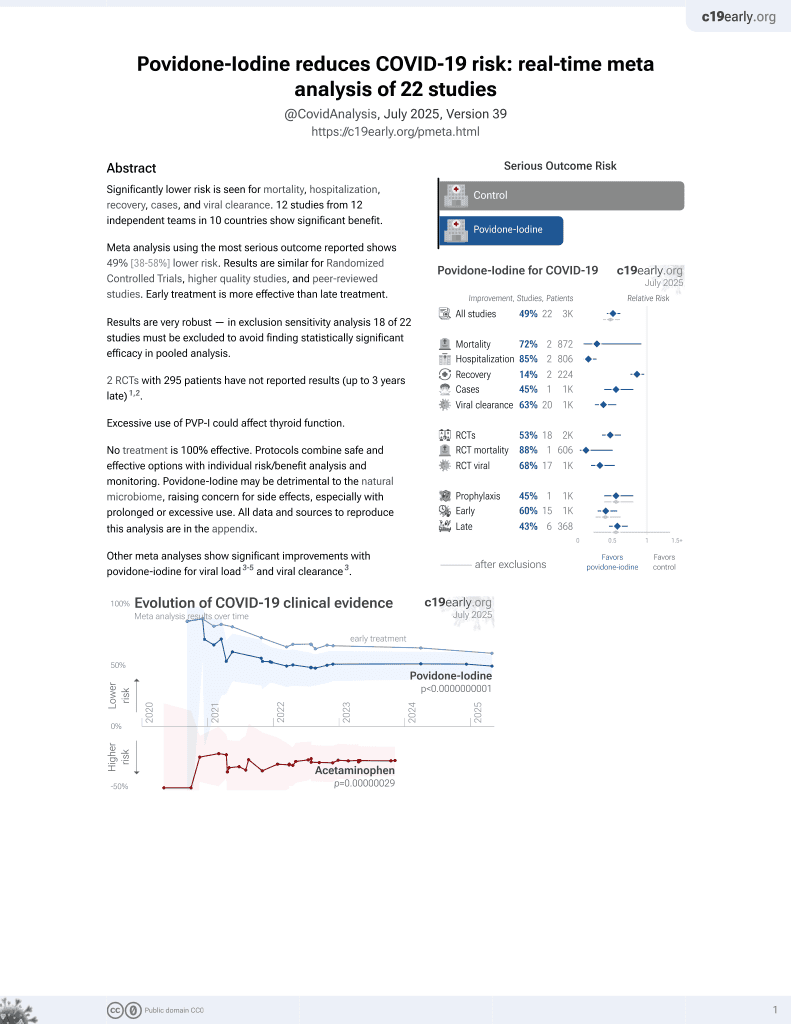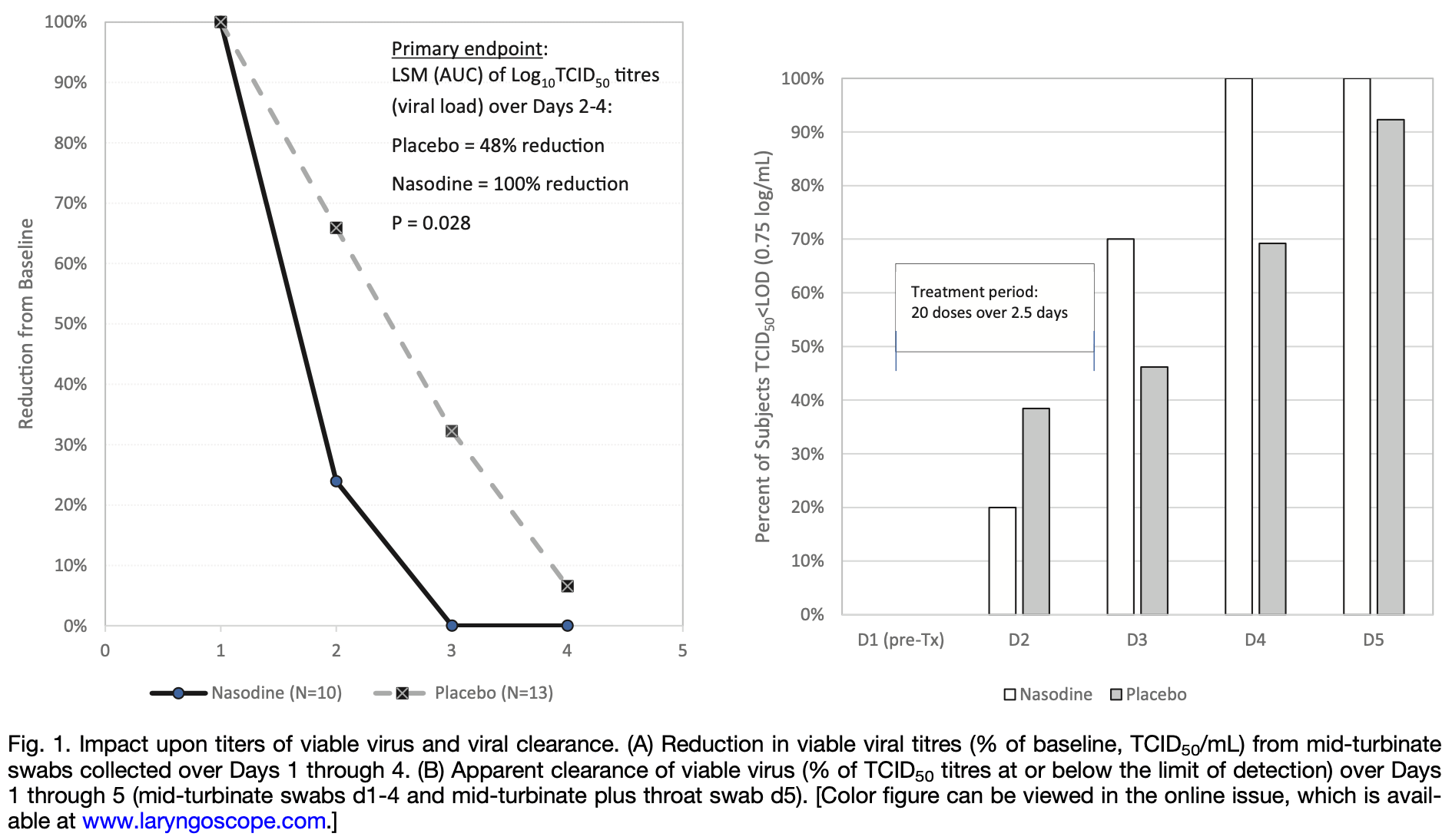
Phase II Trial of the Impact 0.5% Povidone‐Iodine Nasal Spray (Nasodine®) on Shedding of SARS‐CoV‐2
et al., The Laryngoscope, doi:10.1002/lary.31430, ACTRN12618001244291, Mar 2024
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 23 early COVID-19 outpatients showing significantly improved reduction in viral load and significantly faster viral clearance with povidone-iodine nasal spray compared to placebo. The study was underpowered due to low recruitment, enrolling only 23 patients from a target of 144. Authors report generally mild symptoms and a 6% benefit over placebo on symptom scores (AUC symptom score days 2-5) without statistical significance, but do not provide details.
Notably, no benefit was seen for rapid antigen test positivity, which is unable to distinguish viable and non-viable virus. The relatively poor diagnostic information from viral positivity using methods that cannot distinguish viable virus may present misleading results in many COVID-19 studies.
Treatment 8 times daily for a total of 20 doses.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
|
relative viral clearance rate, 59.5% better, RR 0.40, p = 0.03, treatment 10, control 13.
|
|
relative LSM log10TCID50 AUC2-4 reduction, 52.0% better, RR 0.48, p = 0.03, treatment 10, control 13.
|
|
risk of no recovery, 6.0% lower, RR 0.94, treatment 10, control 13.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Friedland et al., 30 Mar 2024, Double Blind Randomized Controlled Trial, placebo-controlled, South Africa, peer-reviewed, 2 authors, trial ACTRN12618001244291.
Contact: friedland@uwa.edu.au, peter.friedland@health.wa.gov.au.
