In vivo (human) and in vitro inactivation of SARS-CoV-2 with 0.5% povidone-iodine nasal spray
et al., Australian Journal of Otolaryngology, doi:10.21037/ajo-21-40, Feb 2022
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
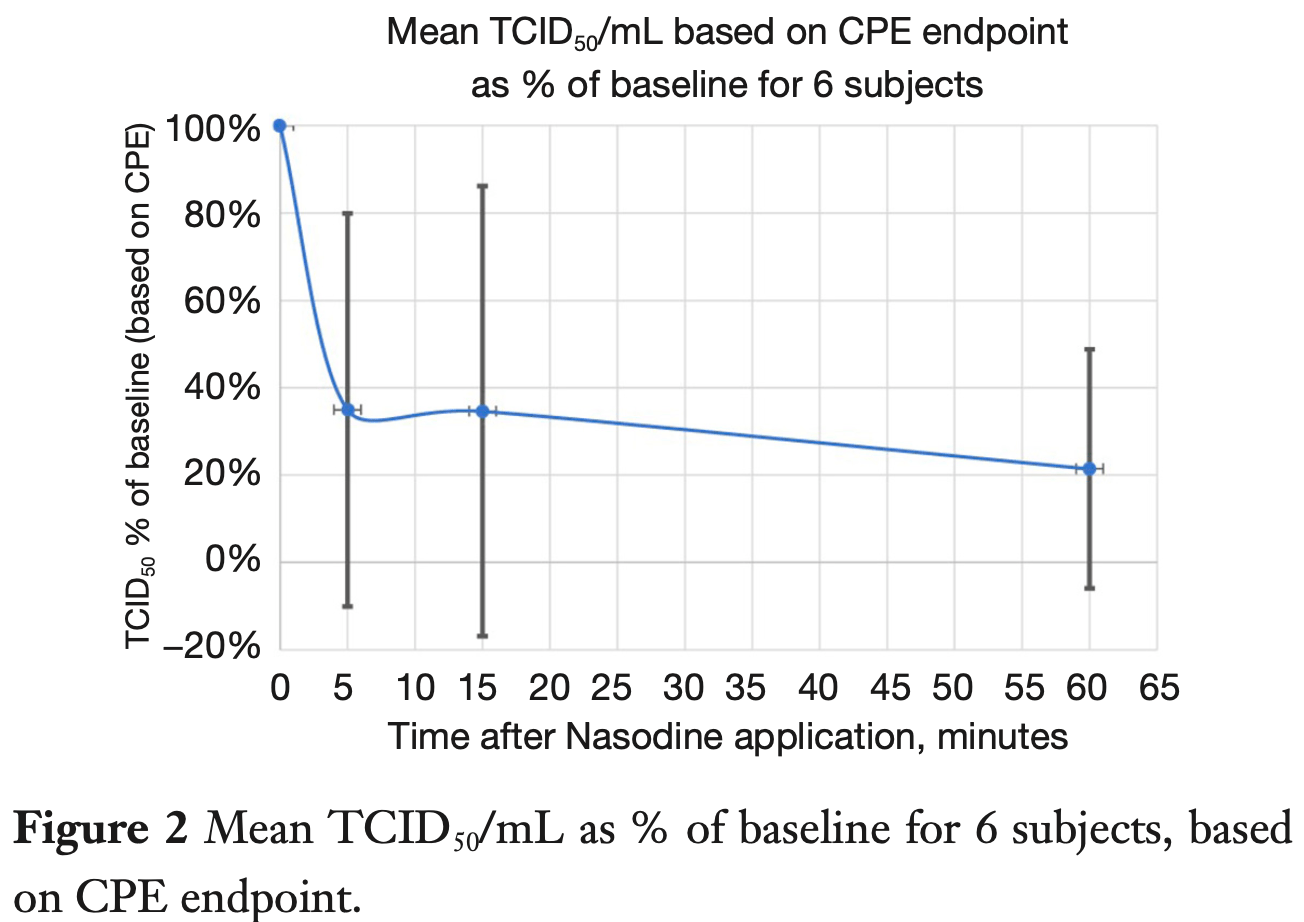
Small study of povidone-iodine nasal spray with 14 patients, showing rapid reduction in viral load for the 6 patients that had culturable virus at baseline. All patients remained PCR+ despite no culturable virus detected for 3 of 6 patients. This study also contains in vitro results showing rapid reduction in viable virus with povidone-iodine.
