Intranasal Chlorpheniramine Maleate for the treatment of COVID-19: Translational and Clinical Evidence
et al., Medical Research Archives, doi:10.18103/mra.v10i3.2752, Dec 2022
47th treatment shown to reduce risk in
December 2022, now with p < 0.00000000001 from 3 studies.
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Small RCT showing significantly improved recovery with intranasal chlorpheniramine maleate. Authors also perform an in vitro study showing efficacy with a highly differentiated three-dimensional model of normal, human-derived tracheal/bronchial epithelial cells.
3 preclinical studies support the efficacy of chlorpheniramine for COVID-19:
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments6.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers antihistamine H1RAs and chlorpheniramine.
|
risk of hospitalization, 87.4% lower, RR 0.13, p = 0.08, treatment 0 of 32 (0.0%), control 2 of 13 (15.4%), NNT 6.5, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Elshaier et al., Chlorpheniramine Maleate Displaying Multiple Modes of Antiviral Action Against SARS-CoV-2: An Initial Mechanistic Study, Cureus, doi:10.7759/cureus.92375.
2.
Sanchez-Gonzalez et al., Intranasal Chlorpheniramine Maleate for the treatment of COVID-19: Translational and Clinical Evidence, Medical Research Archives, doi:10.18103/mra.v10i3.2752.
3.
Valerio-Pascua et al., Mitigating the risks of post-acute sequelae of SARS-CoV-2 infection (PASC) with intranasal chlorpheniramine: perspectives from the ACCROS studies, BMC Infectious Diseases, doi:10.1186/s12879-024-10211-8.
4.
Valerio-Pascua (B) et al., Chlorpheniramine Intranasal Spray to Accelerate COVID-19 Clinical Recovery in an Outpatient Setting: The ACCROS Trials, Research Square, doi:10.21203/rs.3.rs-2167465/v1.
Sanchez-Gonzalez et al., 31 Dec 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, mean age 44.5, 5 authors.
Contact: gferrer@pulmonary-institute.com.
Intranasal Chlorpheniramine Maleate for the treatment of COVID-19: Translational and Clinical Evidence
Medical Research Archives, doi:10.18103/mra.v10i3.2752
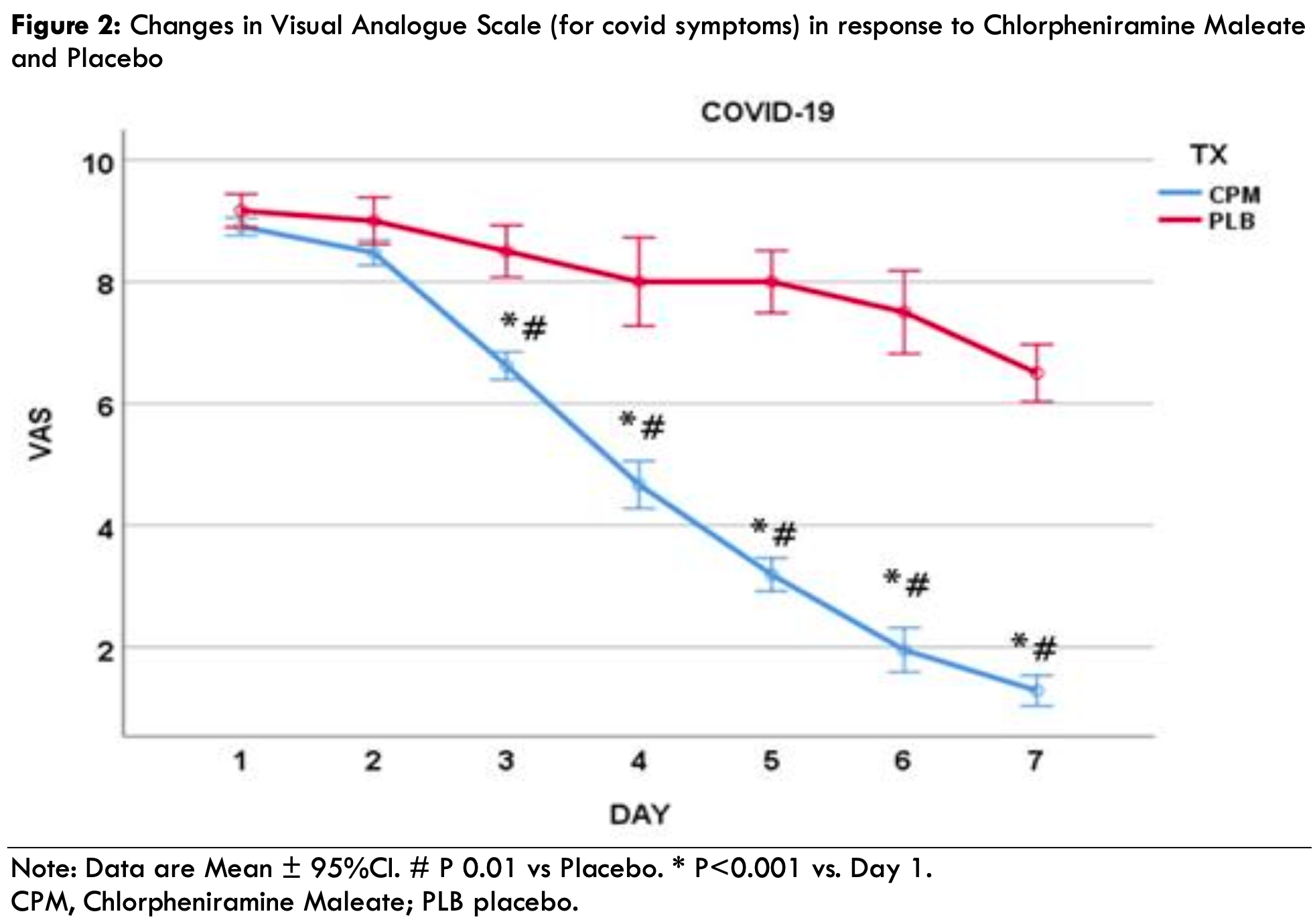
