Effect of Sinamaz nasal drop on asymptomatic family members of COVID 19 patients: An open-label randomized controlled trial
et al., Phytotherapy Research, doi:10.1002/ptr.7915, IRCT20210515051305N1, Jul 2023
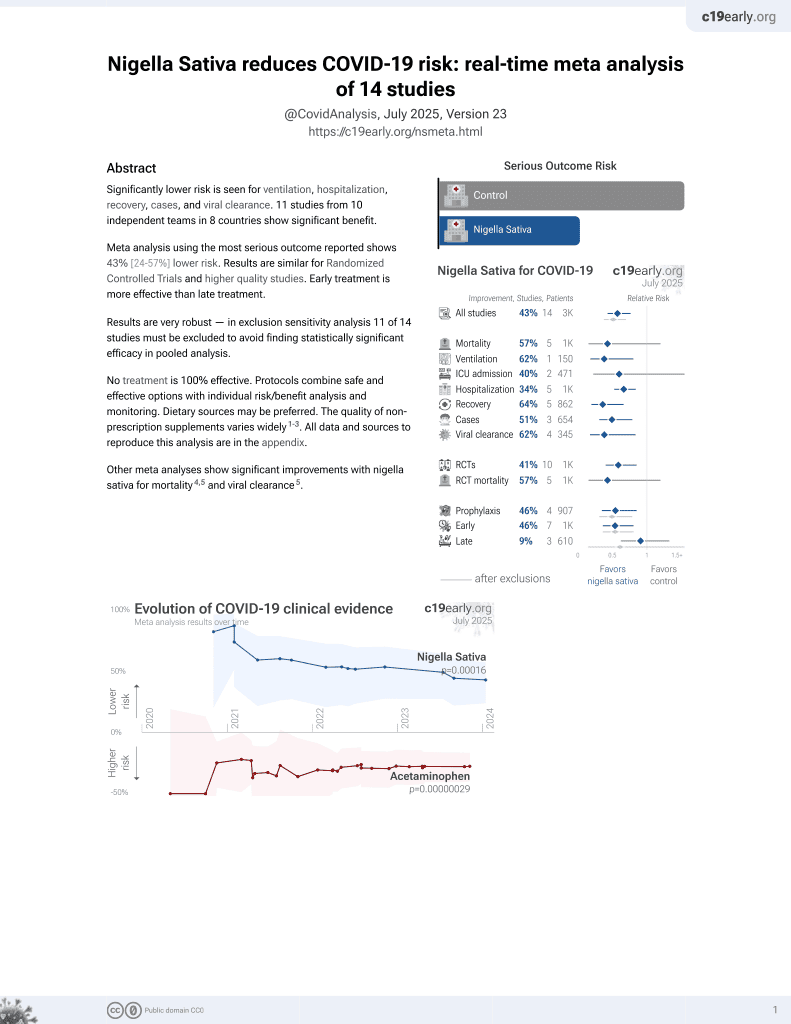
14th treatment shown to reduce risk in
January 2021, now with p = 0.00016 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 173 family members of COVID-19 patients, showing lower incidence of COVID-19 symptoms with nasal drops containing nigella sativa oil and olea europaea oil. One drop in each nostril twice daily for 7 days.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
|
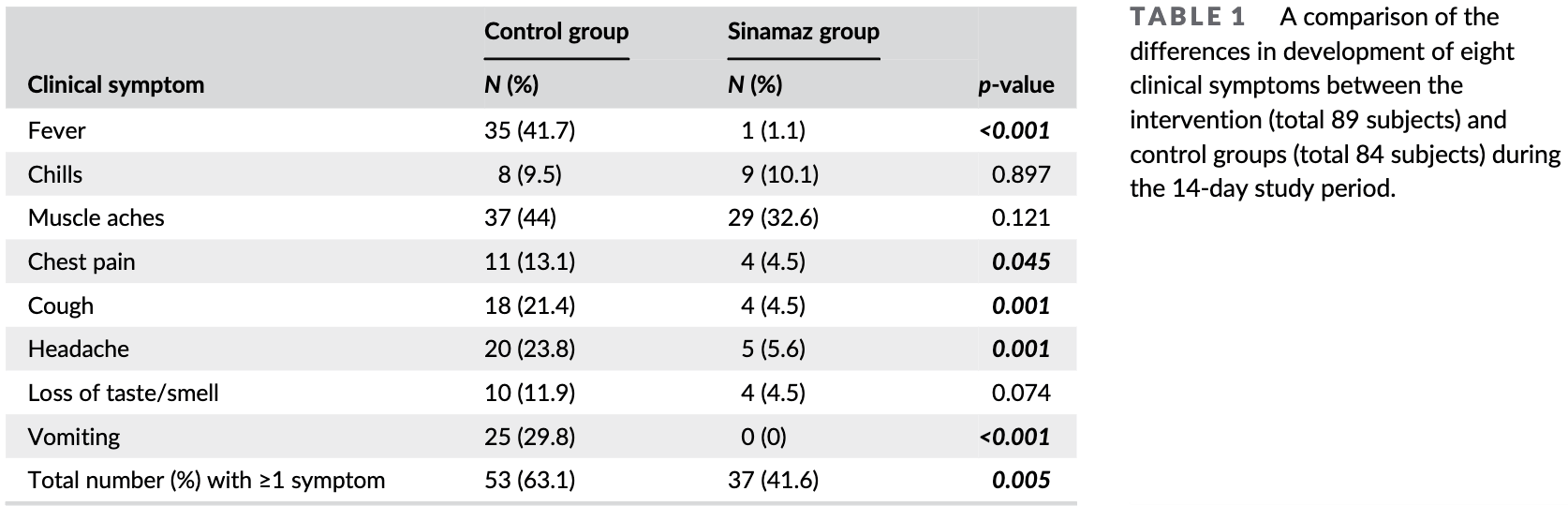
risk of symptomatic case, 34.1% lower, RR 0.66, p = 0.006, treatment 37 of 89 (41.6%), control 53 of 84 (63.1%), NNT 4.6, any symptom.
