Hydroxyzine inhibits SARS-CoV-2 Spike protein binding to ACE2 in a qualitative in vitro assay
et al., bioRxiv, doi:10.1101/2021.01.04.424792, Jan 2021
11th treatment shown to reduce risk in
December 2020, now with p = 0.000052 from 17 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
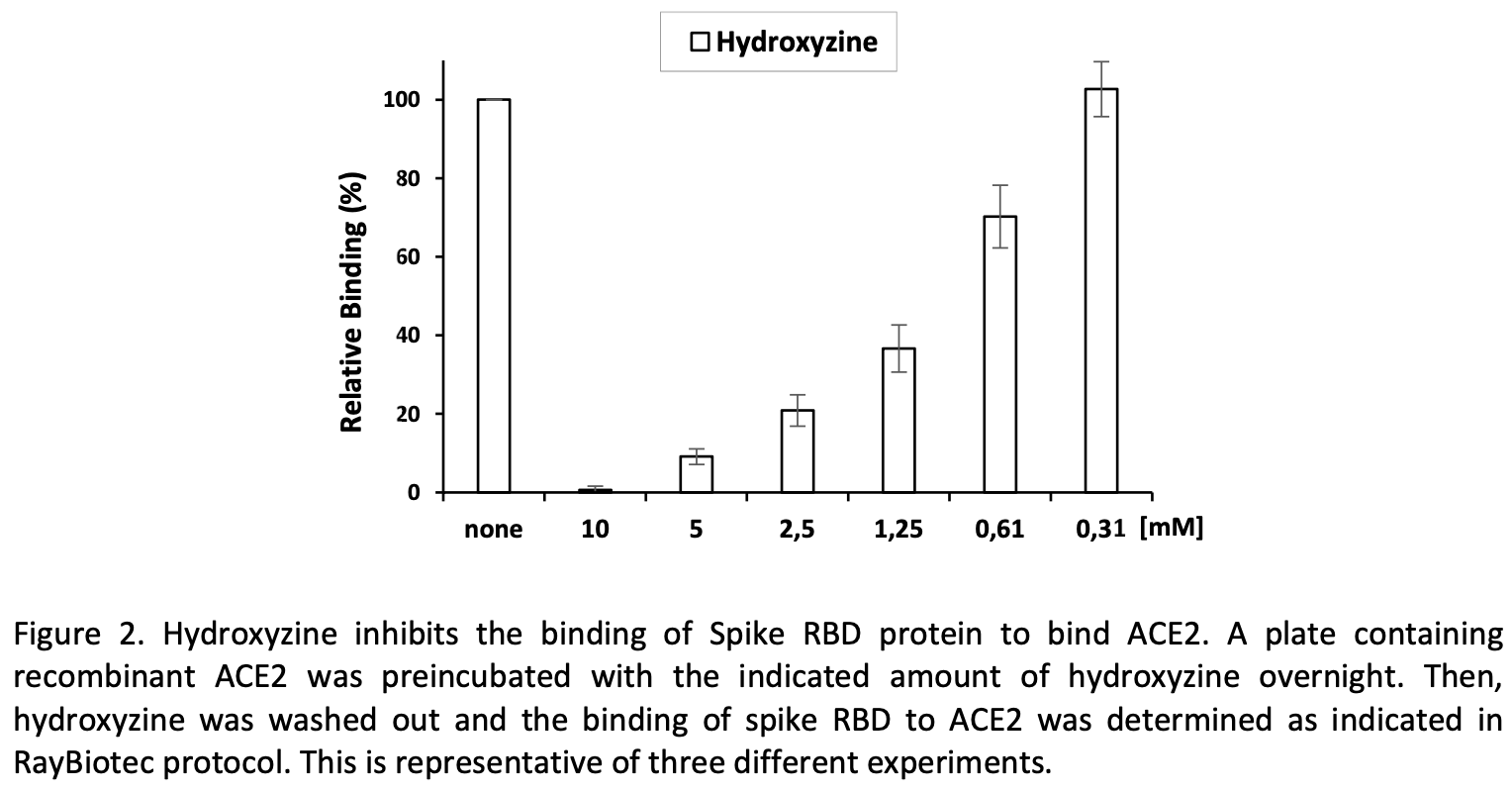
In vitro study showing that hydroxyzine inhibits the binding of SARS-CoV-2 Spike protein receptor-binding domain (RBD) to ACE2 in a qualitative assay. Hydroxyzine inhibited RBD binding to ACE2 in a dose-dependent manner, while labetalol, another drug reported to interact with ACE2, did not affect RBD/ACE2 interaction. Preincubation of ACE2 with hydroxyzine inhibited the subsequent binding of RBD to a greater extent. The findings suggest hydroxyzine could interfere with viral entry by blocking spike binding to ACE2, potentially explaining its reported clinical benefits in COVID-19 patients. Authors propose the efficacy of hydroxyzine for COVID-19 is worth further investigation given its well-characterized properties and affordable cost.
