Povidone iodine, hydrogen peroxide and chlorhexidine mouthwashes reduce SARS-CoV2 burden in whole mouth fluid and respiratory droplets
et al., medRxiv, doi:10.1101/2021.02.25.21252488 , Mar 2021
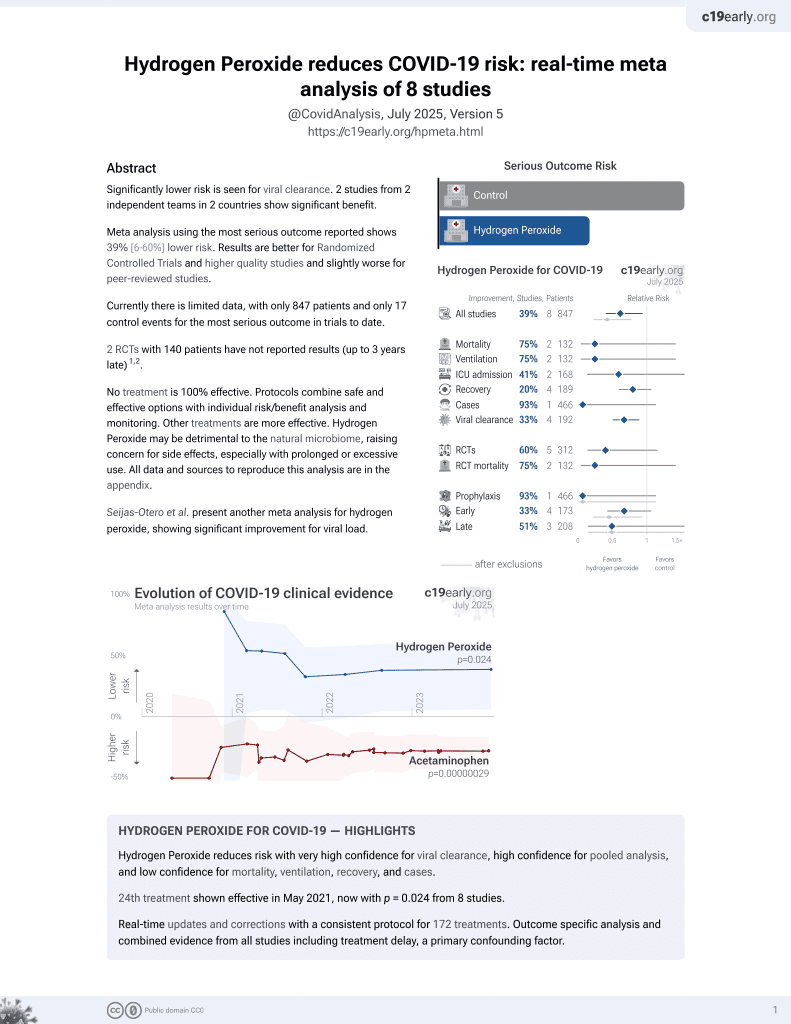
24th treatment shown to reduce risk in
May 2021, now with p = 0.024 from 8 studies.
Lower risk for viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Study of SARS-CoV-2 burden in whole mouth fluid and respiratory droplets with povidone iodine, hydrogen peroxide, and chlorhexidine mouthwashes in 36 hospitalized COVID-19 patients using PCR and rapid antigen testing. There were significant reductions in SARS-CoV-2 burden with all treatments in both respiratory droplets and whole mouth fluid.
Analysis of short-term changes in viral load using PCR may not detect
effective treatments because PCR is unable to differentiate between intact
infectious virus and non-infectious or destroyed virus particles. For example
Tarragó-Gil, Alemany perform RCTs with cetylpyridinium chloride
(CPC) mouthwash that show no difference in PCR viral load, however there was
significantly increased detection of SARS-CoV-2 nucleocapsid protein,
indicating viral lysis. CPC inactivates SARS-CoV-2 by degrading its membrane,
exposing the nucleocapsid of the virus. To better estimate changes in viral
load and infectivity, methods like viral culture that can
differentiate intact vs. degraded virus are preferred.
.
