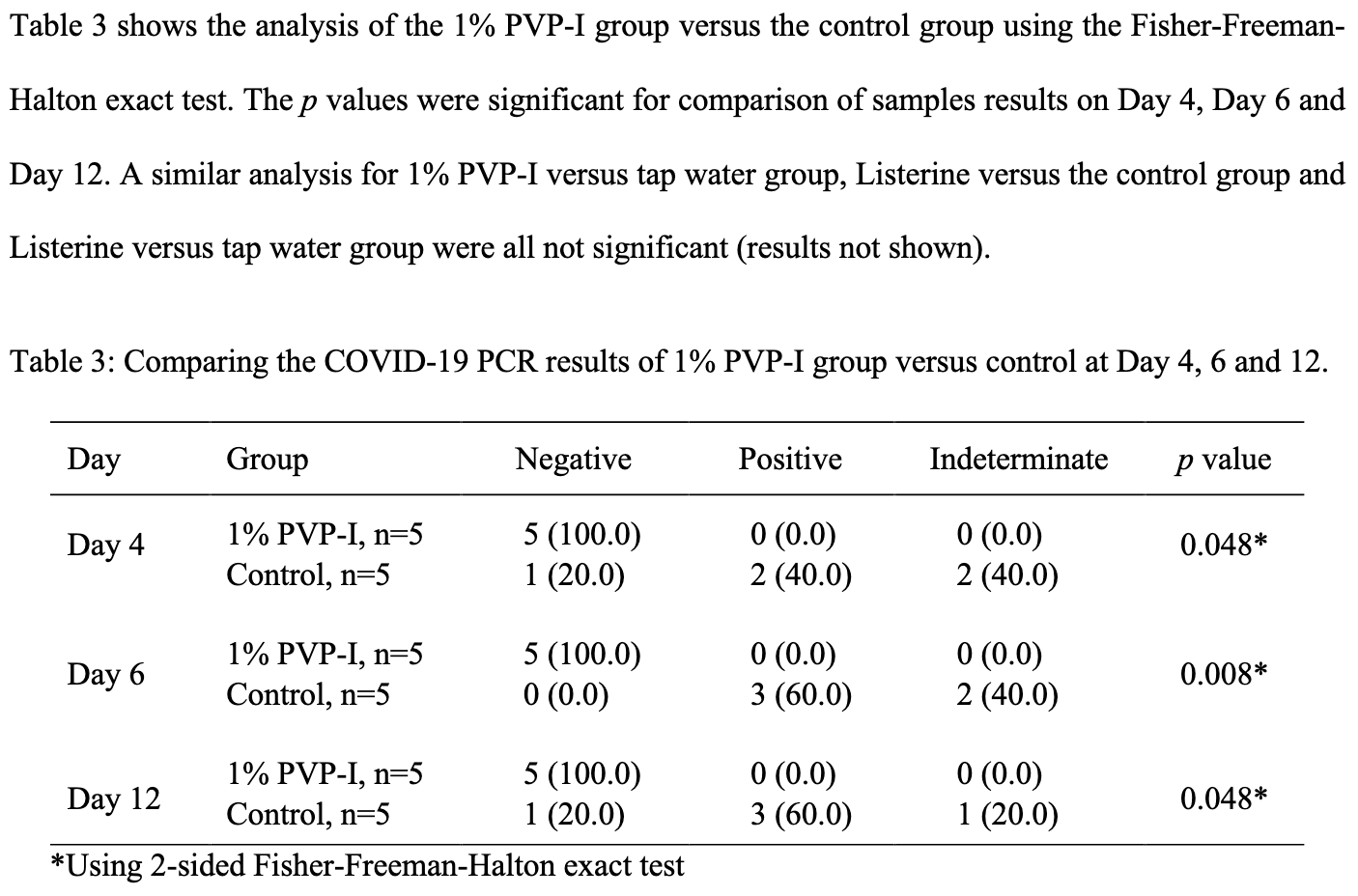
Early viral clearance among COVID-19 patients when gargling with povidone-iodine and essential oils: a pilot clinical trial
et al., medRxiv, doi:10.1101/2020.09.07.20180448, NCT04410159, Sep 2020
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Tiny RCT with 5 PVP-I patients, gargling 30 seconds, 3x per day, and 5 control patients (essential oils and tap water were also tested), showing improved viral clearance with PVP-I.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
|
risk of no viral clearance, 85.7% lower, RR 0.14, p = 0.17, treatment 0 of 5 (0.0%), control 3 of 5 (60.0%), NNT 1.7, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 12.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mohamed et al., 9 Sep 2020, Randomized Controlled Trial, Malaysia, preprint, 16 authors, study period 22 June, 2020 - 29 June, 2020, trial NCT04410159 (history).