Phase II Trial of the Impact 0.5% Povidone‐Iodine Nasal Spray (Nasodine®) on Shedding of SARS‐CoV‐2
The Laryngoscope, doi:10.1002/lary.31430
Objective: A Phase II trial was conducted to determine if nasal disinfection with a commercial Good Manufacturing Practice-manufactured 0.5% povidone-iodine nasal spray (Nasodine ® ) may be a useful adjunct in the management of COVID-19 by reducing viral shedding and prevention of transmission of SARS-CoV-2. The aim was to confirm the results from a human single-dose pilot study by assessing repeated and frequent doses on nasal shedding of SARS-CoV-2 from adult subjects with confirmed COVID-19. Methods: A multicenter, randomized, double-blinded, placebo-controlled Phase II clinical trial involving adults with early COVID-19 symptoms. Baseline nasal swabs were collected to quantify pretreatment SARS-CoV-2 nasal viral load, followed by Nasodine treatment eight times daily over 3 calendar days. Daily nasal swabs were collected post-dose to assess the impact of treatment on nasal viral load, measured by log10 TCID50 in quantitative culture. Results: Nasodine subjects exhibited significantly improved reduction in viral load (log10 TCID50) on Days 2-4 compared to placebo recipients (p = 0.028), rate of nasal clearance of viable virus (p = 0.032), and complete (100%) nasal and throat clearance of the virus by Day 5. No difference was seen in antigen shedding as measured by time transition from Rapid Antigen Test (RAT) positivity to RAT negativity. Conclusion: A total of 20 doses of Nasodine ® nasal spray administered over 2.5 days significantly reduced the titers of viable SARS-CoV-2 virus in the nasal passages of COVID-19 subjects. This is the first study demonstrating the efficacy of a tolerable intranasal formulation of povidone-iodine on viral shedding in COVID-19 subjects. Nasal disinfection may diminish viral transmission to others.
References
Anderson, Sivalingam, Kang, Povidone-iodine demonstrates rapid in vitro Virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease, Infect Dis Ther, doi:10.1007/s40121-020-00316-3
Arefin, Rumi, Uddin, Virucidal effect of povidone iodine on COVID-19 in the nasopharynx: an open-label randomized clinical trial, Indian J Otolaryngol Head Neck Surg, doi:10.1007/s12070-021-02616-7
Baxter, Schwartz, Johnson, Rapid initiation of nasal saline irrigation to reduce severity in high-risk COVID+ outpatients: a randomized clinical trial compared to a national dataset observational arm, doi:10.1101/2021.08.16.21262044
Bidra, Pelletier, Westover, Frank, Brown et al., Rapid in-vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using povidone-iodine Oral antiseptic rinse, J Prosthodont, doi:10.1111/jopr.13209
Campbell, SARS-CoV-2 and the nose: risks and implications for primary care, Aust J Gen Pract
Challacombe, Kirk-Bayley, Sunkaraneni, Combes, Povidone iodine, Br Dent J, doi:10.1038/s41415-020-1589-4
Choudhury, Effect of 1% povidone iodine mouthwash/gargle, nasal and eye drop in COVID-19 patient, Biores Commun
Elsersy, Zahran, Elbakry, Combined nasal, oropharyngeal povidone iodine plus Glycyrrhizic acid sprays, accelerate clinical and laboratory recovery and reduces household transmission of SARS-CoV-2: a randomized placebo-controlled clinical trial, Front Med, doi:10.3389/fmed.2022.863917
Frank, Brown, Capriotti, Westover, Pelletier et al., In vitro efficacy of a povidone-iodine nasal antiseptic for rapid inactivation of SARS-CoV-2, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2020.3053
Friedland, Tucker, Goodall, In vivo (human) and in vitro inactivation of SARS-CoV-2 with 0.5% povidone-iodine nasal spray, Aust J Otolaryngol, doi:10.21037/ajo-21-40
Gallo, Locatello, Mazzoni, Novelli, Annunziato, The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection, Mucosal Immunol, doi:10.1038/s41385-020-00359-2
Guenezan, Garcia, Strasters, Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19: a randomized clinical trial, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2020.5490
Hale, Lux, Kim, In vitro Nasodine can be an effective Antibiofilm agent for biofilms that may cause CRS, Laryngoscope, doi:10.1002/lary.30558
Hou, Okuda, Edwards, SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract, doi:10.1016/j.cell.2020.05.042
Jamir, Tripathi, Shankar, Kakkar, Ayyanar et al., Determinants of outcome among critically ill police personnel with COVID-19: a retrospective observational study from Andhra Pradesh, India, Cureus, doi:10.7759/cureus.20394
Kawana, Kitamura, Nakagomi, Inactivation of human viruses by povidone-iodine in comparison with other antiseptics, Dermatology, doi:10.1159/000246027
