Molecular Modeling and Preliminary Clinical Data Suggesting Antiviral Activity for Chlorpheniramine (Chlorphenamine) Against COVID-19
, S., Cureus, doi:10.7759/cureus.20980, Jan 2022
11th treatment shown to reduce risk in
December 2020, now with p = 0.000052 from 17 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
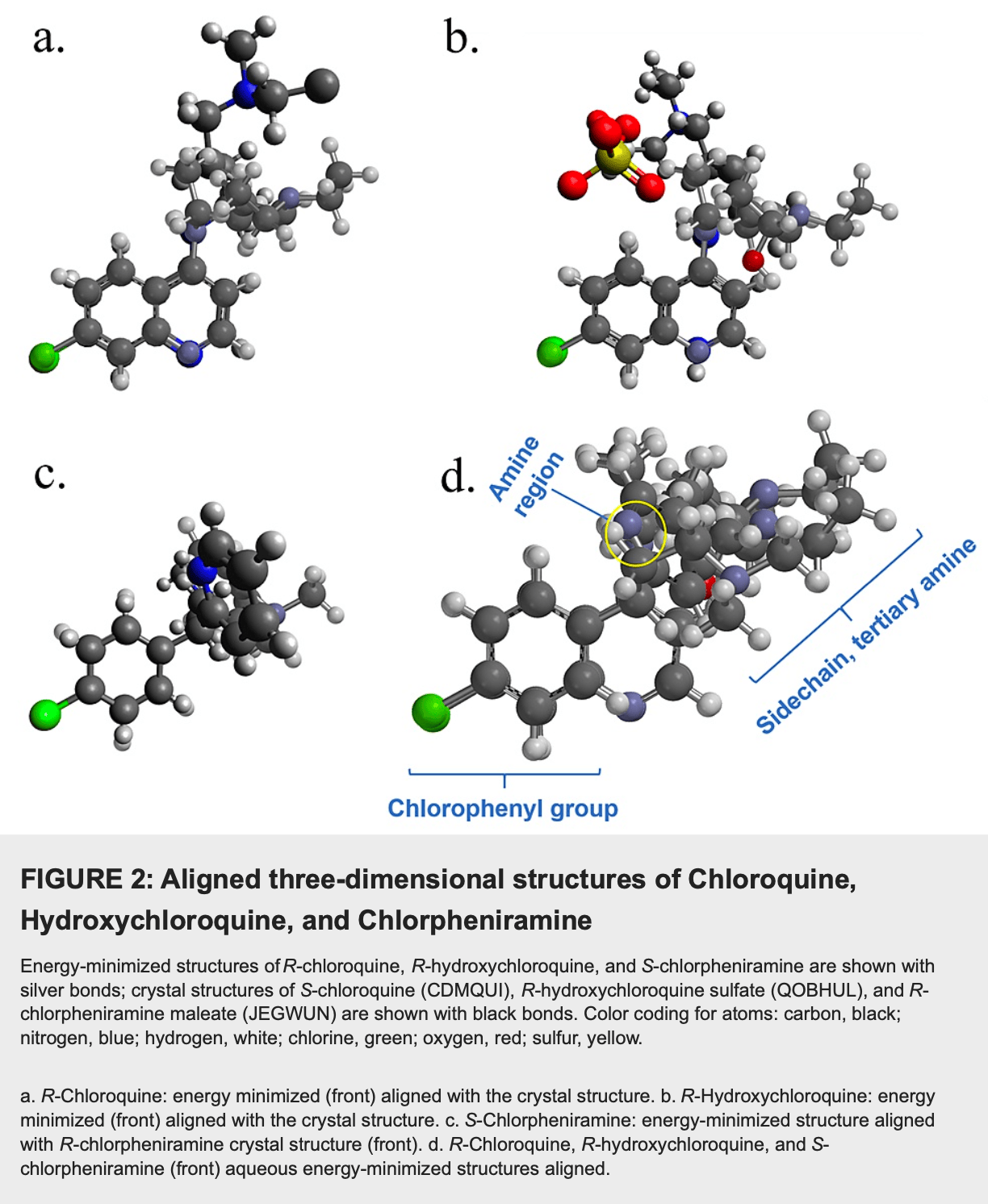
In silico study suggesting that the over-the-counter antihistamine chlorpheniramine may have antiviral activity against SARS-CoV-2. Using structural database searches and molecular modeling, authors found that chlorpheniramine shares similar structural features with chloroquine and hydroxychloroquine, including a chlorophenyl group, pyridine ring, alkyl sidechain, and terminal tertiary amine. Energy-minimized three-dimensional structures showed significant similarity between the drugs. In a preliminary retrospective study of 13 participants who took chlorpheniramine preventively or post-exposure, none were hospitalized or died from COVID-19. Authors propose that chlorpheniramine may prevent SARS-CoV-2 entry into cells in a similar manner as chloroquine and hydroxychloroquine.