EARLY VIRAL CLEARANCE AMONG COVID-19 PATIENTS WHEN GARGLING WITH POVIDONE-IODINE AND ESSENTIAL OILS – A CLINICAL TRIAL
doi:10.1101/2020.09.07.20180448
Background: Gargling had been reported to have significant roles in the prevention and treatment of respiratory tract infections. The purpose of this study was to assess the ability of regular gargling to eliminate SARS-CoV-2 in the oropharynx and nasopharynx. Methodology: This pilot, open labeled, randomized, parallel study compared the effect of 30 seconds, 3 times/day gargling using 1% povidone-iodine (PVP-I), essential oils and tap water on SARS-CoV-2 viral clearance among COVID-19 patients in a tertiary hospital in Kuala Lumpur. Progress was monitored by day 4,6 and 12 PCR (Ct value), gargling and symptoms diary as well as clinical observations. Results: Five confirmed Stage 1 COVID-19 patients were recruited for each arm. The age range was from 22 to 56 years old. The majority were males. Two respondents had co-morbidities, which were asthma and obesity. Viral clearance was achieved at day 6 in 100%, 80%, 20% and 0% for 1% PVP-I, essential oils, tap water and control group respectively. Analysis of 1% PVP-I group versus control group showed significant p-value for comparison of PCR results on Day 4, Day 6 and Day 12. Conclusions: This preliminary study showed that gargling with 1% PVP-I and essential oils show great potential to be part of the treatment and management of Stage 1 COVID-19. Larger studies are required to ascertain the benefit of gargling for different stages of COVID-19 patients. This study was registered in clinicaltrial.gov (NCT04410159).
Author's Contribution NAM and NK were the co-principal investigator, NB and WSWS designed the study and analysed the data, PP and NAZ supervised the study implementation, NEMK, CXK, ANKS and SMS monitored participants and collected data, UKA, SNO and WKK involved in molecular investigations, ZZR and MNAS wrote the draft, II supervised the study and proofread the manuscript.
