-title": "J Clin Exp Pharmacol",
"key": "ref29",
"unstructured": "Torres, J., et al., Chlorpheniramine Maleate Nasal Spray In COVID-19 Patients: Case Series. J Clin Exp Pharmacol, 2021. 10(2): p. 3.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2020.01409",
"article-title": "Beyond Anti-viral Effects of Chloroquine/Hydroxychloroquine",
"author": "Gies V",
"doi-asserted-by": "crossref",
"first-page": "1409",
"journal-title": "Front Immunol",
"key": "ref30",
"unstructured": "Gies, V., et al., Beyond Anti-viral Effects of Chloroquine/Hydroxychloroquine. Front Immunol, 2020. 11: p. 1409.",
"volume": "11",
"year": "2020"
},
{
"article-title": "Sars-Cov-2 and risk of antiviral drug resistance",
"author": "Vitiello A",
"first-page": "1",
"journal-title": "Ir J Med Sci",
"key": "ref31",
"unstructured": "Vitiello, A., Sars-Cov-2 and risk of antiviral drug resistance. Ir J Med Sci, 2021: p. 1–2.",
"year": "2021"
},
{
"DOI": "10.1096/fj.202000502",
"article-title": "Existing bitter medicines for fighting 2019-nCoV-associated infectious diseases",
"author": "Li X",
"doi-asserted-by": "crossref",
"first-page": "6008",
"issue": "5",
"journal-title": "Faseb j",
"key": "ref32",
"unstructured": "Li, X., et al., Existing bitter medicines for fighting 2019-nCoV-associated infectious diseases. Faseb j, 2020. 34(5): p. 6008–6016.",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1002/lio2.26",
"article-title": "Taste Receptors: Regulators of Sinonasal Innate Immunity",
"author": "Carey RM",
"doi-asserted-by": "crossref",
"first-page": "88",
"issue": "4",
"journal-title": "Laryngoscope Investig Otolaryngol",
"key": "ref33",
"unstructured": "Carey, R.M., et al., Taste Receptors: Regulators of Sinonasal Innate Immunity. Laryngoscope Investig Otolaryngol, 2016. 1(4): p. 88–95.",
"volume": "1",
"year": "2016"
},
{
"article-title": "Bitter Taste Receptors (TAS2Rs) in Human Lung Macrophages: Receptor Expression and Inhibitory Effects of TAS2R Agonists",
"author": "Grassin-Delyle S",
"issue": "1267",
"journal-title": "Frontiers in Physiology",
"key": "ref34",
"unstructured": "Grassin-Delyle, S., et al., Bitter Taste Receptors (TAS2Rs) in Human Lung Macrophages: Receptor Expression and Inhibitory Effects of TAS2R Agonists. Frontiers in Physiology, 2019. 10(1267).",
"volume": "10",
"year": "2019"
},
{
"article-title": "A hypothesis: Bitter taste receptors as a therapeutic target for the clinical symptoms of SARS-CoV-2",
"author": "Kumar SA",
"first-page": "43",
"issue": "2",
"journal-title": "Pharmazie",
"key": "ref35",
"unstructured": "Kumar, S.A. and W. Cheng, A hypothesis: Bitter taste receptors as a therapeutic target for the clinical symptoms of SARS-CoV-2. Pharmazie, 2021. 76(2): p. 43–54.",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1016/j.ejphar.2004.11.006",
"article-title": "Comparative anticholinergic activities of 10 histamine H1 receptor antagonists in two functional models",
"author": "Orzechowski RF",
"doi-asserted-by": "crossref",
"first-page": "257",
"issue": "3",
"journal-title": "Eur J Pharmacol",
"key": "ref36",
"unstructured": "Orzechowski, R.F., D.S. Currie, and C.A. Valancius, Comparative anticholinergic activities of 10 histamine H1 receptor antagonists in two functional models. Eur J Pharmacol, 2005. 506(3): p. 257–64.",
"volume": "506",
"year": "2005"
},
{
"DOI": "10.1016/0091-6749(77)90177-4",
"article-title": "Bronchodilating activity of an H1 blocker, chlorpheniramine",
"author": "Popa VT",
"doi-asserted-by": "crossref",
"first-page": "54",
"issue": "1",
"journal-title": "J Allergy Clin Immunol",
"key": "ref37",
"unstructured": "Popa, V.T., Bronchodilating activity of an H1 blocker, chlorpheniramine. J Allergy Clin Immunol, 1977. 59(1): p. 54–63.",
"volume": "59",
"year": "1977"
},
{
"DOI": "10.2500/105065898781390271",
"article-title": "Anticholinergic properties of brompheniramine, chlorpheniramine, and atropine in human nasal mucosa in vitro",
"author": "Fang SY",
"doi-asserted-by": "crossref",
"first-page": "131",
"issue": "2",
"journal-title": "Am J Rhinol",
"key": "ref38",
"unstructured": "Fang, S.Y., H.M. Druce, and J.N. Baraniuk, Anticholinergic properties of brompheniramine, chlorpheniramine, and atropine in human nasal mucosa in vitro. Am J Rhinol, 1998. 12(2): p. 131–3.",
"volume": "12",
"year": "1998"
},
{
"DOI": "10.7150/ijms.21573",
"article-title": "Chlorpheniramine attenuates histamine-mediated aquaporin 5 downregulation in human nasal epithelial cells via suppression of NF-κB activation",
"author": "Chang YL",
"doi-asserted-by": "crossref",
"first-page": "1268",
"issue": "12",
"journal-title": "Int J Med Sci",
"key": "ref39",
"unstructured": "Chang, Y.L., et al., Chlorpheniramine attenuates histamine-mediated aquaporin 5 downregulation in human nasal epithelial cells via suppression of NF-κB activation. Int J Med Sci, 2017. 14(12): p. 1268–1275.",
"volume": "14",
"year": "2017"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.researchsquare.com/article/rs-2167465/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Chlorpheniramine Intranasal Spray to Accelerate COVID-19 Clinical Recovery in an Outpatient Setting: The ACCROS Trials",
"type": "posted-content"
}
valeriopascua3b