13 preclinical studies support the efficacy of antihistamine H1RAs for COVID-19:
1.
Hamdan et al., In silico Evaluation of H1-Antihistamine as Potential Inhibitors of SARS-CoV-2 RNA-dependent RNA Polymerase: Repurposing Study of COVID-19 Therapy, Turkish Journal of Pharmaceutical Sciences, doi:10.4274/tjps.galenos.2024.49768.
2.
Elshaier et al., Chlorpheniramine Maleate Displaying Multiple Modes of Antiviral Action Against SARS-CoV-2: An Initial Mechanistic Study, Cureus, doi:10.7759/cureus.92375.
3.
Black, S., Molecular Modeling and Preliminary Clinical Data Suggesting Antiviral Activity for Chlorpheniramine (Chlorphenamine) Against COVID-19, Cureus, doi:10.7759/cureus.20980.
4.
Hou et al., Testing of the inhibitory effects of loratadine and desloratadine on SARS-CoV-2 spike pseudotyped virus viropexis, Chemico-Biological Interactions, doi:10.1016/j.cbi.2021.109420.
5.
Yu et al., The histamine receptor H1 acts as an alternative receptor for SARS-CoV-2, mBio, doi:10.1128/mbio.01088-24.
6.
Sanchez-Gonzalez et al., Intranasal Chlorpheniramine Maleate for the treatment of COVID-19: Translational and Clinical Evidence, Medical Research Archives, doi:10.18103/mra.v10i3.2752.
7.
Morin-Dewaele et al., Desloratadine, an FDA-approved cationic amphiphilic drug, inhibits SARS-CoV-2 infection in cell culture and primary human nasal epithelial cells by blocking viral entry, Scientific Reports, doi:10.1038/s41598-022-25399-5.
8.
Reznikov et al., Identification of antiviral antihistamines for COVID-19 repurposing, Biochemical and Biophysical Research Communications, doi:10.1016/j.bbrc.2020.11.095.
Black et al., 6 Jan 2022, peer-reviewed, 1 author.
Contact: sblack@uttyler.edu.
In silico studies are an important part of preclinical research, however results may be very different in vivo.
Molecular Modeling and Preliminary Clinical Data Suggesting Antiviral Activity for Chlorpheniramine (Chlorphenamine) Against COVID-19
Cureus, doi:10.7759/cureus.20980
Chlorpheniramine maleate, a widely used over-the-counter antihistamine, has been identified as a structural analog of aminoquinolines known to possess antiviral activity against the Betacoronavirus severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19). Structural similarities include the chlorophenyl group, pyridine ring, alkyl sidechain, and terminal tertiary amine; the comparison of aqueous energy-minimized structures indicates significant three-dimensional similarity as well. Preliminary clinical evidence supports these conclusions. The present study suggests that chlorpheniramine possesses antiviral activity against COVID-19.
Additional Information Disclosures Human subjects: Consent was obtained or waived by all participants in this study. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References
Bienvenu, Marty, Jones, Picot, Systematic review of registered trials of hydroxychloroquine prophylaxis for COVID-19 health-care workers at the first third of 2020, One Health, doi:10.1016/j.onehlt.2020.100141
Bolton, Chen, Kim, PubChem3D: a new resource for scientists, J Cheminform, doi:10.1186/1758-2946-3-32
Booth, Moniri, Bakker, Choksi, Nix et al., A novel phenylaminotetralin radioligand reveals a subpopulation of histamine H(1) receptors, J Pharmacol Exp Ther, doi:10.1124/jpet.302.1.328
Courseille, Busetta, Hospital M: 7-Chloro-4(4-diethylamino-1-methylbutyl-amino) quinoline, C18H26ClN3 (Chloroquine Base), Cryst Struct Commun
De Oliveira, Rocha, Paluch, Costa, Repurposing approved drugs as inhibitors of SARS-CoV-2 Sprotein from molecular modeling and virtual screening, J Biomol Struct Dyn, doi:10.1080/07391102.2020.1772885
Dhama, Khan, Tiwari, Coronavirus disease 2019-COVID-19, Clin Microbiol Rev, doi:10.1128/CMR.00028-20
Drosten, Günther, Preiser, Identification of a novel coronavirus in patients with severe acute respiratory syndrome, N Engl J Med, doi:10.1056/NEJMoa030747
Dyall, Coleman, Hart, Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection, Antimicrob Agents Chemother, doi:10.1128/AAC.03036-14