Recently, the nasal cavity has been highlighted as an ideal route of administration for interventions as it is the portal of entry of the severe acute respiratory syndrome coronavirus (SARS-CoV-2). The present study aimed to demonstrate the feasibility and efficacy of intranasally administered Chlorpheniramine Maleate (CPM) spray to treat coronavirus disease 2019 (COVID-19). Methods: The present study used a two-phase, non-clinical to clinical approach. The non-clinical phase evaluated CPM's antiviral activity against SARS-CoV-2 delta (B.1.617.2) strain via a highly differentiated three-dimensional in vitro model of normal, human-derived tracheal/bronchial epithelial cells. CPM was tested in duplicate inserts of the tissue models of the human airway. Virus yield reduction assays measured antiviral activity on day six after infection. For the clinical phase, COVID-19 symptomatic (polymerase chain reaction positive) patients were recruited and assigned to a 7-day CPM treatment (n=32) or placebo (PLB; n=13). Close safety monitoring of all patients was conducted before and after administering the drug. The primary outcomes monitored were time to symptom resolution (days), progression to hospitalization, emergency room visits, and symptoms of the severity of the disease using a visual analog scale (VAS) on a scale of 1-10 (no symptoms to worst symptoms). Results: The virus yielded a reduction in the assay such that the CPM solution log reduction value was 2.69 and Remdesivir 0.12, demonstrating much high antiviral activity of CPM. Results of the clinical phase demonstrate that VAS scores between the groups were evident after using CPM for two days (day 3). The CPM group VAS were significantly lower (P<0.001) starting from day three compared with day one. In contrast, there were no statistically significant (P>0.05) changes in the PLB during the 7-day treatment window. No subjects in the intervention group were hospitalized, while two in the PLB required hospitalization (15.4%; X2=5.15, P=0.023). Besides some mild discomfort felt by subjects immediately after applying the spray, the participants reported neither adverse reactions nor side effects.
Conclusion: If taken together, the results of the present two-phase study point towards the conclusion that CPM is an antiviral agent that can be administered intranasally to treat COVID-19 effectively.