References
Astani, Reichling, Schnitzler, Comparative Study on the Antiviral Activity of Selected Monoterpenes Derived from Essential Oils, Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives
Bigliardi, Lorenz, Abdul Latiff Alsagoff, Yehia El-Kafrawi, Pyon et al., Povidone Iodine in Wound Healing: A Review of Current Concepts and Practices, International Journal of Surgery
Cascella, Rajnik, Cuomo, Scott C Dulebohn, Di, Features, Evaluation and Treatment Coronavirus (COVID-19)
Challacombe, Kirk-Bayley, Sunkaraneni, Combes, Povidone Iodine, British Dental Journal
Chen, Rui, Wang, Zhao, Cui et al., A Mathematical Model for Simulating the Phase-Based Transmissibility of a Novel Coronavirus, Infectious Diseases of Poverty
Ecdc, Novel Coronavirus (SARS-CoV-2) Discharge Criteria for Confirmed COVID-19 Cases -When Is It Safe to Discharge COVID-19 Cases from the Hospital or End Home Isolation
Eggers, Eickmann, Zorn, Rapid and Effective Virucidal Activity of Povidone-Iodine Products against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA), Infectious Diseases and Therapy
Eggers, Koburger-Janssen, Eickmann, Zorn, In Vitro Bactericidal and Virucidal Efficacy of Povidone-Iodine Gargle/Mouthwash Against Respiratory and Oral Tract Pathogens, Infectious Diseases and Therapy, doi:10.1007/s40121-018-0200-7
Hassandarvish, Tiong, Sazaly, Mohamed, Arumugam et al., Povidone Iodine Gargle and Mouthwash, British Dental Journal, doi:10.1038/s41415-020-1794-1
Hu, Song, Xu, Jin, Chen et al., Clinical Characteristics of 24 Asymptomatic Infections with COVID-19 Screened among Close Contacts in Nanjing, China, Science China Life Sciences
Jin, Yang, Ji, Wu, Chen et al., Virology, Epidemiology, Pathogenesis, and Control of COVID-19, Viruses
Kanagalingam, Feliciano, Hah, Labib, Le et al., Practical Use of Povidone-Iodine Antiseptic in the Maintenance of Oral Health and in the Prevention and Treatment of Common Oropharyngeal Infections, International Journal of Clinical Practice, doi:10.1111/ijcp.12707
Kitamura, Satomura, Kawamura, Yamada, Takashima et al., Can We Prevent Influenza-like Illnesses by Gargling?, Internal Medicine, doi:10.2169/internalmedicine.46.0104
Mady, Kubik, Baddour, Snyderman, Nicholas R Rowan, Consideration of Povidone-Iodine as a Public Health Intervention for COVID-19: Utilization as 'Personal Protective Equipment' for Frontline Providers Exposed in High-Risk Head and Neck and Skull Base Oncology Care, Oral Oncology
Manger, Walshaw, Fitzgerald, Doughty, Wanyonyi et al., Evidence Summary: The Relationship between Oral Health and Pulmonary Disease, British Dental Journal
Mason, Pathogenesis of COVID-19 from a Cell Biology Perspective, Eur Respiratory Soc
Meiller, Silva, Ferreira, Jabra-Rizk, Kelley et al., Efficacy of Listerine® Antiseptic in Reducing Viral Contamination of Saliva, Journal of Clinical Periodontology
Nadjib, Mohamed, Effective Antiviral Activity of Essential Oils and Their Characteristic Terpenes against Coronaviruses: An Update, J. Pharmacol. Clin. Toxicol
Pattanshetty, Narayana, Radhakrishnan, Povidone-iodine Gargle as a Prophylactic Intervention to Interrupt the Transmission of SARS-CoV-2, Oral Diseases
Satomura, Kitamura, Kawamura, Shimbo, Watanabe et al., Prevention of Upper Respiratory Tract Infections by Gargling: A Randomized Trial, American Journal of Preventive Medicine, doi:10.1016/j.amepre.2005.06.013
Toit, Du, Outbreak of a Novel Coronavirus, Nature Reviews Microbiology, doi:10.1038/s41579-020-0332-0
Toyoizumi, Yamada, Matsumoto, Sameshima, Gargling with Green Tea for Influenza Prophylaxis: A Pilot Clinical Study, Japanese Journal of Clinical Pharmacology and Therapeutics, doi:10.3999/jscpt.44.459
Uhm, Sun, Young Ahn, Hoon Hyun, Sohn et al., Patterns of Viral Clearance in the Natural Course of Asymptomatic Coronavirus Disease 2019 (COVID-19): Comparison with Symptomatic Nonsevere COVID-19, International Journal of Infectious Diseases
Wölfel, Victor M Corman, Guggemos, Seilmaier, Zange et al., Virological Assessment of Hospitalized Patients with COVID-2019, Nature
DOI record:
{
"DOI": "10.1101/2020.09.07.20180448",