Ekins, Coffee, FDA approved drugs as potential Ebola treatments, F1000Res, doi:10.12688/f1000research.6164.2
Ekins, Freundlich, Coffee, A common feature pharmacophore for FDA-approved drugs inhibiting the Ebola virus, F1000Res, doi:10.12688/f1000research.5741.2
Gordon, Jang, Bouhaddou, A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature, doi:10.1038/s41586-020-2286-9
Horby, Mafham, Linsell, Effect of hydroxychloroquine in hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2022926
James, Williams, Structural studies of histamine H1 effector molecules: the crystal structure of the antihistaminic drug (+)-Chlorpheniramine Maleate; [(+)-S-1-(p-Chlorophenyl)-1-(2-pyridyl)-3-NNdimethylpropylamine Maleate, Can J Chem, doi:10.1139/v74-267
Macrae, Sovago, Cottrell, Mercury 4.0: from visualization to analysis, design and prediction, J Appl Crystallogr, doi:10.1107/S1600576719014092
Nathan, Papa, Schwenk, None, U.S. Patent
Olliaro, Torreele, Vaillant, COVID-19 vaccine efficacy and effectiveness-the elephant (not) in the room, Lancet Microbe, doi:10.1016/S2666-5247(21)00069-0
Parvez, Structure of an anhistaminic drug, racemic chlorpheniramine hydrogen maleate, Acta Cryst, doi:10.1107/S0108270189013958
Patil, Singhal, Masand, A systematic review on use of aminoquinolines for the therapeutic management of COVID-19: Efficacy, safety and clinical trials, Life Sci, doi:10.1016/j.lfs.2020.117775
Petersen, Koopmans, Go, Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30484-9
Planas, Veyer, Baidaliuk, Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization, Nature, doi:10.1038/s41586-021-03777-9
Pooladanda, Thatikonda, Godugu, The current understanding and potential therapeutic options to combat COVID-19, Life Sci, doi:10.1016/j.lfs.2020.117765
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review, JAMA, doi:10.1001/jama.2020.6019
Sandor, Sturdivant, Ting, Influenza virus and SARS-CoV-2 vaccines, J Immunol, doi:10.4049/jimmunol.2001287
Semeniuk, Kalinowska-Tluscik, Nitek, Oleksyn, Intermolecular interactions in crystalline hydroxychloroquine sulfate in comparison with those in selected antimalarial drugs, J Chem Crystallogr, doi:10.1007/s10870-008-9327-9
Serre, Buob, Boffa, Hydroxychloroquine-induced podocytopathy mimicking Fabry disease, BMJ Case Rep, doi:10.1136/bcr-2018-228876
Shanmugam, Muralidharan, Velmurugan, Gromiha, Therapeutic targets and computational approaches on drug development for COVID-19, Curr Top Med Chem, doi:10.2174/1568026620666200710105507
Singh, Gupta, SARS-CoV-2 therapeutics: how far do we stand from a remedy?, Pharmacol Rep, doi:10.1007/s43440-020-00204-0
Sinha, Dola, Soni, Synthesis of chiral chloroquine and its analogues as antimalarial agents, Bioorg Med Chem, doi:10.1016/j.bmc.2014.09.009
Tambyah, SARS: responding to an unknown virus, Eur J Clin Microbiol Infect Dis, doi:10.1007/s10096-004-1175-8
Tazikeh-Lemeski, Moradi, Raoufi, Shahlaei, Janlou et al., Targeting SARS-COV-2 nonstructural protein 16: a virtual drug repurposing study, J Biomol Struct Dyn, doi:10.1080/07391102.2020.1779133
Tse, Meganck, Graham, Baric, The current and future state of vaccines, antivirals and gene therapies against emerging coronaviruses, Front Microbiol, doi:10.3389/fmicb.2020.00658
Wang, Fast identification of possible drug treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study, J Chem Inf Model, doi:10.1021/acs.jcim.0c00179
Westover, Ferrer, Vazquez, Bethencourt-Mirabal, Go, In vitro virucidal effect of intranasally delivered chlorpheniramine maleate compound against severe acute respiratory syndrome coronavirus 2, Cureus, doi:10.7759/cureus.10501
Wikipedia, List of COVID-19 Vaccine Authorizations
Wishart, Feunang, Guo, DrugBank 5.0: a major update to the DrugBank database for 2018, Nucleic Acids Res, doi:10.1093/nar/gkx1037
Wolber, Langer, LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters, J Chem Inf Model, doi:10.1021/ci049885e
Wong, Li, Lau, Woo, Global Epidemiology of Bat Coronaviruses, Viruses, doi:10.3390/v11020174