|
|
risk of symptomatic case, 97.3% lower, RR 0.03, p < 0.001, treatment 1 of 89 (1.1%), control 35 of 84 (41.7%), NNT 2.5, fever.
|
|
risk of symptomatic case, 65.7% lower, RR 0.34, p = 0.06, treatment 4 of 89 (4.5%), control 11 of 84 (13.1%), NNT 12, chest pain.
|
|
risk of symptomatic case, 62.2% lower, RR 0.38, p = 0.10, treatment 4 of 89 (4.5%), control 10 of 84 (11.9%), NNT 13, loss of taste/smell.
|
|
risk of symptomatic case, 26.0% lower, RR 0.74, p = 0.16, treatment 29 of 89 (32.6%), control 37 of 84 (44.0%), NNT 8.7, muscle ache.
|
|
risk of symptomatic case, 6.2% higher, RR 1.06, p = 1.00, treatment 9 of 89 (10.1%), control 8 of 84 (9.5%), chills.
|
|
risk of symptomatic case, 79.0% lower, RR 0.21, p = 0.001, treatment 4 of 89 (4.5%), control 18 of 84 (21.4%), NNT 5.9, cough.
|
|
risk of symptomatic case, 76.4% lower, RR 0.24, p < 0.001, treatment 5 of 89 (5.6%), control 20 of 84 (23.8%), NNT 5.5, headache.
|
|
risk of symptomatic case, 98.1% lower, RR 0.02, p < 0.001, treatment 0 of 89 (0.0%), control 25 of 84 (29.8%), NNT 3.4, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), vomiting.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Daneshfard et al., 16 Jul 2023, Randomized Controlled Trial, Iran, peer-reviewed, mean age 39.5 (treatment) 34.0 (control), 16 authors, study period 16 June, 2021 - 22 May, 2022, this trial uses multiple treatments in the treatment arm (combined with olea europaea oil) - results of individual treatments may vary, trial IRCT20210515051305N1.
DOI record:
{
"DOI": "10.1002/ptr.7915",
"ISSN": [
"0951-418X",
"1099-1573"
],
"URL": "http://dx.doi.org/10.1002/ptr.7915",
"alternative-id": [
"10.1002/ptr.7915"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-12-07"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-05-27"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-07-16"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6729-9113",
"affiliation": [
{
"name": "Chronic Respiratory Diseases Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD) Shahid Beheshti University of Medical Sciences Tehran Iran"
},
{
"name": "Traditional Medicine Clinical Trial Research Center Shahed University Tehran Iran"
},
{
"name": "Persian Medicine Network (PMN) Universal Scientific Education and Research Network (USERN) Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Daneshfard",
"given": "Babak",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Scientific Research Kian Asa Center for Preventive Medicine (SBMU Supervised Area) & Scientific Authority Center for Countering Biological Treats Tehran Iran"
}
],
"family": "Aghanouri",
"given": "Reza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, Faculty of Medical Sciences Tarbiat Modares University Tehran Iran"
}
],
"family": "Kazemnejad",
"given": "Anoshiravan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5998-7303",
"affiliation": [
{
"name": "School of Life Sciences University of Technology Sydney Ultimo New South Wales Australia"
},
{
"name": "Ingham Institute for Applied Medical Research Liverpool New South Wales Australia"
}
],
"authenticated-orcid": false,
"family": "Fatima Shad",
"given": "Kaneez",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ingham Institute for Applied Medical Research Liverpool New South Wales Australia"
},
{
"name": "Department of Neurophysiology Liverpool Hospital Liverpool New South Wales Australia"
}
],
"family": "Cordato",
"given": "Dennis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Life Sciences University of Technology Sydney Ultimo New South Wales Australia"
},
{
"name": "Ingham Institute for Applied Medical Research Liverpool New South Wales Australia"
}
],
"family": "Soubra",
"given": "Wissam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Traditional Medicine Clinical Trial Research Center Shahed University Tehran Iran"
},
{
"name": "Department of Traditional Persian Medicine, School of Medicine Shahed University Tehran Iran"
}
],
"family": "Bahaeddin",
"given": "Zahra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Molecular Biology Research Center, Systems Biology and Poisonings Institute Bagiyatallah University of Medical Science Tehran Iran"
}
],
"family": "Hosseini",