Lachapelle, Casado, Antiseptics in the era of bacterial resistance: a focus on povidone iodine, Future Med
Lepelletier, Maillard, Pozzetto, Simon, Povidone iodine: properties, mechanisms of action, and role in infection control and Staphylococcus aureus decolonization, Antimicrob Agents Chemother, doi:10.1128/AAC.00682-20
Ma, Prevention of infection by highly pathogenic viruses using topical application of povidone-iodine on mucous membranes
Mady, Kubik, Baddour, Snyderman, Rowan, Consideration of povidone-iodine as a public health intervention for COVID-19: utilization as "personal protective equipment" for frontline providers exposed in high-risk head and neck and skull base oncology care, Oral Oncol, doi:10.1016/j.oraloncology.2020.104724
Naqvi, Citardi, Cattano, Ostrosky-Zeichner, Knackstedt et al., Povidone-iodine solution as SARS-CoV-2 prophylaxis for procedures of the upper aerodigestive tract a theoretical framework, J Otolaryngol Head Neck Surg, doi:10.1186/s40463-020-00474-x
Nuckolls, Chemical considerations related to the dilution of commercial 10% Povidone-iodine for use in the COVID-19 Pandemic
Ramezanpour, Smith, Psaltis, Wormald, Vreugde, In vitro safety evaluation of a povidone-iodine solution applied to human nasal epithelial cells, Int Forum Allergy Rhinol, doi:10.1002/alr.22575
Sirijatuphat, Leelarasamee, Puangpet, Thitithanyanont, A pilot study of 0.4% povidone-iodine nasal spray to eradicate SARS-CoV-2 in the nasopharynx, Infect Drug Resist, doi:10.2147/IDR.S391630
Zarabanda, Vukkadala, Phillips, The effect of povidone-iodine nasal spray on nasopharyngeal SARS-CoV-2 viral load: a randomized control trial, Laryngoscope, doi:10.1002/lary.29935
DOI record:
{
"DOI": "10.1002/lary.31430",
"ISSN": [
"0023-852X",
"1531-4995"
],
"URL": "http://dx.doi.org/10.1002/lary.31430",
"abstract": "<jats:sec><jats:title>Objective</jats:title><jats:p>A Phase II trial was conducted to determine if nasal disinfection with a commercial Good Manufacturing Practice‐manufactured 0.5% povidone‐iodine nasal spray (Nasodine®) may be a useful adjunct in the management of COVID‐19 by reducing viral shedding and prevention of transmission of SARS‐CoV‐2. The aim was to confirm the results from a human single‐dose pilot study by assessing repeated and frequent doses on nasal shedding of SARS‐CoV‐2 from adult subjects with confirmed COVID‐19.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>A multicenter, randomized, double‐blinded, placebo‐controlled Phase II clinical trial involving adults with early COVID‐19 symptoms. Baseline nasal swabs were collected to quantify pretreatment SARS‐CoV‐2 nasal viral load, followed by Nasodine treatment eight times daily over 3 calendar days. Daily nasal swabs were collected post‐dose to assess the impact of treatment on nasal viral load, measured by log10 TCID50 in quantitative culture.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Nasodine subjects exhibited significantly improved reduction in viral load (log10 TCID50) on Days 2–4 compared to placebo recipients (<jats:italic>p</jats:italic> = 0.028), rate of nasal clearance of viable virus (<jats:italic>p</jats:italic> = 0.032), and complete (100%) nasal and throat clearance of the virus by Day 5. No difference was seen in antigen shedding as measured by time transition from Rapid Antigen Test (RAT) positivity to RAT negativity.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>A total of 20 doses of Nasodine® nasal spray administered over 2.5 days significantly reduced the titers of viable SARS‐CoV‐2 virus in the nasal passages of COVID‐19 subjects. This is the first study demonstrating the efficacy of a tolerable intranasal formulation of povidone‐iodine on viral shedding in COVID‐19 subjects. Nasal disinfection may diminish viral transmission to others.</jats:p></jats:sec><jats:sec><jats:title>Level of Evidence</jats:title><jats:p>Level 2 <jats:italic>Laryngoscope</jats:italic>, 2024</jats:p></jats:sec>",
"alternative-id": [
"10.1002/lary.31430"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-11-29"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2024-03-12"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2024-03-30"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2686-8516",
"affiliation": [
{
"name": "Faculty of Medical and Health Sciences University of Western Australia Crawley Western Australia Australia"
},
{
"name": "Department Otorhinolaryngology Head Neck Skull Base Surgery Sir Charles Gairdner Hospital Nedlands Western Australia Australia"
},
{
"name": "Firebrick Pharma Limited Melbourne Victoria Australia"
}
],
"authenticated-orcid": false,
"family": "Friedland",