13 preclinical studies support the efficacy of antihistamine H1RAs for COVID-19:
1.
Hamdan et al., In silico Evaluation of H1-Antihistamine as Potential Inhibitors of SARS-CoV-2 RNA-dependent RNA Polymerase: Repurposing Study of COVID-19 Therapy, Turkish Journal of Pharmaceutical Sciences, doi:10.4274/tjps.galenos.2024.49768.
2.
Elshaier et al., Chlorpheniramine Maleate Displaying Multiple Modes of Antiviral Action Against SARS-CoV-2: An Initial Mechanistic Study, Cureus, doi:10.7759/cureus.92375.
3.
Black, S., Molecular Modeling and Preliminary Clinical Data Suggesting Antiviral Activity for Chlorpheniramine (Chlorphenamine) Against COVID-19, Cureus, doi:10.7759/cureus.20980.
4.
Hou et al., Testing of the inhibitory effects of loratadine and desloratadine on SARS-CoV-2 spike pseudotyped virus viropexis, Chemico-Biological Interactions, doi:10.1016/j.cbi.2021.109420.
5.
Yu et al., The histamine receptor H1 acts as an alternative receptor for SARS-CoV-2, mBio, doi:10.1128/mbio.01088-24.
6.
Sanchez-Gonzalez et al., Intranasal Chlorpheniramine Maleate for the treatment of COVID-19: Translational and Clinical Evidence, Medical Research Archives, doi:10.18103/mra.v10i3.2752.
7.
Morin-Dewaele et al., Desloratadine, an FDA-approved cationic amphiphilic drug, inhibits SARS-CoV-2 infection in cell culture and primary human nasal epithelial cells by blocking viral entry, Scientific Reports, doi:10.1038/s41598-022-25399-5.
8.
Reznikov et al., Identification of antiviral antihistamines for COVID-19 repurposing, Biochemical and Biophysical Research Communications, doi:10.1016/j.bbrc.2020.11.095.
Rivas et al., 5 Jan 2021, Spain, preprint, 3 authors.
Contact: jose.zamorano@salud-juntaex.es.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Hydroxyzine inhibits SARS-CoV-2 Spike protein binding to ACE2 in a qualitative in vitro assay
doi:10.1101/2021.01.04.424792
COVID-19 currently represents a major public health problem. Multiple efforts are being performed to control this disease. Vaccinations are already in progress. However, no effective treatments have been found so far. The disease is caused by the SARS-CoV-2 coronavirus that through the Spike protein interacts with its cell surface receptor ACE2 to enter into the host cells. Therefore, compounds able to block this interaction may help to stop disease progression. In this study, we have analyzed the effect of compounds reported to interact and modify the activity of ACE2 on the binding of the Spike protein. Among the compounds tested, we found that hydroxyzine could inhibit the binding of the receptor-binding domain of Spike protein to ACE2 in a qualitative in vitro assay. This finding supports the reported clinical data showing the benefits of hydroxyzine on COVID-19 patients, raising the need for further investigation into its effectiveness in the treatment of COVID-19 given its well-characterized medical properties and affordable cost.