Friedland et al., 9 Feb 2022, Australia, peer-reviewed, 12 authors.
In vivo (human) and in vitro inactivation of SARS-CoV-2 with 0.5% povidone-iodine nasal spray
Australian Journal of Otolaryngology, doi:10.21037/ajo-21-40
Background: Nasal disinfection with 0.5% povidone-iodine (PVP-I) may be a useful adjunct in the management of COVID-19. The purpose of this article is to confirm the in vitro activity of the PVP-I nasal spray against SARS-CoV-2 and whether that may translate into reduced nasal shedding in vivo. Methods: Two SARS-CoV-2 virus isolates were exposed to 0.5% PVP-nasal spray (Nasodine®) for different times in vitro, with PCR and cell culture used to assess impact on viral infectivity and RNA copies. An open label in vivo single arm pilot study of 14 subjects with positive COVID-19 PCR diagnosis was undertaken. Baseline nasal swabs were collected to quantify SARS-CoV-2 pre-treatment, followed by a single 0.5% PVP nasal spray application (1.12 mL). Nasal swabs were collected at 5, 15, and 60 minutes post-dose to assess immediate and residual impact of treatment. Results: In vitro, the nasal spray reduced infectivity by 3.5 log10 TCID 50 /mL (99.97%) after 15 seconds exposure and eliminated detectable viral infectivity after 60 seconds; there was no effect on viral RNA detection by PCR. In vivo, culturable virus (VOC beta/B.1.351 variant) was obtained from 6 of 14 PCRconfirmed positive subjects; in these subjects, 5 minutes after the single PVP-I dose, the mean viral titre was reduced by 65% versus baseline and by 79% versus baseline at 60 minutes post-dose. 5 of the 6 subjects (83%), had reduction or cessation of viral shedding at 5 minutes in all 6 subjects, virus titers 60 minutes post-dose were below baseline value. 0.5% PVP-I treatment didn't interfere with the laboratory diagnosis of COVID-19 via PCR-detection of viral RNA in humans. Conclusions: 0.5% PVP-I nasal spray is rapidly virucidal to SARS-CoV-2 in vitro using exposure times consistent with nasal residence; single in vivo nasal administration reduced infectious viral titers in COVID-19 subjects with culturable virus. A single application of 0.5% PVP-I nasal spray does not interfere with PCR-mediated laboratory diagnosis of COVID-19. We are undertaking a large double blinded randomized controlled trial to confirm if repeated application of 0.5% PVP-I nasal spray over a longer period could be useful in suppressing viral shedding and transmission risk in COVID-positive patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the noncommercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
Abu-Raddad, Chemaitelly, Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants, N Engl J Med
Anderson, Sivalingam, Kang, Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus
Bidra, Pelletier, Westover, Rapid In-Vitro Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Using Povidone-Iodine Oral Antiseptic Rinse, J Prosthodont
Campbell, SARS-CoV-2 and the nose: Risks and implications for primary care, Aust J Gen Pract
Challacombe, Kirk-Bayley, Sunkaraneni, Povidone iodine, Br Dent J
Frank, Brown, Capriotti, In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2, JAMA Otolaryngol Head Neck Surg
Friedland, Tucker, Goodall, Julander, Mendenhall et al., In vivo (human) and in vitro inactivation of SARS-CoV-2 with 0.5% povidone-iodine nasal spray, J Otolaryngol
Gallo, Locatello, Mazzoni, The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection, Mucosal Immunol
Hou, Okuda, Edwards, SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract, Cell
Kawana, Kitamura, Nakagomi, Inactivation of human viruses by povidone-iodine in comparison with other antiseptics, Dermatology
Kim, Rimmer, Mrad, Betadine has a ciliotoxic effect on ciliated human respiratory cells, J Laryngol Otol
Lachapelle, Castel, Casado, Antiseptics in the era of bacterial resistance: a focus on povidone iodine, Clin Pract