"URL": "http://dx.doi.org/10.1101/2020.09.07.20180448",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Gargling had been reported to have significant roles in the prevention and treatment of respiratory tract infections. The purpose of this study was to assess the ability of regular gargling to eliminate SARS-CoV-2 in the oropharynx and nasopharynx.</jats:p></jats:sec><jats:sec><jats:title>Methodology</jats:title><jats:p>This pilot, open labeled, randomized, parallel study compared the effect of 30 seconds, 3 times/day gargling using 1% povidone-iodine (PVP-I), essential oils and tap water on SARS-CoV-2 viral clearance among COVID-19 patients in a tertiary hospital in Kuala Lumpur. Progress was monitored by day 4,6 and 12 PCR (Ct value), gargling and symptoms diary as well as clinical observations.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Five confirmed Stage 1 COVID-19 patients were recruited for each arm. The age range was from 22 to 56 years old. The majority were males. Two respondents had co-morbidities, which were asthma and obesity. Viral clearance was achieved at day 6 in 100%, 80%, 20% and 0% for 1% PVP-I, essential oils, tap water and control group respectively. Analysis of 1% PVP-I group versus control group showed significant p-value for comparison of PCR results on Day 4, Day 6 and Day 12.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>This preliminary study showed that gargling with 1% PVP-I and essential oils show great potential to be part of the treatment and management of Stage 1 COVID-19. Larger studies are required to ascertain the benefit of gargling for different stages of COVID-19 patients. This study was registered in clinicaltrial.gov (<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04410159\">NCT04410159</jats:ext-link>).</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2020,
9,
9
]
]
},
"author": [
{
"affiliation": [],
"family": "Mohamed",
"given": "Nurul Azmawati",
"sequence": "first"
},
{
"affiliation": [],
"family": "Baharom",
"given": "Nizam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sulaiman",
"given": "Wan Shahida Wan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rashid",
"given": "Zetti Zainol",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ken",
"given": "Wong Kon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ali",
"given": "Umi Kalsom",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Othman",
"given": "Siti Norlia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Samat",
"given": "Muttaqillah Najihan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kori",
"given": "Najma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Periyasamy",
"given": "Petrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zakaria",
"given": "Nor Azizan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sugurmar",
"given": "Agni Nhirmal Kumar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kazmin",
"given": "Nur Ezzaty Mohammad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khee",
"given": "Cheong Xiong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saniman",
"given": "Siti Mariyam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Isahak",
"given": "Ilina",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
9,
9
]
],
"date-time": "2020-09-09T22:35:27Z",
"timestamp": 1599690927000
},
"deposited": {
"date-parts": [
[
2021,
2,
6
]
],
"date-time": "2021-02-06T11:37:33Z",
"timestamp": 1612611453000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T07:29:53Z",
"timestamp": 1698823793180
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 17,
"issued": {
"date-parts": [
[
2020,
9,
9
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2020.09.07.20180448",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2020,
9,
9
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2020,
9,
9
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1002/ptr.2955",
"article-title": "Comparative Study on the Antiviral Activity of Selected Monoterpenes Derived from Essential Oils",
"doi-asserted-by": "crossref",
"first-page": "673",
"issue": "5",
"journal-title": "Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives",
"key": "2021020411450666000_2020.09.07.20180448v1.1",
"volume": "24",
"year": "2010"
},
{
"DOI": "10.1016/j.ijsu.2017.06.073",
"article-title": "Povidone Iodine in Wound Healing: A Review of Current Concepts and Practices",
"doi-asserted-by": "crossref",
"first-page": "260",
"journal-title": "International Journal of Surgery",
"key": "2021020411450666000_2020.09.07.20180448v1.2",
"volume": "44",
"year": "2017"
},
{
"key": "2021020411450666000_2020.09.07.20180448v1.3",
"unstructured": "Cascella, Marco , Michael Rajnik , Arturo Cuomo , Scott C Dulebohn , and Raffaela Di Napoli . 2020. “Features, Evaluation and Treatment Coronavirus (COVID-19).” In Statpearls [Internet]. StatPearls Publishing."
