A prospective, randomized, open-label trial of early versus late povidone-iodine gargling in patients with COVID-19
et al., Scientific Reports, doi:10.1038/s41598-022-24683-8, jRCT1051200078, Nov 2022
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 430 COVID+ patients in Japan, showing significantly lower viral infectivity from culture, and significantly faster PCR viral clearance with PVP-I.
For days 2-4 the study compares treatment with PVP-I vs. water (on day 5 both groups received PVP-I). Most patients were asymptomatic. 4 times per day mouthwashing and gargling with 20mL of 15-fold diluted PVP-I 7% or water.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
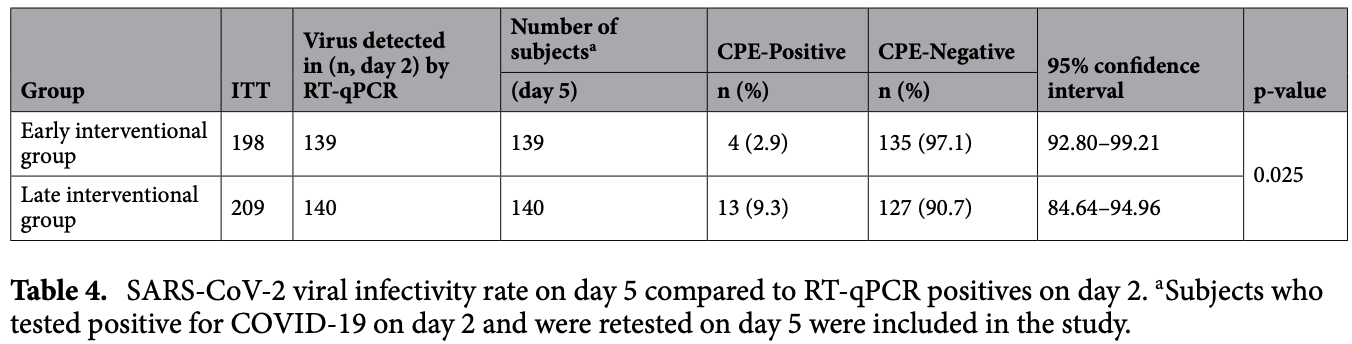
viral infectivity, 69.0% lower, RR 0.31, p = 0.03, treatment 4 of 139 (2.9%), control 13 of 140 (9.3%), NNT 16, viral infectivity from culture, day 5.
|
|
risk of no viral clearance, 38.0% lower, HR 0.62, p = 0.01, treatment 139, control 140, inverted to make HR<1 favor treatment, day 5, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Matsuyama et al., 28 Nov 2022, Randomized Controlled Trial, Japan, peer-reviewed, mean age 45.1, 4 authors, study period 30 November, 2020 - 17 March, 2021, trial jRCT1051200078.
Contact: matsuyamaak@opho.jp.
A prospective, randomized, open-label trial of early versus late povidone-iodine gargling in patients with COVID-19
Scientific Reports, doi:10.1038/s41598-022-24683-8
Povidone-iodine (PVP-I) is a broad-spectrum antiseptic reagent that has been used for over 50 years. The purpose of this study is to look into the effect of gargling with PVP-I gargling on virus clearance and saliva infectivity in COVID-19. A prospective, randomized, open-label trial of intervention with PVP-I was conducted at three quarantine facilities in Osaka, Japan, enrolling adolescents and adults with asymptomatic-to-mild COVID-19. Patients were randomly allocated to the early and late intervention group at a 1:1 ratio. The early group gargled with PVP-I from days 2 to day 6; the late group gargled with water first, then with PVP-I from day 5 after sampling till day 6. The primary and secondary endpoints were viral clearance for SARS-CoV-2 using RT-qPCR at days 5 and 6, respectively, and the investigational endpoint was saliva infectivity clearance on day5. We enrolled 430 participants, with 215 assigned to each group, and 139 in the early group and 140 in the late had a positive saliva RT-qPCR test on day 2. On day 5, the early group had a significantly higher RT-qPCR negative rate than that of the late group (p = 0.015), and the early had a significantly higher clearance rate of infectivity (p = 0.025). During the PVP-I intervention, one participant reported oropharyngeal discomfort. Gargling with PVP-I may hasten virus clearance and reduce viral transmission via salivary droplets and aerosols in patients with asymptomatic-to-mild COVID-19. (Clinical trial registration numbers: jRCT1051200078 and dateof registration: 24/11/2020). The salivary SARS-CoV-2 viral load is thought to play a significant role in the accelerated transmission of coronavirus disease 2019 (COVID-19) 1 . A reduction in salivary viral load is thought to suppress transmission 2 , and some studies have suggested that mouthwash and/or gargling with povidone-iodine (PVP-I) may have an antiseptic effect for SARS-CoV-2, reducing its viral load 3 . However, it is unknown whether gargling with PVP-I would eliminate salivary viral load and infectivity in COVID-19 patients in vivo. PVP-I is a polyvinylpyrrolidone (PVP) and iodine complex that has an antiseptic effect by releasing iodine. Its mechanism of action involves the use of iodine to oxidize microbial components. PVP-I has previously been shown to have an antiseptic effect on SARS-CoV and MERS in vitro 4, 5 , and it was also effective against SARS-CoV-2 6 . A single in vitro gargle with PVP-I reduced the salivary viral load in two of four patients with a high salivary viral load 7 . Although recent studies have confirmed the short-term effects of reducing salivary viral load in a small number of patients with COVID-19 8 , there have been no prospective randomized intervention studies involving PVP-I gargling. The purpose of this study was to see if gargling with PVP-I will reduce viral load and SARS-CoV-2 infectivity in patients with COVID-19.