"given": "Seyed Mohammad Javad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Virology, Faculty of Medical Sciences Tarbiat Modares University Tehran Iran"
}
],
"family": "Ravanshad",
"given": "Mehrdad",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6607-0640",
"affiliation": [
{
"name": "Traditional Medicine Clinical Trial Research Center Shahed University Tehran Iran"
},
{
"name": "Department of Traditional Persian Medicine, School of Medicine Shahed University Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Alijaniha",
"given": "Fatemeh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Pharmacy Shahid Beheshti University of Medical Sciences Tehran Iran"
}
],
"family": "Kamalinejad",
"given": "Mohammad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Traditional Medicine Clinical Trial Research Center Shahed University Tehran Iran"
}
],
"family": "Jafari Hajati",
"given": "Razieh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Traditional Medicine Clinical Trial Research Center Shahed University Tehran Iran"
}
],
"family": "Rafie Tari",
"given": "Amir",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Traditional Medicine Shahid Beheshti University of Medical Sciences Tehran Iran"
}
],
"family": "Ghaffari",
"given": "Farzaneh",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9239-768X",
"affiliation": [
{
"name": "Traditional Medicine Clinical Trial Research Center Shahed University Tehran Iran"
},
{
"name": "Department of Traditional Persian Medicine, School of Medicine Shahed University Tehran Iran"
},
{
"name": "Hikmat, Islamic and Traditional Medicine Department The Academy of Medical Sciences Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Naseri",
"given": "Mohsen",
"sequence": "additional"
}
],
"container-title": "Phytotherapy Research",
"container-title-short": "Phytotherapy Research",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
7,
17
]
],
"date-time": "2023-07-17T04:44:09Z",
"timestamp": 1689569049000
},
"deposited": {
"date-parts": [
[
2024,
4,
9
]
],
"date-time": "2024-04-09T12:12:22Z",
"timestamp": 1712664742000
},
"indexed": {
"date-parts": [
[
2024,
4,
9
]
],
"date-time": "2024-04-09T12:40:29Z",
"timestamp": 1712666429086
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2023,
7,
16
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2024,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
7,
16
]
],
"date-time": "2023-07-16T00:00:00Z",
"timestamp": 1689465600000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7915",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "1727-1730",
"prefix": "10.1002",
"published": {
"date-parts": [
[
2023,
7,
16
]
]
},
"published-online": {
"date-parts": [
[
2023,
7,
16
]
]
},
"published-print": {
"date-parts": [
[
2024,
4
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.3109/14764172.2014.968583",
"doi-asserted-by": "publisher",
"key": "e_1_2_2_2_1"
},
{
"article-title": "Prolegomena to a true integrative medical paradigm",
"author": "Daneshfard B.",
"first-page": "50",
"issue": "2",
"journal-title": "Alternative Therapies in Health and Medicine",
"key": "e_1_2_2_3_1",
"volume": "25",
"year": "2019"
},
{
"DOI": "10.1111/1440-1681.13553",
"doi-asserted-by": "publisher",
"key": "e_1_2_2_4_1"
},
{
"DOI": "10.1002/ptr.6895",
"doi-asserted-by": "publisher",
"key": "e_1_2_2_5_1"
},
{
"article-title": "SARS‐CoV‐2‐neutralising monoclonal antibodies for treatment of COVID‐19",
"author": "Kreuzberger N.",
"first-page": "D013825",
"issue": "9",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_2_6_1",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1073/pnas.2109229118",
"doi-asserted-by": "publisher",
"key": "e_1_2_2_7_1"
},
{
"DOI": "10.1016/j.cmi.2020.10.036",
"doi-asserted-by": "publisher",
"key": "e_1_2_2_8_1"
},
{
"key": "e_1_2_2_9_1",
"unstructured": "WHO. (n.d.).WHO (COVID‐19) Homepage. Retrieved fromhttps://covid19.who.int"
}
],
"reference-count": 8,
"references-count": 8,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/ptr.7915"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology"
],
"subtitle": [],
"title": "Effect of Sinamaz nasal drop on asymptomatic family members of <scp>COVID</scp> 19 patients: An open‐label randomized controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "38"
}