Xu, Xia, Pu, Wang, Li et al., The antihistamine drugs carbinoxamine maleate and chlorpheniramine maleate exhibit potent antiviral activity against a broad spectrum of influenza viruses, Front Microbiol, doi:10.3389/fmicb.2018.02643
Zumla, Hui, Perlman, Middle East respiratory syndrome, Lancet, doi:10.1016/S0140-6736(15)60454-8
DOI record:
{
"DOI": "10.7759/cureus.20980",
"ISSN": [
"2168-8184"
],
"URL": "http://dx.doi.org/10.7759/cureus.20980",
"author": [
{
"affiliation": [],
"family": "Black",
"given": "Shaun D",
"sequence": "first"
}
],
"container-title": [
"Cureus"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
6
]
],
"date-time": "2022-01-06T08:58:25Z",
"timestamp": 1641459505000
},
"deposited": {
"date-parts": [
[
2022,
1,
6
]
],
"date-time": "2022-01-06T08:58:35Z",
"timestamp": 1641459515000
},
"indexed": {
"date-parts": [
[
2022,
1,
7
]
],
"date-time": "2022-01-07T06:14:51Z",
"timestamp": 1641536091266
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "2168-8184"
}
],
"issued": {
"date-parts": [
[
2022,
1,
6
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.cureus.com/articles/81059-molecular-modeling-and-preliminary-clinical-data-suggesting-antiviral-activity-for-chlorpheniramine-chlorphenamine-against-covid-19",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "4492",
"original-title": [],
"prefix": "10.7759",
"published": {
"date-parts": [
[
2022,
1,
6
]
]
},
"published-print": {
"date-parts": [
[
2022,
1,
6
]
]
},
"publisher": "Cureus, Inc.",
"reference": [
{
"key": "ref1",
"unstructured": "Johns Hopkins Coronavirus Resource Center Dashboard. (2021). Accessed. 12/15/2021: https://coronavirus.jhu.edu/map.html."
},
{
"DOI": "10.1128/CMR.00028-20",
"article-title": "Coronavirus disease 2019-COVID-19",
"author": "Dhama K",
"doi-asserted-by": "publisher",
"journal-title": "Clin Microbiol Rev",
"key": "ref2",
"unstructured": "Dhama K, Khan S, Tiwari R, et al.. Coronavirus disease 2019-COVID-19. Clin Microbiol Rev. 2020, 33:10.1128/CMR.00028-20",
"volume": "33",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa030747",
"article-title": "Identification of a novel coronavirus in patients with severe acute respiratory syndrome",
"author": "Drosten C",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref3",
"unstructured": "Drosten C, Günther S, Preiser W, et al.. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003, 348:1967-76. 10.1056/NEJMoa030747",
"volume": "348",
"year": "2003"
},
{
"DOI": "10.1007/s10096-004-1175-8",
"article-title": "SARS: responding to an unknown virus",
"author": "Tambyah PA",
"doi-asserted-by": "publisher",
"journal-title": "Eur J Clin Microbiol Infect Dis",
"key": "ref4",
"unstructured": "Tambyah PA. SARS: responding to an unknown virus. Eur J Clin Microbiol Infect Dis. 2004, 23:589-95. 10.1007/s10096-004-1175-8",
"volume": "23",
"year": "2004"
},
{
"DOI": "10.1016/S1473-3099(20)30484-9",
"article-title": "Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics",
"author": "Petersen E",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Infect Dis",
"key": "ref5",
"unstructured": "Petersen E, Koopmans M, Go U, et al.. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020, 20:e238-44. 10.1016/S1473-3099(20)30484-9",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.3390/v11020174",
"article-title": "Global Epidemiology of Bat Coronaviruses",
"author": "Wong AC",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "ref6",
"unstructured": "Wong AC, Li X, Lau SK, Woo PC. Global Epidemiology of Bat Coronaviruses. Viruses. 2019, 11:10.3390/v11020174",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1016/S0140-6736(15)60454-8",
"article-title": "Middle East respiratory syndrome",
"author": "Zumla A",
"doi-asserted-by": "publisher",
"journal-title": "Lancet",
"key": "ref7",
"unstructured": "Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015, 386:995-1007. 10.1016/S0140-6736(15)60454-8",
"volume": "386",
"year": "2015"
},
{
"key": "ref8",
"unstructured": "World Health Organization, MERS-CoV Cases and Mortality. (2021). Accessed. 12/15/2021: http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html."
