Intensive Treatment With Ivermectin and Iota-Carrageenan as Pre-exposure Prophylaxis for COVID-19 in Health Care Workers From Tucuman, Argentina
et al., American Journal of Therapeutics, doi:10.1097/MJT.0000000000001433, NCT04701710, Jan 2021
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
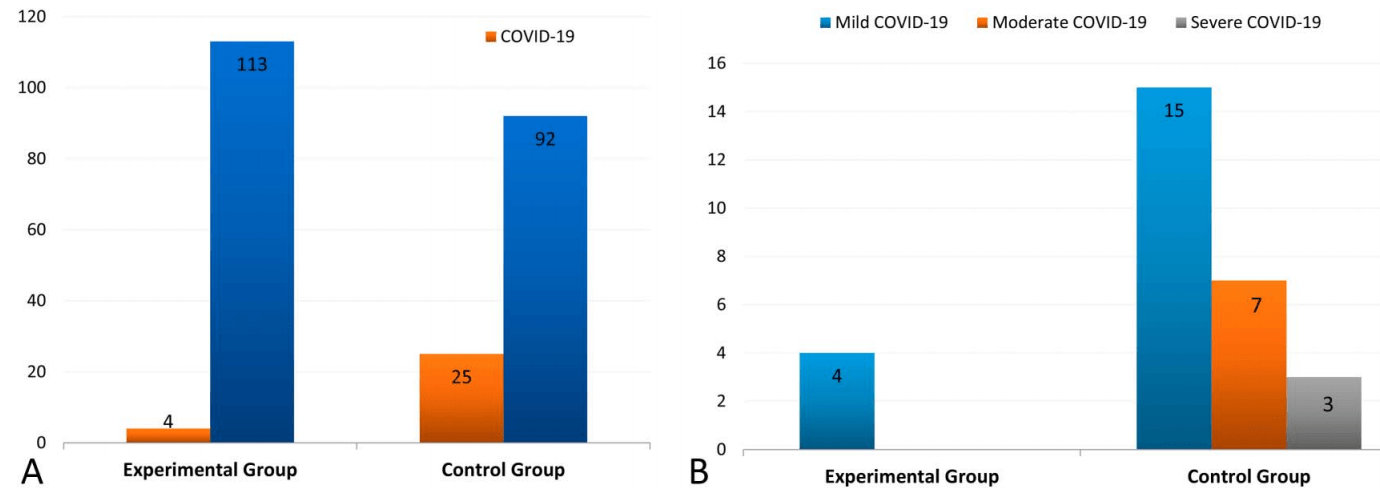
Prophylaxis RCT for ivermectin and iota-carrageenan in Argentina, 117 healthcare workers treated with ivermectin and iota-carrageenan, and 117 controls, showing significantly lower cases with treatment. There were no moderate/severe cases with treatment vs. 10 in the control group. There were 4 cases with treatment (all mild) vs. 25 for the control group.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
This is the 12th of 53 COVID-19 RCTs for ivermectin, which collectively show efficacy with p=0.000000087.
This is the 31st of 106 COVID-19 controlled studies for ivermectin, which collectively show efficacy with p<0.0000000001.
Study covers iota-carrageenan and ivermectin.
|
risk of moderate/severe case, 95.2% lower, RR 0.05, p = 0.002, treatment 0 of 117 (0.0%), control 10 of 117 (8.5%), NNT 12, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), moderate/severe COVID-19.
|
|
risk of case, 84.0% lower, RR 0.16, p = 0.004, treatment 4 of 117 (3.4%), control 25 of 117 (21.4%), NNT 5.6, adjusted per study, odds ratio converted to relative risk, all cases, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Aref et al., Possible Role of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Recovery of Post-COVID-19 Anosmia, Infection and Drug Resistance, doi:10.2147/IDR.S381715.
2.
Aref (B) et al., Clinical, Biochemical and Molecular Evaluations of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Reducing Upper Respiratory Symptoms of Mild COVID-19, International Journal of Nanomedicine, doi:10.2147/IJN.S313093.
3.
Chahla et al., Intensive Treatment With Ivermectin and Iota-Carrageenan as Pre-exposure Prophylaxis for COVID-19 in Health Care Workers From Tucuman, Argentina, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001433.
Chahla et al., 11 Jan 2021, Randomized Controlled Trial, Argentina, peer-reviewed, 11 authors, study period 15 October, 2020 - 31 December, 2020, dosage 12mg weekly, this trial uses multiple treatments in the treatment arm (combined with iota-carrageenan) - results of individual treatments may vary, trial NCT04701710 (history).
Intensive Treatment With Ivermectin and Iota-Carrageenan as Pre-exposure Prophylaxis for COVID-19 in Health Care Workers From Tucuman, Argentina
tive effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes.