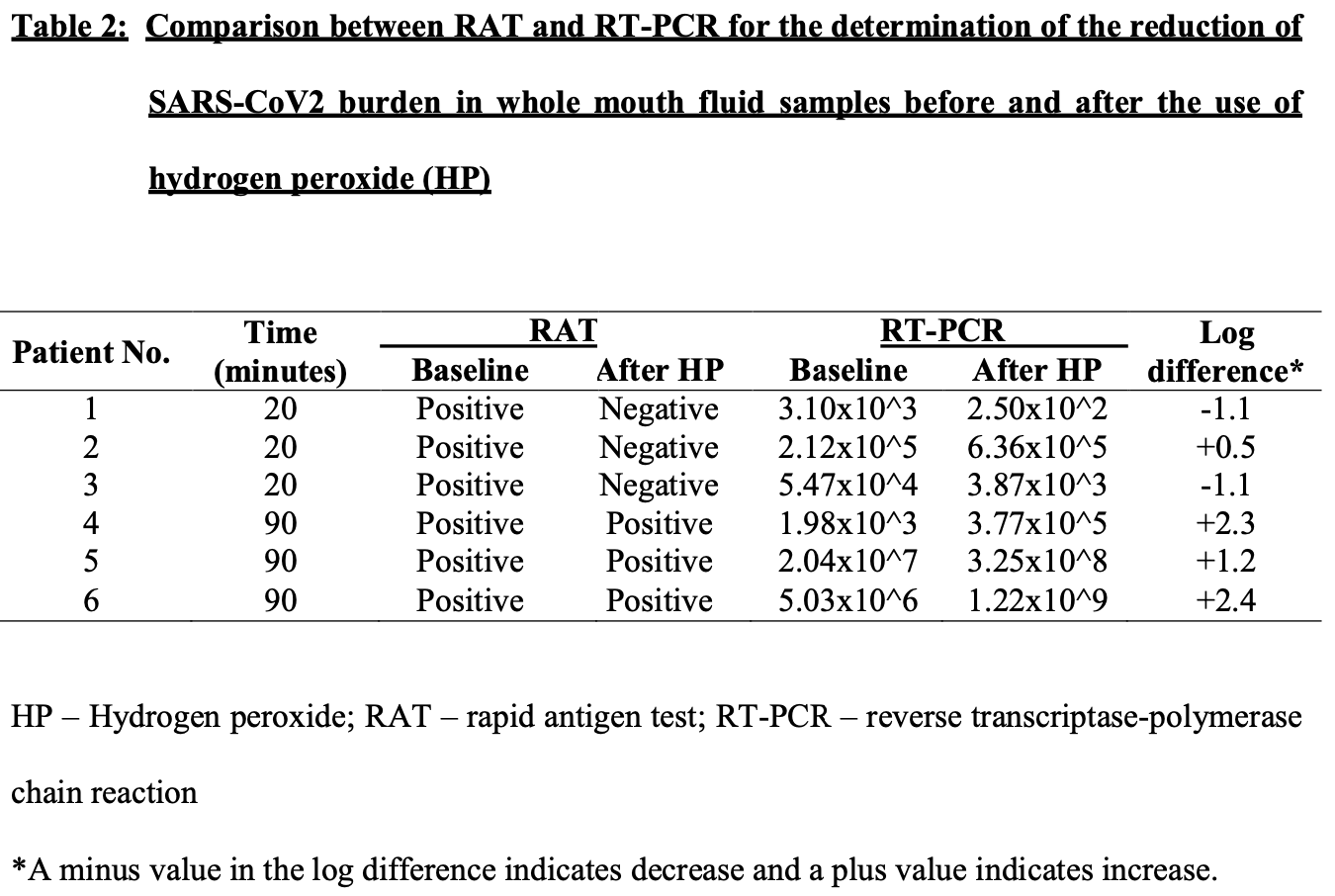
Authors perform antigen testing for 6 hydrogen peroxide patients, showing that 50% became negative after treatment.
Study covers chlorhexidine, hydrogen peroxide, and povidone-iodine.
|
risk of no viral clearance, 50.0% lower, RR 0.50, p = 0.18, treatment 3 of 6 (50.0%), control 6 of 6 (100.0%), NNT 2.0, antigen results.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Jayaraman et al., 1 Mar 2021, prospective, India, preprint, 12 authors.
Abstract: medRxiv preprint doi: https://doi.org/10.1101/2021.02.25.21252488; this version posted March 1, 2021. The copyright holder for this preprint
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
It is made available under a CC-BY-NC-ND 4.0 International license .
Povidone iodine, hydrogen peroxide and chlorhexidine mouthwashes reduce SARS-CoV2
burden in whole mouth fluid and respiratory droplets
Bagavad Gita Jayaraman1*, Gunaseelan Rajan1, Priya Kannian2*, Chandra Lavanya4, Krittika
Ravichandran1, Nagalingeswaran Kumarasamy3, Kannan Ranganathan4, Veeraraghavan Aswini2,
Pasuvaraj Mahanathi2, Stephen Challacombe5, Jennifer Webster-Cyriaque6, Newell W
Johnson1,3,5,7
1
2
Chennai Dental Research Foundation, Chennai, India
VHS Laboratory Services, Department of Clinical Research, VHS Hospital, Chennai, India
3
VHS-Infectious Diseases Medical Centre, VHS Hospital, Chennai, India
4
Department of Oral Pathology, Ragas Dental College and Hospital, Chennai, India
5
Faculty of Dentistry, Oral & Craniofacial Sciences, King’s College London, UK
6
University of North Carolina, Chapel Hill, USA
7
Menzies Health Institute Queensland, Griffith University, Queensland, Australia
Address for Correspondence:
* Dr. Bagavad Gita Jayaraman
Research Scientist
Chennai Dental Research Foundation
No. 56, Dr. R. K. Salai
6th Floor, Mylapore
Chennai – 600004
Tamil Nadu, India
Ph: 91-44-42103440
Email: gita70.geetha@gmail.com
* Dr. Priya Kannian
Scientist & Head
Department of Clinical Research
VHS Hospital
Rajiv Gandhi Salai
Taramani, Chennai – 600113
Tamil Nadu, India
Ph: 91-44-22541972
Email: priyakannian@gmail.com
Word count: 599 words
1
NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice.
medRxiv preprint doi: https://doi.org/10.1101/2021.02.25.21252488; this version posted March 1, 2021. The copyright holder for this preprint
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
It is made available under a CC-BY-NC-ND 4.0 International license .