},
{
"key": "ref9",
"unstructured": "World Health Organization, Information on SARS-CoV-1. (2021). Accessed. 12/15/2021. https://www.who.int/health-topics/severe-acute-respiratory-syndrome."
},
{
"DOI": "10.1038/s41586-021-03777-9",
"article-title": "Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization",
"author": "Planas D",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "ref10",
"unstructured": "Planas D, Veyer D, Baidaliuk A, et al.. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021, 596:276-80. 10.1038/s41586-021-03777-9",
"volume": "596",
"year": "2021"
},
{
"DOI": "10.4049/jimmunol.2001287",
"article-title": "Influenza virus and SARS-CoV-2 vaccines",
"author": "Sandor AM",
"doi-asserted-by": "publisher",
"journal-title": "J Immunol",
"key": "ref11",
"unstructured": "Sandor AM, Sturdivant MS, Ting JP. Influenza virus and SARS-CoV-2 vaccines. J Immunol. 2021, 206:2509-20. 10.4049/jimmunol.2001287",
"volume": "206",
"year": "2021"
},
{
"key": "ref12",
"unstructured": "Wikipedia, List of COVID-19 Vaccine Authorizations. (2021). Accessed. 12/15/2021: https://en.wikipedia.org/wiki/List_of_COVID-19_vaccine_authorizations."
},
{
"DOI": "10.1016/S2666-5247(21)00069-0",
"article-title": "COVID-19 vaccine efficacy and effectiveness-the elephant (not) in the room",
"author": "Olliaro P",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Microbe",
"key": "ref13",
"unstructured": "Olliaro P, Torreele E, Vaillant M. COVID-19 vaccine efficacy and effectiveness-the elephant (not) in the room. Lancet Microbe. 2021, 2:e279-80. 10.1016/S2666-5247(21)00069-0",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"article-title": "A SARS-CoV-2 protein interaction map reveals targets for drug repurposing",
"author": "Gordon DE",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "ref14",
"unstructured": "Gordon DE, Jang GM, Bouhaddou M, et al.. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020, 583:459-68. 10.1038/s41586-020-2286-9",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1016/j.lfs.2020.117765",
"article-title": "The current understanding and potential therapeutic options to combat COVID-19",
"author": "Pooladanda V",
"doi-asserted-by": "publisher",
"journal-title": "Life Sci",
"key": "ref15",
"unstructured": "Pooladanda V, Thatikonda S, Godugu C. The current understanding and potential therapeutic options to combat COVID-19. Life Sci. 2020, 254:117765. 10.1016/j.lfs.2020.117765",
"volume": "254",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6019",
"article-title": "Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review",
"author": "Sanders JM",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "ref16",
"unstructured": "Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020, 323:1824-36. 10.1001/jama.2020.6019",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.3389/fmicb.2020.00658",
"article-title": "The current and future state of vaccines, antivirals and gene therapies against emerging coronaviruses",
"author": "Tse LV",
"doi-asserted-by": "publisher",
"journal-title": "Front Microbiol",
"key": "ref17",
"unstructured": "Tse LV, Meganck RM, Graham RL, Baric RS. The current and future state of vaccines, antivirals and gene therapies against emerging coronaviruses. Front Microbiol. 2020, 11:658. 10.3389/fmicb.2020.00658",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1021/acs.jcim.0c00179",
"article-title": "Fast identification of possible drug treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study",
"author": "Wang J",
"doi-asserted-by": "publisher",
"journal-title": "J Chem Inf Model",
"key": "ref18",
"unstructured": "Wang J. Fast identification of possible drug treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study. J Chem Inf Model. 2020, 60:3277-86. 10.1021/acs.jcim.0c00179",
"volume": "60",
"year": "2020"
},
{
"DOI": "10.1007/s43440-020-00204-0",
"article-title": "SARS-CoV-2 therapeutics: how far do we stand from a remedy?",
"author": "Singh A",
"doi-asserted-by": "publisher",
"journal-title": "Pharmacol Rep",
"key": "ref19",
"unstructured": "Singh A, Gupta V. SARS-CoV-2 therapeutics: how far do we stand from a remedy?. Pharmacol Rep. 2021, 73:750-68. 10.1007/s43440-020-00204-0",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.2174/1568026620666200710105507",
"article-title": "Therapeutic targets and computational approaches on drug development for COVID-19",
"author": "Shanmugam A",
"doi-asserted-by": "publisher",