},
{
"DOI": "10.1038/s41415-020-1589-4",
"article-title": "Povidone Iodine",
"doi-asserted-by": "crossref",
"first-page": "656",
"issue": "9",
"journal-title": "British Dental Journal",
"key": "2021020411450666000_2020.09.07.20180448v1.4",
"volume": "228",
"year": "2020"
},
{
"DOI": "10.1186/s40249-020-00646-x",
"doi-asserted-by": "publisher",
"key": "2021020411450666000_2020.09.07.20180448v1.5"
},
{
"key": "2021020411450666000_2020.09.07.20180448v1.6",
"unstructured": "ECDC. 2020. “Technical Report: Novel Coronavirus (SARS-CoV-2) Discharge Criteria for Confirmed COVID-19 Cases – When Is It Safe to Discharge COVID-19 Cases from the Hospital or End Home Isolation?” Https://Www.Ecdc.Europa.Eu/Sites/Default/Files/Documents/COVID-19-Discharge-Criteria.Pdf. Page 3. 2020."
},
{
"DOI": "10.1007/s40121-015-0091-9",
"article-title": "Rapid and Effective Virucidal Activity of Povidone-Iodine Products against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA)",
"doi-asserted-by": "crossref",
"first-page": "491",
"issue": "4",
"journal-title": "Infectious Diseases and Therapy",
"key": "2021020411450666000_2020.09.07.20180448v1.7",
"volume": "4",
"year": "2015"
},
{
"DOI": "10.1007/s40121-018-0200-7",
"article-title": "In Vitro Bactericidal and Virucidal Efficacy of Povidone-Iodine Gargle/Mouthwash Against Respiratory and Oral Tract Pathogens",
"doi-asserted-by": "crossref",
"first-page": "249",
"issue": "2",
"journal-title": "Infectious Diseases and Therapy",
"key": "2021020411450666000_2020.09.07.20180448v1.8",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1038/s41415-020-1794-1",
"article-title": "Povidone Iodine Gargle and Mouthwash",
"doi-asserted-by": "crossref",
"first-page": "900",
"issue": "12",
"journal-title": "British Dental Journal",
"key": "2021020411450666000_2020.09.07.20180448v1.9",
"volume": "228",
"year": "2020"
},
{
"DOI": "10.1007/s11427-020-1661-4",
"article-title": "Clinical Characteristics of 24 Asymptomatic Infections with COVID-19 Screened among Close Contacts in Nanjing, China",
"doi-asserted-by": "crossref",
"first-page": "706",
"issue": "5",
"journal-title": "Science China Life Sciences",
"key": "2021020411450666000_2020.09.07.20180448v1.10",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.3390/v12040372",
"doi-asserted-by": "publisher",
"key": "2021020411450666000_2020.09.07.20180448v1.11"
},
{
"DOI": "10.1111/ijcp.12707",
"article-title": "Practical Use of Povidone-Iodine Antiseptic in the Maintenance of Oral Health and in the Prevention and Treatment of Common Oropharyngeal Infections",
"doi-asserted-by": "crossref",
"first-page": "1247",
"issue": "11",
"journal-title": "International Journal of Clinical Practice",
"key": "2021020411450666000_2020.09.07.20180448v1.12",
"volume": "69",
"year": "2015"
},
{
"DOI": "10.2169/internalmedicine.46.0104",
"doi-asserted-by": "publisher",
"key": "2021020411450666000_2020.09.07.20180448v1.13"
},
{
"DOI": "10.1016/j.oraloncology.2020.104724",
"article-title": "Consideration of Povidone-Iodine as a Public Health Intervention for COVID-19: Utilization as ‘Personal Protective Equipment’ for Frontline Providers Exposed in High-Risk Head and Neck and Skull Base Oncology Care",
"doi-asserted-by": "crossref",
"first-page": "104724",
"journal-title": "Oral Oncology",
"key": "2021020411450666000_2020.09.07.20180448v1.14",
"volume": "105",
"year": "2020"
},
{
"DOI": "10.1038/sj.bdj.2017.315",
"article-title": "Evidence Summary: The Relationship between Oral Health and Pulmonary Disease",
"doi-asserted-by": "crossref",
"first-page": "527",
"issue": "7",
"journal-title": "British Dental Journal",
"key": "2021020411450666000_2020.09.07.20180448v1.15",
"volume": "222",
"year": "2017"
},
{
"DOI": "10.1183/13993003.00607-2020",
"doi-asserted-by": "crossref",
"key": "2021020411450666000_2020.09.07.20180448v1.16",
"unstructured": "Mason, Robert J. 2020. “Pathogenesis of COVID-19 from a Cell Biology Perspective.” Eur Respiratory Soc."