Rossana E. Chahla, MD, PhD 1 Luis Medina Ruiz, MD 2 Eugenia S. Ortega, MSc 3 Marcelo F. Morales, RN 4 Francisco Barreiro, MD 5 Alexia George, MD 5 Cesar Mancilla, RN 4 Sylvia D' Amato, RN 4 Guillermo Barrenechea, MSc 3 Daniel G. Goroso, PhD
References
Abd-Elsalam, Noor, Badawi, Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: A randomized controlled study
Alam, Murshed, Gomes, Ivermectin as pre-exposure prophylaxis for COVID-19 among healthcare providers in a selected tertiary hospital in dhakaan observational study, Eur J Med Heal Sci
Ashburn, Thor, Drug repositioning: identifying and developing new uses for existing drugs, Nat Rev Drug Discov
Caly, Druce, Catton, Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect , the company' s public news and information, Antivir Res
Caly, Wagstaff, Jans, Nuclear trafficking of proteins from RNA viruses: potential target for antivirals?, Antivir Res
Chaccour, Casellas, Matteo, The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with nonsevere COVID-19: a pilot, double-blind, placebocontrolled, randomized clinical trial, EClinicalMedicine
Chaccour, Ruiz-Castillo, Richardson, The sars-cov-2 ivermectin navarra-isglobal trial (saint) to evaluate the potential of ivermectin to reduce covid-19 transmission in low risk, non-severe covid-19 patients in the first 48 hours after symptoms onset: a structured summary of a study protocol, Trials
Chahla, Prophylaxis covid-19 in health workers through intensive treatment with ivermectin and iotacarrageenan (Ivercar-Tuc), ClinicalTrials
Chong, Sullivan, New uses for old drugs, Nature
H Ector, Roberto, Psaltis, Study of the efficacy and safety of topical ivermectin + iota-carrageenan in the prophylaxis against COVID-19 in health personnel, J Biomed Res Clin Investig
Hirsch, Hector, Covid 19 and ivermectin prevention and treatment update, J Infect Dis Trav Med
Kory, Meduri, Varon, Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19, Am J Ther
Krolewiecki, Lifschitz, Moragas, Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial, EClinicalMedicine
Liu, Fang, Reagan, In silico drug repositioning: what we need to know, Drug Discov Today
Shouman, Hegazy, Nafae, Use of ivermectin as a potential chemoprophylaxis for COVID-19 in Egypt: a randomized clinical trial, J Clin Diagn Res
DOI record:
{
"DOI": "10.1097/mjt.0000000000001433",
"ISSN": [
"1075-2765"
],
"URL": "http://dx.doi.org/10.1097/MJT.0000000000001433",
"author": [
{
"affiliation": [],
"family": "Chahla",
"given": "Rossana E.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Medina Ruiz",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ortega",
"given": "Eugenia S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morales, RN",
"given": "Marcelo F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barreiro",
"given": "Francisco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "George",
"given": "Alexia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mancilla, RN",
"given": "Cesar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "D' Amato, RN",
"given": "Sylvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barrenechea",
"given": "Guillermo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goroso",
"given": "Daniel G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peral de Bruno",
"given": "Maria",
"sequence": "additional"
}
],
"container-title": "American Journal of Therapeutics",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
9,
2
]
],
"date-time": "2021-09-02T00:35:39Z",
"timestamp": 1630542939000
},
"deposited": {
"date-parts": [
[
2021,
9,
7
]
],
"date-time": "2021-09-07T20:00:46Z",
"timestamp": 1631044846000
},
"indexed": {
"date-parts": [
[
2022,
7,
11
]
],
"date-time": "2022-07-11T06:51:07Z",
"timestamp": 1657522267235
},
"is-referenced-by-count": 5,
"issue": "5",
"issued": {
"date-parts": [
[
2021,
8,
16
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2021
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://journals.lww.com/10.1097/MJT.0000000000001433",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "e601-e604",
"prefix": "10.1097",
"published": {
"date-parts": [
[
2021,
8,
16
]
]
},
"published-online": {
"date-parts": [
[
2021,
9
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1038/448645a",
"article-title": "New uses for old drugs",
"author": "Chong",
"doi-asserted-by": "crossref",
"first-page": "645",
"journal-title": "Nature.",
"key": "R1-20210907",
"volume": "448",
"year": "2007"
},
{
"DOI": "10.1016/j.drudis.2012.08.005",
"article-title": "In silico drug repositioning: what we need to know",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "110",
"journal-title": "Drug Discov Today",
"key": "R2-20210907",
"volume": "18",
"year": "2013"
},
{
"DOI": "10.1038/nrd1468",
"article-title": "Drug repositioning: identifying and developing new uses for existing drugs",
"author": "Ashburn",
"doi-asserted-by": "crossref",
"first-page": "673",