"journal-title": "Curr Top Med Chem",
"key": "ref20",
"unstructured": "Shanmugam A, Muralidharan N, Velmurugan D, Gromiha MM. Therapeutic targets and computational approaches on drug development for COVID-19. Curr Top Med Chem. 2020, 20:2210-20. 10.2174/1568026620666200710105507",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1080/07391102.2020.1772885",
"article-title": "Repurposing approved drugs as inhibitors of SARS-CoV-2 S-protein from molecular modeling and virtual screening",
"author": "de Oliveira OV",
"doi-asserted-by": "publisher",
"journal-title": "J Biomol Struct Dyn",
"key": "ref21",
"unstructured": "de Oliveira OV, Rocha GB, Paluch AS, Costa LT. Repurposing approved drugs as inhibitors of SARS-CoV-2 S-protein from molecular modeling and virtual screening. J Biomol Struct Dyn. 2021, 39:3924-33. 10.1080/07391102.2020.1772885",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.1080/07391102.2020.1779133",
"article-title": "Targeting SARS-COV-2 non-structural protein 16: a virtual drug repurposing study",
"author": "Tazikeh-Lemeski E",
"doi-asserted-by": "publisher",
"journal-title": "J Biomol Struct Dyn",
"key": "ref22",
"unstructured": "Tazikeh-Lemeski E, Moradi S, Raoufi R, Shahlaei M, Janlou MA, Zolghadri S. Targeting SARS-COV-2 non-structural protein 16: a virtual drug repurposing study. J Biomol Struct Dyn. 2021, 39:4633-46. 10.1080/07391102.2020.1779133",
"volume": "39",
"year": "2021"
},
{
"key": "ref23",
"unstructured": "COVID-19 Treatment - Analysis of 302 Global Studies Showing High Effectiveness for Early Treatment. (2021). Accessed. 12/15/2021: https://c19study.com."
},
{
"DOI": "10.1016/j.lfs.2020.117775",
"article-title": "A systematic review on use of aminoquinolines for the therapeutic management of COVID-19: Efficacy, safety and clinical trials",
"author": "Patil VM",
"doi-asserted-by": "publisher",
"journal-title": "Life Sci",
"key": "ref24",
"unstructured": "Patil VM, Singhal S, Masand N. A systematic review on use of aminoquinolines for the therapeutic management of COVID-19: Efficacy, safety and clinical trials. Life Sci. 2020, 254:117775. 10.1016/j.lfs.2020.117775",
"volume": "254",
"year": "2020"
},
{
"DOI": "10.1016/j.onehlt.2020.100141",
"article-title": "Systematic review of registered trials of hydroxychloroquine prophylaxis for COVID-19 health-care workers at the first third of 2020",
"author": "Bienvenu AL",
"doi-asserted-by": "publisher",
"journal-title": "One Health",
"key": "ref25",
"unstructured": "Bienvenu AL, Marty AM, Jones MK, Picot S. Systematic review of registered trials of hydroxychloroquine prophylaxis for COVID-19 health-care workers at the first third of 2020. One Health. 2020, 10:100141. 10.1016/j.onehlt.2020.100141",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2022926",
"article-title": "Effect of hydroxychloroquine in hospitalized patients with Covid-19",
"author": "Horby P",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref26",
"unstructured": "Horby P, Mafham M, Linsell L, et al.. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020, 383:2030-40. 10.1056/NEJMoa2022926",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1136/bcr-2018-228876",
"article-title": "Hydroxychloroquine-induced podocytopathy mimicking Fabry disease",
"author": "Serre J",
"doi-asserted-by": "publisher",
"journal-title": "BMJ Case Rep",
"key": "ref27",
"unstructured": "Serre J, Buob D, Boffa JJ. Hydroxychloroquine-induced podocytopathy mimicking Fabry disease. BMJ Case Rep. 2019, 12:10.1136/bcr-2018-228876",
"volume": "12",
"year": "2019"
},
{
"article-title": "",
"author": "Nathan S",
"key": "ref28",
"unstructured": "Nathan S, Papa D, Schwenk E. U.S. Patent 2,567,245. 1951. https://patents.google.com/patent/US2567245A/en.",
"year": "1951"
},
{
"DOI": "10.12688/f1000research.6164.2",
"article-title": "FDA approved drugs as potential Ebola treatments",
"author": "Ekins S",
"doi-asserted-by": "publisher",
"journal-title": "F1000Res",
"key": "ref29",
"unstructured": "Ekins S, Coffee M. FDA approved drugs as potential Ebola treatments. F1000Res. 2015, 4:48. 10.12688/f1000research.6164.2",
"volume": "4",
"year": "2015"
},
{
"DOI": "10.3389/fmicb.2018.02643",
"article-title": "The antihistamine drugs carbinoxamine maleate and chlorpheniramine maleate exhibit potent antiviral activity against a broad spectrum of influenza viruses",
"author": "Xu W",
"doi-asserted-by": "publisher",
"journal-title": "Front Microbiol",