"given": "Peter L.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Firebrick Pharma Limited Melbourne Victoria Australia"
}
],
"family": "Tucker",
"given": "Simon",
"sequence": "additional"
}
],
"container-title": "The Laryngoscope",
"container-title-short": "The Laryngoscope",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2024,
3,
30
]
],
"date-time": "2024-03-30T11:29:42Z",
"timestamp": 1711798182000
},
"deposited": {
"date-parts": [
[
2024,
3,
30
]
],
"date-time": "2024-03-30T11:29:47Z",
"timestamp": 1711798187000
},
"indexed": {
"date-parts": [
[
2024,
3,
30
]
],
"date-time": "2024-03-30T12:13:07Z",
"timestamp": 1711800787343
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
3,
30
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
30
]
],
"date-time": "2024-03-30T00:00:00Z",
"timestamp": 1711756800000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/lary.31430",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2024,
3,
30
]
]
},
"published-online": {
"date-parts": [
[
2024,
3,
30
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.31128/AJGP-05-20-5452",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_2_1"
},
{
"DOI": "10.1016/j.cell.2020.05.042",
"doi-asserted-by": "crossref",
"key": "e_1_2_7_3_1",
"unstructured": "HouYJ OkudaK EdwardsCE et al.SARS‐CoV‐2 reverse genetics reveals a variable infection gradient in the respiratory tract. (1097–4172 (Electronic)) (In eng). 2020;182(2):429‐446.https://doi.org/10.1016/j.cell.2020.05.042"
},
{
"DOI": "10.1038/s41385-020-00359-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_4_1"
},
{
"DOI": "10.1128/AAC.00682-20",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_5_1"
},
{
"article-title": "Antiseptics in the era of bacterial resistance: a focus on povidone iodine",
"author": "Lachapelle JMCO",
"first-page": "579",
"journal-title": "Future Med",
"key": "e_1_2_7_6_1",
"volume": "10",
"year": "2013"
},
{
"DOI": "10.1159/000246027",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_7_1"
},
{
"DOI": "10.1007/s40121-020-00316-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_8_1"
},
{
"DOI": "10.1186/s40463-020-00474-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_9_1"
},
{
"DOI": "10.1038/s41415-020-1589-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_10_1"
},
{
"DOI": "10.1016/j.oraloncology.2020.104724",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_11_1"
},
{
"DOI": "10.1001/jamaoto.2020.3053",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_12_1"
},
{
"DOI": "10.1111/jopr.13209",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_13_1"
},
{
"key": "e_1_2_7_14_1",
"unstructured": "MaG.Prevention of infection by highly pathogenic viruses using topical application of povidone‐iodine on mucous membranes.2020."
},
{
"DOI": "10.1001/jamaoto.2020.5490",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_15_1"
},
{
"DOI": "10.1002/lary.29935",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_16_1"
},
{
"DOI": "10.2147/IDR.S391630",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_17_1"
},
{
"DOI": "10.3329/brc.v7i1.54245",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_18_1"
},
{
"DOI": "10.1007/s12070-021-02616-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_19_1"
},
{
"DOI": "10.1101/2021.08.16.21262044",
"doi-asserted-by": "crossref",
"key": "e_1_2_7_20_1",
"unstructured": "BaxterAL SchwartzKR JohnsonRW et al.Rapid initiation of nasal saline irrigation to reduce severity in high‐risk COVID+ outpatients: a randomized clinical trial compared to a national dataset observational arm.2021https://doi.org/10.1101/2021.08.16.21262044"
},
{
"DOI": "10.7759/cureus.20394",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_21_1"
},
{
"DOI": "10.3389/fmed.2022.863917",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_22_1"
},
{
"DOI": "10.31219/osf.io/8kepw",
"doi-asserted-by": "crossref",
"key": "e_1_2_7_23_1",
"unstructured": "NuckollsC.Chemical considerations related to the dilution of commercial 10% Povidone‐iodine for use in the COVID‐19 Pandemic.2020."
},
{
"DOI": "10.1002/alr.22575",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_24_1"
},
{
"DOI": "10.21037/ajo-21-40",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_25_1"
},
{
"DOI": "10.1002/lary.30558",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_26_1"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/lary.31430"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Otorhinolaryngology"
],
"subtitle": [],
"title": "Phase <scp>II</scp> Trial of the Impact 0.5% Povidone‐Iodine Nasal Spray (Nasodine®) on Shedding of <scp>SARS‐CoV</scp>‐2",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}