},
{
"DOI": "10.1111/j.1600-051X.2005.00673.x",
"article-title": "Efficacy of Listerine® Antiseptic in Reducing Viral Contamination of Saliva",
"doi-asserted-by": "crossref",
"first-page": "341",
"issue": "4",
"journal-title": "Journal of Clinical Periodontology",
"key": "2021020411450666000_2020.09.07.20180448v1.17",
"volume": "32",
"year": "2005"
},
{
"article-title": "Effective Antiviral Activity of Essential Oils and Their Characteristic Terpenes against Coronaviruses: An Update",
"first-page": "1138",
"issue": "1",
"journal-title": "J. Pharmacol. Clin. Toxicol",
"key": "2021020411450666000_2020.09.07.20180448v1.18",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1111/ODI.13378/v2/response1",
"doi-asserted-by": "crossref",
"key": "2021020411450666000_2020.09.07.20180448v1.19",
"unstructured": "Pattanshetty, Sanjay , Aparna Narayana , and Raghu Radhakrishnan . 2020. “Povidone-iodine Gargle as a Prophylactic Intervention to Interrupt the Transmission of SARS-CoV-2.” Oral Diseases."
},
{
"DOI": "10.1016/j.amepre.2005.06.013",
"doi-asserted-by": "publisher",
"key": "2021020411450666000_2020.09.07.20180448v1.20"
},
{
"DOI": "10.1038/s41579-020-0332-0",
"article-title": "Outbreak of a Novel Coronavirus",
"doi-asserted-by": "crossref",
"first-page": "123",
"issue": "3",
"journal-title": "Nature Reviews Microbiology",
"key": "2021020411450666000_2020.09.07.20180448v1.21",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.3999/jscpt.44.459",
"article-title": "Gargling with Green Tea for Influenza Prophylaxis: A Pilot Clinical Study",
"doi-asserted-by": "crossref",
"first-page": "459",
"issue": "6",
"journal-title": "Japanese Journal of Clinical Pharmacology and Therapeutics",
"key": "2021020411450666000_2020.09.07.20180448v1.22",
"volume": "44",
"year": "2013"
},
{
"DOI": "10.1016/j.ijid.2020.07.070",
"doi-asserted-by": "crossref",
"key": "2021020411450666000_2020.09.07.20180448v1.23",
"unstructured": "Uhm, Jae-Sun , Jin Young Ahn , Jong Hoon Hyun , Yujin Sohn , Jung Ho Kim , Su Jin Jeong , Nam Su Ku , Jun Yong Choi , Yu-Kyung Park , and Ho-sung Yi . 2020. “Patterns of Viral Clearance in the Natural Course of Asymptomatic Coronavirus Disease 2019 (COVID-19): Comparison with Symptomatic Nonsevere COVID-19.” International Journal of Infectious Diseases."
},
{
"DOI": "10.1038/s41586-020-2196-x",
"doi-asserted-by": "publisher",
"key": "2021020411450666000_2020.09.07.20180448v1.24"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2020.09.07.20180448"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "EARLY VIRAL CLEARANCE AMONG COVID-19 PATIENTS WHEN GARGLING WITH POVIDONE-IODINE AND ESSENTIAL OILS – A CLINICAL TRIAL",
"type": "posted-content"
}