References
Basu, Computational characterization of inhaled droplet transport to the nasopharynx, Sci Rep, doi:10.1038/s41598-021-85765-7
Basu, Holbrook, Kudlaty, Numerical evaluation of spray position for improved nasal drug delivery, Scientific Reports, doi:10.1038/s41598-020-66716-0
Black, Molecular Modeling and Preliminary Clinical Data Suggesting Antiviral Activity for Chlorpheniramine (Chlorphenamine) Against COVID-19, Cureus, doi:10.7759/cureus.20980
Blanco, Bonilla, Homma, Suzuki, Smith et al., Antihistamines and azithromycin as a treatment for COVID-19 on primary health care -A retrospective observational study in elderly patients, Pulm Pharmacol Ther, doi:10.1016/j.pupt.2021.101989
Branco, Yoshikawa, Pietrobon, Sato, Role of Histamine in Modulating the Immune Response and Inflammation, Mediators of Inflammation, doi:10.1155/2018/9524075
Doyle, Skoner, Gentile, Nasal cytokines as mediators of illness during the common cold, Current Allergy and Asthma Reports, doi:10.1007/s11882-005-0034-8
Ekins, Freundlich, Coffee, A common feature pharmacophore for FDA-approved drugs inhibiting the Ebola virus, Res, doi:10.12688/f1000research.5741
Eldanasory, Eljaaly, Memish, Tawfiq, Histamine release theory and roles of antihistamine in the treatment of cytokines storm of COVID-19, Travel Med Infect Dis, doi:10.1016/j.tmaid.2020.101874
Ferrer, Sanchez-Gonzalez, Effective Nasal Disinfection as an Overlooked Strategy in Our Fight against COVID-19, Ear Nose Throat J, doi:10.1177/01455613211002929
Galal, Hussein, Amin, Determinants of persistent post-COVID-19 symptoms: value of a novel COVID-19 symptom score, The Egyptian Journal of Bronchology, doi:10.1186/s43168-020-00049-4
Graham, Temple, Obar, Mast Cells and Influenza A Virus: Association with Allergic Responses and Beyond, Review. Frontiers in Immunology, doi:10.3389/fimmu.2015.00238
Hou, Okuda, Edwards, SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract, Cell, doi:10.1016/j.cell.2020.05.042
Jia, Look, Shi, ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia, J Virol, doi:10.1128/jvi.79.23.14614-14621.2005
Kirkegaard, Secher, Borum, Mygind, Inhibition of histamine-induced nasal symptoms by the H1 antihistamine chlorpheniramine maleate: demonstration of topical effect, Br J Dis Chest, doi:10.1016/0007-0971(83)90017-7
Kumar, Cheng, A hypothesis: Bitter taste receptors as a therapeutic target for the clinical symptoms of SARS-CoV-2, Pharmazie, doi:10.1691/ph.2021.0840
Li, Zhang, Liu, Gu, Existing bitter medicines for fighting 2019-nCoV-associated infectious diseases, Faseb j, doi:10.1096/fj.202000502
Long, Tang, Shi, Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections, Nat Med, doi:10.1038/s41591-020-0965-6
Mangal, Murari, Vashisht, Olfactory Dysfunction Among Asymptomatic Patients with SARS CoV2 Infection: A Case-Control Study, Indian J Otolaryngol Head Neck Surg, doi:10.1007/s12070-021-02366-6
Mostafa, Kandeil, Amme, FDA-Approved Drugs with Potent In Vitro Antiviral Activity against Severe Acute Respiratory Syndrome Coronavirus 2, Pharmaceuticals, doi:10.3390/ph13120443
Pilicheva, Boyuklieva, Can the Nasal Cavity Help Tackle COVID-19?, Pharmaceutics, doi:10.3390/pharmaceutics1310161
Qin, Zhou, Hu, Dysregulation of immune response in patients with COVID-19 in Wuhan, China, Clin Infect Dis, doi:10.1093/cid/ciaa248
Reed, Muench, A Simple method of estimating fifty per cent endpoints12, American Journal of Epidemiology, doi:10.1093/oxfordjournals.aje.a118408
Reznikov, Norris, Vashisht, Identification of antiviral antihistamines for COVID-19 repurposing, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2020.11.095
Sanchez-Gonzalez, Moskowitz, Issuree, Yatzkan, Rizvi et al., A Pathophysiological Perspective on COVID-19's Lethal Complication: From Viremia to Hypersensitivity Pneumonitis-like Immune Dysregulation, Infect Chemother
Sanchez-Gonzalez, Rizvi, Torres, Ferrer, A Randomized Controlled Pilot Trial to Test the Efficacy of Intranasal Chlorpheniramine Maleate With Xylitol for the Treatment of Allergic Rhinitis, Cureus, doi:10.7759/cureus.14206
Taha, Hall, Shortess, Rathbone, Barham, Treatment Protocol for COVID-19 Based on T2R Phenotype, Viruses, doi:10.3390/v13030503
Torres, Go, Camacho, Sanchez-Gonzalez, Ferrer, Chlorpheniramine Maleate Nasal Spray In COVID-19 Patients: Case Series, J Clin Exp Pharmacol, doi:10.35248/2161-1459.21.10.275
Van Toor, Buchwald, Stengele, Trenk, Gercek et al., Systemic bioavailability of nasally applied chlorphenamine maleate (0.4% nasal spray) relative to tablets administered perorally, International journal of clinical pharmacology and therapeutics, doi:10.5414/cpp39173
Westover, Ferrer, Vazquez, Bethencourt-Mirabal, Go, In Vitro Virucidal Effect of Intranasally Delivered Chlorpheniramine Maleate Compound Against Severe Acute Respiratory Syndrome Coronavirus 2, Cureus, doi:10.7759/cureus.10501
Wilson, Simpson, Ferreira, Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis, JCI Insight, doi:10.1172/jci.insight.140289