References
Baden, Sahly, Essink, Kotloff, Frey et al., Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine, Engl J Med, doi:10.1056/NEJMoa2035389
Hoertel, Sánchez, Vernet, Beeker, Neuraz et al., Association between Hydroxyzine Use and Reduced Mortality in Patients Hospitalized for Coronavirus Disease 2019: Results from a multicenter observational study, medRxiv October, doi:10.1101/2020.10.23.20154302
Kalil, Patterson, Mehta, Tomashek, Wolfe et al., Baricitinib plus remdesivir for hospitalized adults with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2031994
Kulemina, Ostrov, Prediction of off-target effects on angiotensinconverting enzyme 2, J Biomol Screen
Reznikov, Norris, Vashisht, Bluhm, Li et al., Identification of antiviral antihistamines for COVID-19 repurposing, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2020.11.095
Stone, Frigault, Serling-Boyd, Fernandes, Harvey et al., Efficacy of tocilizumab in patients hospitalized with COVID-19, N Engl J Med
Vabret, Britton, Gruber, Hegde, Kim et al., Immunology of COVID-19: Current State of the Science, Immunity
DOI record:
{
"DOI": "10.1101/2021.01.04.424792",
"URL": "http://dx.doi.org/10.1101/2021.01.04.424792",
"abstract": "<jats:title>Abstract</jats:title><jats:p>COVID-19 currently represents a major public health problem. Multiple efforts are being performed to control this disease. Vaccinations are already in progress. However, no effective treatments have been found so far. The disease is caused by the SARS-CoV-2 coronavirus that through the Spike protein interacts with its cell surface receptor ACE2 to enter into the host cells. Therefore, compounds able to block this interaction may help to stop disease progression. In this study, we have analyzed the effect of compounds reported to interact and modify the activity of ACE2 on the binding of the Spike protein. Among the compounds tested, we found that hydroxyzine could inhibit the binding of the receptor-binding domain of Spike protein to ACE2 in a qualitative <jats:italic>in vitro</jats:italic> assay. This finding supports the reported clinical data showing the benefits of hydroxyzine on COVID-19 patients, raising the need for further investigation into its effectiveness in the treatment of COVID-19 given its well-characterized medical properties and affordable cost.</jats:p>",
"accepted": {
"date-parts": [
[
2021,
1,
15
]
]
},
"author": [
{
"affiliation": [],
"family": "Rivas",
"given": "Maria Dolores",
"sequence": "first"
},
{
"affiliation": [],
"family": "Saponi-Cortes",
"given": "Jose Maria Rafael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zamorano",
"given": "Jose",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
1,
6
]
],
"date-time": "2021-01-06T07:35:07Z",
"timestamp": 1609918507000
},
"deposited": {
"date-parts": [
[
2021,
1,
18
]
],
"date-time": "2021-01-18T10:50:44Z",
"timestamp": 1610967044000
},
"group-title": "Immunology",
"indexed": {
"date-parts": [
[
2022,
4,
1
]
],
"date-time": "2022-04-01T14:43:54Z",
"timestamp": 1648824234422
},
"institution": [
{
"name": "bioRxiv"
}
],
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2021,
1,
5
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.01.04.424792",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
1,
5
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
1,
5
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1016/j.immuni.2020.05.002",
"doi-asserted-by": "publisher",
"key": "2021011802350654000_2021.01.04.424792v2.1"
},
{
"DOI": "10.1056/NEJMoa2035389",
"doi-asserted-by": "publisher",
"key": "2021011802350654000_2021.01.04.424792v2.2"
},
{
"DOI": "10.1056/NEJMoa2028836",
"doi-asserted-by": "publisher",
"key": "2021011802350654000_2021.01.04.424792v2.3"
},
{
"DOI": "10.1056/NEJMoa2031994",
"doi-asserted-by": "publisher",
"key": "2021011802350654000_2021.01.04.424792v2.4"
},
{
"DOI": "10.1177/1087057111413919",
"doi-asserted-by": "publisher",
"key": "2021011802350654000_2021.01.04.424792v2.5"
},
{
"DOI": "10.1016/j.bbrc.2020.11.095",
"doi-asserted-by": "publisher",
"key": "2021011802350654000_2021.01.04.424792v2.6"
},
{
"DOI": "10.1101/2020.10.23.20154302",
"doi-asserted-by": "crossref",
"key": "2021011802350654000_2021.01.04.424792v2.7",
"unstructured": "Hoertel N , M Sánchez , R Vernet , N Beeker , A Neuraz , C Blanco , M Olfson , C Lemogne , P Meneton , C Daniel , N Paris , A Gramfort , G Lemaitre , E Salamanca , M Bernaux , A Bellamine , A Burgun , and Limosin. Association between Hydroxyzine Use and Reduced Mortality in Patients Hospitalized for Coronavirus Disease 2019: Results from a multicenter observational study. medRxiv October 27, 2020 doi: https://doi.org/10.1101/2020.10.23.20154302."
}
],
"reference-count": 7,
"references-count": 7,
"relation": {},
"resource": {
"primary": {
"URL": "http://biorxiv.org/lookup/doi/10.1101/2021.01.04.424792"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Hydroxyzine inhibits SARS-CoV-2 Spike protein binding to ACE2 in a qualitative <i>in vitro</i> assay",
"type": "posted-content"
}