Lamas, Dios, Rodríguez, Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests, Oral Dis, doi:10.1111/odi.13526
Lepelletier, Maillard, Pozzetto, Povidone Iodine: Properties, Mechanisms of Action, and Role in Infection Control and Staphylococcus aureus Decolonization, Antimicrob Agents Chemother
Mady, Kubik, Baddour, Consideration of povidone-iodine as a public health intervention for COVID-19: Utilization as "Personal Protective Equipment" for frontline providers exposed in high-risk head and neck and skull base oncology care, Oral Oncol
Molloy, Goodall, Prevention of infection by highly pathogenic viruses using topical application of povidoneiodine on mucous membranes
Naqvi, Citardi, Cattano, Povidone-iodine solution as SARS-CoV-2 prophylaxis for procedures of the upper aerodigestive tract a theoretical framework, J Otolaryngol Head Neck Surg
Ramezanpour, Smith, Psaltis, In vitro safety evaluation of a povidone-iodine solution applied to human nasal epithelial cells, Int Forum Allergy Rhinol
Sato, Miyake, Hazama, Povidone-iodineinduced cell death in cultured human epithelial HeLa cells and rat oral mucosal tissue, Drug Chem Toxicol
Sikazwe, Levy, Speers, An efficient, reproducible and accurate RT-qPCR based method to determine mumps specific neutralizing antibody, J Virol Methods
Speake, Phillips, Chong, Flight-Associated Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 Corroborated by Whole-Genome Sequencing, Emerg Infect Dis
Zhou, Dejnirattisai, Supasa, Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera, Cell
DOI record:
{
"DOI": "10.21037/ajo-21-40",
"ISSN": [
"2616-2792"
],
"URL": "http://dx.doi.org/10.21037/ajo-21-40",
"author": [
{
"affiliation": [],
"family": "Friedland",
"given": "Peter",
"sequence": "first"
},
{
"affiliation": [],
"family": "Tucker",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goodall",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Julander",
"given": "Justin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mendenhall",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Molloy",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Jong",
"given": "Douwe Marcus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Musungaie",
"given": "Dany Badibanga",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sikazwe",
"given": "Chisha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Panta",
"given": "Kirtu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Levy",
"given": "Avram",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "David",
"sequence": "additional"
}
],
"container-title": [
"Australian Journal of Otolaryngology"
],
"content-domain": {
"crossmark-restriction": false,
"domain": [
"amegroups.com"
]
},
"created": {
"date-parts": [
[
2022,
2,
9
]
],
"date-time": "2022-02-09T07:18:55Z",
"timestamp": 1644391135000
},
"deposited": {
"date-parts": [
[
2022,
2,
9
]
],
"date-time": "2022-02-09T07:18:56Z",
"timestamp": 1644391136000
},
"indexed": {
"date-parts": [
[
2022,
2,
10
]
],
"date-time": "2022-02-10T07:40:08Z",
"timestamp": 1644478808511
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "2616-2792"
}
],
"issued": {
"date-parts": [
[
2022,
2
]
]
},
"link": [
{
"URL": "https://www.theajo.com/article/download/4466/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8611",
"original-title": [],
"page": "2-2",
"prefix": "10.21037",
"published": {
"date-parts": [
[
2022,
2
]
]
},
"published-online": {
"date-parts": [
[
2022,
2
]
]
},
"publisher": "AME Publishing Company",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [
"Aust J Otolaryngol"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Otorhinolaryngology"
],
"subtitle": [],
"title": [
"In vivo (human) and in vitro inactivation of SARS-CoV-2 with 0.5% povidone-iodine nasal spray"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.21037/ame_crossmark_policy",
"volume": "5"
}