"journal-title": "Nat Rev Drug Discov.",
"key": "R3-20210907",
"volume": "3",
"year": "2004"
},
{
"DOI": "10.1016/j.antiviral.2012.06.008",
"article-title": "Nuclear trafficking of proteins from RNA viruses: potential target for antivirals?",
"author": "Caly",
"doi-asserted-by": "crossref",
"first-page": "202",
"journal-title": "Antivir Res",
"key": "R4-20210907",
"volume": "95",
"year": "2012"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect , the company' s public news and information",
"author": "Caly",
"doi-asserted-by": "crossref",
"first-page": "104787",
"journal-title": "Antivir Res",
"key": "R5-20210907",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1097/MJT.0000000000001377",
"article-title": "Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19",
"author": "Kory",
"doi-asserted-by": "crossref",
"first-page": "e299",
"journal-title": "Am J Ther.",
"key": "R6-20210907",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2020.100720",
"article-title": "The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial",
"author": "Chaccour",
"doi-asserted-by": "crossref",
"first-page": "100720",
"journal-title": "EClinicalMedicine.",
"key": "R7-20210907",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.100959",
"article-title": "Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial",
"author": "Krolewiecki",
"doi-asserted-by": "crossref",
"first-page": "100959",
"journal-title": "EClinicalMedicine.",
"key": "R8-20210907",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.31546/2633-8653.1007",
"article-title": "Study of the efficacy and safety of topical ivermectin + iota-carrageenan in the prophylaxis against COVID-19 in health personnel",
"author": "Héctor",
"doi-asserted-by": "crossref",
"first-page": "1007",
"journal-title": "J Biomed Res Clin Investig.",
"key": "R9-20210907",
"volume": "2",
"year": "2020"
},
{
"article-title": "Ivermectin as pre-exposure prophylaxis for COVID-19 among healthcare providers in a selected tertiary hospital in dhaka—an observational study",
"author": "Alam",
"first-page": "1",
"journal-title": "Eur J Med Heal Sci.",
"key": "R10-20210907",
"volume": "2",
"year": "2020"
},
{
"article-title": "Use of ivermectin as a potential chemoprophylaxis for COVID-19 in Egypt: a randomized clinical trial",
"author": "Shouman",
"first-page": "27",
"journal-title": "J Clin Diagn Res.",
"key": "R11-20210907",
"volume": "15",
"year": "2021"
},
{
"article-title": "Prophylaxis covid-19 in health workers through intensive treatment with ivermectin and iota-carrageenan (Ivercar-Tuc)",
"author": "Chahla",
"first-page": "NCT047017",
"journal-title": "ClinicalTrials.gov.",
"key": "R12-20210907",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1186/s13063-020-04421-z",
"article-title": "The sars-cov-2 ivermectin navarra-isglobal trial (saint) to evaluate the potential of ivermectin to reduce covid-19 transmission in low risk, non-severe covid-19 patients in the first 48 hours after symptoms onset: a structured summary of a study protocol",
"author": "Chaccour",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Trials",
"key": "R13-20210907",
"volume": "21",
"year": "2020"
},
{
"article-title": "Covid 19 and ivermectin prevention and treatment update",
"author": "Hirsch",
"first-page": "1",
"journal-title": "J Infect Dis Trav Med.",
"key": "R14-20210907",
"volume": "4",
"year": "2020"
},
{
"article-title": "Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: A randomized controlled study [published online ahead of print, 2021 Jun 2]",
"author": "Abd-Elsalam",
"journal-title": "J Med Virol.",
"key": "R15-20210907",
"year": "2021"
},
{
"DOI": "10.1186/s12941-020-00368-w",
"article-title": "Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19",
"author": "Sharun",
"doi-asserted-by": "crossref",
"first-page": "23",
"journal-title": "Ann Clin Microbiol Antimicrob.",
"key": "R16-20210907",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.3906/sag-2004-145",
"article-title": "Antiviral treatment of covid-19",
"author": "Şimşek Yavuz",
"doi-asserted-by": "crossref",
"first-page": "611",
"journal-title": "Turkish J Med Sci.",
"key": "R17-20210907",
"volume": "50",
"year": "2020"
},
{
"DOI": "10.1097/MJT.0000000000001402",
"article-title": "Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-analysis, and Trial Sequential Analysis to Inform Clinical Guidelines",
"author": "Bryant",
"doi-asserted-by": "crossref",
"first-page": "e434",
"journal-title": "Am J Ther.",
"key": "R18-20210907",
"volume": "28",
"year": "2021"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.1097/MJT.0000000000001433"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology",
"General Medicine"
],
"subtitle": [],
"title": "Intensive Treatment With Ivermectin and Iota-Carrageenan as Pre-exposure Prophylaxis for COVID-19 in Health Care Workers From Tucuman, Argentina",
"type": "journal-article",
"volume": "28"
}