"key": "ref30",
"unstructured": "Xu W, Xia S, Pu J, Wang Q, Li P, Lu L, Jiang S. The antihistamine drugs carbinoxamine maleate and chlorpheniramine maleate exhibit potent antiviral activity against a broad spectrum of influenza viruses. Front Microbiol. 2018, 9:2643. 10.3389/fmicb.2018.02643",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1128/AAC.03036-14",
"article-title": "Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection",
"author": "Dyall J",
"doi-asserted-by": "publisher",
"journal-title": "Antimicrob Agents Chemother",
"key": "ref31",
"unstructured": "Dyall J, Coleman CM, Hart BJ, et al.. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014, 58:4885-93. 10.1128/AAC.03036-14",
"volume": "58",
"year": "2014"
},
{
"DOI": "10.1093/nar/gkx1037",
"article-title": "DrugBank 5.0: a major update to the DrugBank database for 2018",
"author": "Wishart DS",
"doi-asserted-by": "publisher",
"journal-title": "Nucleic Acids Res",
"key": "ref32",
"unstructured": "Wishart DS, Feunang YD, Guo AC, et al.. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46:D1074-82. 10.1093/nar/gkx1037",
"volume": "46",
"year": "2018"
},
{
"key": "ref33",
"unstructured": "United States Food and Drug Administration, Animal Drugs@FDA. (2021). Accessed. 12/15/2021: https://animaldrugsatfda.fda.gov/adafda/views/#/search."
},
{
"key": "ref34",
"unstructured": "Wavefunction Spartan software. (2021). Accessed. 2021: http://www.wavefun.com."
},
{
"key": "ref35",
"unstructured": "Avogadro Software. (2021). Accessed. 2021: http://avogadro.cc/."
},
{
"DOI": "10.1186/1758-2946-3-32",
"article-title": "PubChem3D: a new resource for scientists",
"author": "Bolton EE",
"doi-asserted-by": "publisher",
"journal-title": "J Cheminform",
"key": "ref36",
"unstructured": "Bolton EE, Chen J, Kim S, et al.. PubChem3D: a new resource for scientists. J Cheminform. 2011, 3:32. 10.1186/1758-2946-3-32",
"volume": "3",
"year": "2011"
},
{
"DOI": "10.1021/ci049885e",
"article-title": "LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters",
"author": "Wolber G",
"doi-asserted-by": "publisher",
"journal-title": "J Chem Inf Model",
"key": "ref37",
"unstructured": "Wolber G, Langer T. LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J Chem Inf Model. 2005, 45:160-9. 10.1021/ci049885e",
"volume": "45",
"year": "2005"
},
{
"DOI": "10.1107/S1600576719014092",
"article-title": "Mercury 4.0: from visualization to analysis, design and prediction",
"author": "Macrae CF",
"doi-asserted-by": "publisher",
"journal-title": "J Appl Crystallogr",
"key": "ref38",
"unstructured": "Macrae CF, Sovago I, Cottrell SJ, et al.. Mercury 4.0: from visualization to analysis, design and prediction. J Appl Crystallogr. 2020, 53:226-35. 10.1107/S1600576719014092",
"volume": "53",
"year": "2020"
},
{
"DOI": "10.1124/jpet.302.1.328",
"article-title": "A novel phenylaminotetralin radioligand reveals a subpopulation of histamine H(1) receptors",
"author": "Booth RG",
"doi-asserted-by": "publisher",
"journal-title": "J Pharmacol Exp Ther",
"key": "ref39",
"unstructured": "Booth RG, Moniri NH, Bakker RA, Choksi NY, Nix WB, Timmerman H, Leurs R. A novel phenylaminotetralin radioligand reveals a subpopulation of histamine H(1) receptors. J Pharmacol Exp Ther. 2002, 302:328-36. 10.1124/jpet.302.1.328",
"volume": "302",
"year": "2002"
},
{
"DOI": "10.1016/j.bmc.2014.09.009",
"article-title": "Synthesis of chiral chloroquine and its analogues as antimalarial agents",
"author": "Sinha M",
"doi-asserted-by": "publisher",
"journal-title": "Bioorg Med Chem",
"key": "ref40",
"unstructured": "Sinha M, Dola VR, Soni A, et al.. Synthesis of chiral chloroquine and its analogues as antimalarial agents. Bioorg Med Chem. 2014, 22:5950-60. 10.1016/j.bmc.2014.09.009",
"volume": "22",
"year": "2014"
},
{
"DOI": "10.12688/f1000research.5741.2",
"article-title": "A common feature pharmacophore for FDA-approved drugs inhibiting the Ebola virus",
"author": "Ekins S",
"doi-asserted-by": "publisher",
"journal-title": "F1000Res",
"key": "ref41",
"unstructured": "Ekins S, Freundlich JS, Coffee M. A common feature pharmacophore for FDA-approved drugs inhibiting the Ebola virus. F1000Res. 2014, 3:277. 10.12688/f1000research.5741.2",
"volume": "3",
"year": "2014"
},
{
"key": "ref42",
"unstructured": "Cambridge Structural Database (CSD), Chloroquine (CDMQUI). (2021). Accessed. 12/15/2021: https://www.ccdc.cam.ac.uk/structures/Search?Compound=Chloroquine&DatabaseToSearch=Published."