Wu, Liu, Sun, SARS-CoV-2triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury, Signal Transduction and Targeted Therapy, doi:10.1038/s41392-021-00849-0
Xu, Xia, Pu, The Antihistamine Drugs Carbinoxamine Maleate and Chlorpheniramine Maleate Exhibit Potent Antiviral Activity Against a Broad Spectrum of Influenza Viruses, Front Microbiol, doi:10.3389/fmicb.2018.02643
Yang, Pei, Li, Identification of SARS-CoV-2 entry inhibitors among already approved drugs, Acta Pharmacologica Sinica, doi:10.1038/s41401-020-00556-6
Zarkoob, Allué-Guardia, Chen, Modeling SARS-CoV-2 and Influenza Infections and Antiviral Treatments in Human Lung Epithelial Tissue Equivalents, bioRxiv, doi:10.1101/2021.05.11.443693
DOI record:
{
"DOI": "10.18103/mra.v10i3.2752",
"ISSN": [
"2375-1916",
"2375-1924"
],
"URL": "http://dx.doi.org/10.18103/mra.v10i3.2752",
"abstract": "<jats:p>Recently, the nasal cavity has been highlighted as an ideal route of administration for interventions as it is the portal of entry of the severe acute respiratory syndrome coronavirus (SARS-CoV-2). The present study aimed to demonstrate the feasibility and efficacy of intranasally administered Chlorpheniramine Maleate (CPM) spray to treat coronavirus disease 2019 (COVID-19). Methods: The present study used a two-phase, non-clinical to clinical approach. The non-clinical phase evaluated CPM’s antiviral activity against SARS-CoV-2 delta (B.1.617.2) strain via a highly differentiated three-dimensional in vitro model of normal, human-derived tracheal/bronchial epithelial cells. CPM was tested in duplicate inserts of the tissue models of the human airway. Virus yield reduction assays measured antiviral activity on day six after infection. For the clinical phase, COVID-19 symptomatic (polymerase chain reaction positive) patients were recruited and assigned to a 7-day CPM treatment (n=32) or placebo (PLB; n=13). Close safety monitoring of all patients was conducted before and after administering the drug. The primary outcomes monitored were time to symptom resolution (days), progression to hospitalization, emergency room visits, and symptoms of the severity of the disease using a visual analog scale (VAS) on a scale of 1-10 (no symptoms to worst symptoms). Results: The virus yielded a reduction in the assay such that the CPM solution log reduction value was 2.69 and Remdesivir 0.12, demonstrating much high antiviral activity of CPM. Results of the clinical phase demonstrate that VAS scores between the groups were evident after using CPM for two days (day 3). The CPM group VAS were significantly lower (P<0.001) starting from day three compared with day one. In contrast, there were no statistically significant (P>0.05) changes in the PLB during the 7-day treatment window. No subjects in the intervention group were hospitalized, while two in the PLB required hospitalization (15.4%; X2=5.15, P=0.023). Besides some mild discomfort felt by subjects immediately after applying the spray, the participants reported neither adverse reactions nor side effects. Conclusion: If taken together, the results of the present two-phase study point towards the conclusion that CPM is an antiviral agent that can be administered intranasally to treat COVID-19 effectively.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Sanchez-Gonzalez",
"given": "Marcos",
"sequence": "first"
},
{
"affiliation": [],
"family": "Westover",
"given": "Jonna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rizvi",
"given": "Syed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Torres",
"given": "Joselit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferrer",
"given": "Gustavo",
"sequence": "additional"
}
],
"container-title": "Medical Research Archives",
"container-title-short": "MRAJ",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
4,
2
]
],
"date-time": "2022-04-02T04:22:10Z",
"timestamp": 1648873330000
},
"deposited": {
"date-parts": [
[
2022,
4,
2
]
],
"date-time": "2022-04-02T04:22:34Z",
"timestamp": 1648873354000
},
"indexed": {
"date-parts": [
[
2024,
2,
15
]
],
"date-time": "2024-02-15T12:01:00Z",
"timestamp": 1707998460658
},
"is-referenced-by-count": 4,
"issue": "3",
"issued": {
"date-parts": [
[
2022
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2022
]
]
},
"published-print": {
"date-parts": [
[
2022
]
]
}
},
"member": "7483",
"original-title": [],
"prefix": "10.18103",
"published": {
"date-parts": [
[
2022
]
]
},
"published-online": {
"date-parts": [
[
2022
]
]
},
"published-print": {
"date-parts": [
[
2022
]
]
},
"publisher": "Knowledge Enterprise Journals",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://esmed.org/MRA/index.php/mra/article/view/2752"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Intranasal Chlorpheniramine Maleate for the treatment of COVID-19: Translational and Clinical Evidence",
"type": "journal-article",
"volume": "10"
}
sanchezgonzalez2