DOI record:
{
"DOI": "10.1101/2021.02.25.21252488",
"URL": "http://dx.doi.org/10.1101/2021.02.25.21252488",
"abstract": "<jats:p>SARS-CoV2 is transmitted primarily through oral mouth secretions and respiratory droplets. Commercial mouthwashes, povidone iodine (PI), hydrogen peroxide (HP) and chlorhexidine (CHX) have been tested in cell culture and RT-PCR studies for their efficacy to reduce SARS-CoV2 burden. Here, we evaluated SARS-CoV2 burden in whole mouth fluid (WMF) and respiratory droplets (RD) samples before and after the use of PI, HP or CHX mouthwashes in hospitalized COVID-19 patients using RT-PCR and rapid antigen test (RAT). Thirty-six SARS-CoV2 RT-PCR-positive in-patients were randomly assigned to one of the four groups: 20 and 60 minutes after 1% w/v PI or 1.5% HP; 90 and 180 minutes after 1.5% HP or 0.2% w/v CHX. WMF and RD samples were collected concurrently at baseline and after the two different time points. RD (92%) showed a higher reduction in SARS-CoV2 burden than WMF samples (50%; p=0.008). SARS-CoV2 burden was statistically lower at both 20 minutes (p=0.02) and 60 minutes (p=0.03) with PI; at 20 minutes with HP (p=0.0001); and 90 minutes with CHX (p=0.04). The overall and individual mean logarithmic reductions in the WMF and RD samples were greater than 1.0 at 20, 60 and 90 minutes after PI, HP or CHX. RAT-positive patients at 90 minutes post-treatment (n=3) demonstrated a one log increase in virus copies. Among the three RAT-negative post-treatment patients, SARS-CoV2 burden declined by one log in two while the third patient had a slight increase in RNA copies. In conclusion, we have shown for the first time that the mouthwashes, PI, HP and CHX can reduce the SARS-CoV2 burden in the concurrently collected RD and WMF samples. RAT is more appropriate than RT-PCR to evaluate the efficacy of the mouthwashes.</jats:p>",
"accepted": {
"date-parts": [
[
2021,
3,
1
]
]
},
"author": [
{
"affiliation": [],
"family": "Jayaraman",
"given": "Bagavad Gita",
"sequence": "first"
},
{
"affiliation": [],
"family": "Rajan",
"given": "Gunaseelan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2790-6676",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kannian",
"given": "Priya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lavanya",
"given": "Chandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ravichandran",
"given": "Krittika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumarasamy",
"given": "Nagalingeswaran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ranganathan",
"given": "Kannan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aswini",
"given": "Veeraraghavan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahanathi",
"given": "Pasuvaraj",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Challacombe",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Webster-Cyriaque",
"given": "Jennifer",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5866-262X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Johnson",
"given": "Newell W",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
3,
1
]
],
"date-time": "2021-03-01T17:25:17Z",
"timestamp": 1614619517000
},
"deposited": {
"date-parts": [
[
2021,
3,
3
]
],
"date-time": "2021-03-03T16:05:25Z",
"timestamp": 1614787525000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2023,
8,
22
]
],
"date-time": "2023-08-22T17:16:08Z",
"timestamp": 1692724568451
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 4,
"issued": {
"date-parts": [
[
2021,
3,
1
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.02.25.21252488",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
3,
1
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
3,
1
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1177/0022034520967933",
"doi-asserted-by": "crossref",
"key": "2021030308050679000_2021.02.25.21252488v1.1",
"unstructured": "Carrouel F , Goncalves LS , Conte MP , et al. Antiviral Activity of Reagents in Mouth Rinses against SARS-CoV-2. J Dent Res. 2020 Oct 22 : 0022034520967933."
},
{
"DOI": "10.1001/jamaoto.2020.5490",
"doi-asserted-by": "publisher",
"key": "2021030308050679000_2021.02.25.21252488v1.2"
},
{
"DOI": "10.1111/odi.13793",
"doi-asserted-by": "publisher",
"key": "2021030308050679000_2021.02.25.21252488v1.3"
},
{
"DOI": "10.1111/odi.13526",
"doi-asserted-by": "publisher",
"key": "2021030308050679000_2021.02.25.21252488v1.4"
},
{
"DOI": "10.1101/2020.09.14.20186494",
"doi-asserted-by": "publisher",
"key": "2021030308050679000_2021.02.25.21252488v1.5"
}
],
"reference-count": 5,
"references-count": 5,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2021.02.25.21252488"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Povidone iodine, hydrogen peroxide and chlorhexidine mouthwashes reduce SARS-CoV2 burden in whole mouth fluid and respiratory droplets",
"type": "posted-content"
}
jayaraman