},
{
"article-title": "Structural studies of histamine H1 effector molecules: the crystal structure of the antihistaminic drug (+)-Chlorpheniramine Maleate; [(+)-S-1-(p-Chlorophenyl)-1-(2-pyridyl)-3-NN-dimethylpropylamine Maleate]",
"author": "James MNG",
"journal-title": "Can J Chem",
"key": "ref43",
"unstructured": "James MNG, Williams GJB. Structural studies of histamine H1 effector molecules: the crystal structure of the antihistaminic drug (+)-Chlorpheniramine Maleate; [(+)-S-1-(p-Chlorophenyl)-1-(2-pyridyl)-3-NN-dimethylpropylamine Maleate]. Can J Chem. 1974, 52:1872-9.",
"volume": "52",
"year": "1974"
},
{
"article-title": "7-Chloro-4(4-diethylamino-1-methylbutyl-amino) quinoline, C18H26ClN3 (Chloroquine Base)",
"author": "Courseille C",
"journal-title": "Cryst Struct Commun",
"key": "ref44",
"unstructured": "Courseille C, Busetta B, and Hospital M. 7-Chloro-4(4-diethylamino-1-methylbutyl-amino) quinoline, C18H26ClN3 (Chloroquine Base). Cryst Struct Commun. 1973, 2:283.",
"volume": "2",
"year": "1973"
},
{
"article-title": "Structure of an anhistaminic drug, racemic chlorpheniramine hydrogen maleate",
"author": "Parvez M",
"journal-title": "Acta Cryst",
"key": "ref45",
"unstructured": "Parvez M. Structure of an anhistaminic drug, racemic chlorpheniramine hydrogen maleate. Acta Cryst. 1990, 46:943-5.",
"volume": "46",
"year": "1990"
},
{
"DOI": "10.1007/s10870-008-9327-9",
"article-title": "Intermolecular interactions in crystalline hydroxychloroquine sulfate in comparison with those in selected antimalarial drugs",
"author": "Semeniuk A",
"doi-asserted-by": "publisher",
"journal-title": "J Chem Crystallogr",
"key": "ref46",
"unstructured": "Semeniuk A, Kalinowska-Tluscik J, Nitek W, Oleksyn BJ. Intermolecular interactions in crystalline hydroxychloroquine sulfate in comparison with those in selected antimalarial drugs. J Chem Crystallogr. 2008, 38:333-8. 10.1007/s10870-008-9327-9",
"volume": "38",
"year": "2008"
},
{
"DOI": "10.7759/cureus.10501",
"article-title": "In vitro virucidal effect of intranasally delivered chlorpheniramine maleate compound against severe acute respiratory syndrome coronavirus 2",
"author": "Westover JB",
"doi-asserted-by": "publisher",
"journal-title": "Cureus",
"key": "ref47",
"unstructured": "Westover JB, Ferrer G, Vazquez H, Bethencourt-Mirabal A, Go CC. In vitro virucidal effect of intranasally delivered chlorpheniramine maleate compound against severe acute respiratory syndrome coronavirus 2. Cureus. 2020, 12:e10501. 10.7759/cureus.10501",
"volume": "12",
"year": "2020"
}
],
"reference-count": 47,
"references-count": 47,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subject": [
"Aerospace Engineering"
],
"subtitle": [],
"title": [
"Molecular Modeling and Preliminary Clinical Data Suggesting Antiviral Activity for Chlorpheniramine (Chlorphenamine) Against COVID-19"
],
"type": "journal-article"
}