Materials and methods Study design. This was a prospective, randomized,..
Author contributions A.M. had full access to all data in the study. A.M., S.H., and T.T. were responsible for the trial design. A.M. was responsible for the acquisition and analysis of data. H.O. acquired the laboratory data. A.M. drafted the paper. SH. and T.T. revised one. A.M. was responsible for the integrity of the data, the accuracy of the data analysis and decided to publish the manuscript. All authors contributed to conducting the trial.
Competing interests The authors declare no competing interests.
References
Anderson, Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease, Infect. Dis. Ther
Ather, Patel, Ruparel, Diogenes, Hargreaves, Coronavirus Disease 19 (COVID-19): Implications for clinical dental care, J. Endod
Bidra, Rapid in-vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using povidoneiodine oral antiseptic rinse, J. Prosthodont
Burton, Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them, Cochrane Database Syst. Rev
Burton, Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID-19 infection, Cochrane Database Syst. Rev
Eggers, Eickmann, Zorn, Rapid and effective virucidal activity of povidone-iodine products against Middle East respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus Ankara (MVA), Infect. Dis. Ther
Eggers, Koburger-Janssen, Eickmann, Zorn, In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/ mouthwash against respiratory and oral tract pathogens, Infect. Dis. Ther
Huang, SARS-CoV-2 infection of the oral cavity and saliva, Nat. Med
Kariwa, Fujii, Takashima, Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions, and chemical reagents, Jpn. J. Vet. Res
Khan, Parab, Tolerability and usability of 0.5% PVP-I gargles and nasal drops in 6692 patients: Observational study, Am. J. Otolaryngol
Killingley, Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge, Nat. Med, doi:10.21203/rs.3.rs-1121993/v1
Martínez Lamas, Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests, Oral Dis, doi:10.1111/odi.13526
Mateos-Moreno, Mira, Ausina-Márquez, Ferrer, Oral antiseptics against coronavirus: In-vitro and clinical evidence, J. Hosp. Infect
Nobukuni, The influence of long-term treatment with povidone-iodine on thyroid function, Dermatology
Seet, Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial, Int. J. Infect. Dis
Seneviratne, Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: Randomized control trial in Singapore, Infection
Xu, Saliva: Potential diagnostic value and transmission of 2019-nCoV, Int. J. Oral Sci
Yang, Just 2% of SARS-CoV-2-positive individuals carry 90% of the virus circulating in communities, Proc. Natl. Acad. Sci
DOI record:
{
"DOI": "10.1038/s41598-022-24683-8",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-022-24683-8",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Povidone-iodine (PVP–I) is a broad-spectrum antiseptic reagent that has been used for over 50 years. The purpose of this study is to look into the effect of gargling with PVP–I gargling on virus clearance and saliva infectivity in COVID-19. A prospective, randomized, open-label trial of intervention with PVP–I was conducted at three quarantine facilities in Osaka, Japan, enrolling adolescents and adults with asymptomatic-to-mild COVID-19. Patients were randomly allocated to the early and late intervention group at a 1:1 ratio. The early group gargled with PVP–I from days 2 to day 6; the late group gargled with water first, then with PVP–I from day 5 after sampling till day 6. The primary and secondary endpoints were viral clearance for SARS-CoV-2 using RT-qPCR at days 5 and 6, respectively, and the investigational endpoint was saliva infectivity clearance on day5. We enrolled 430 participants, with 215 assigned to each group, and 139 in the early group and 140 in the late had a positive saliva RT-qPCR test on day 2. On day 5, the early group had a significantly higher RT-qPCR negative rate than that of the late group (p = 0.015), and the early had a significantly higher clearance rate of infectivity (p = 0.025). During the PVP–I intervention, one participant reported oropharyngeal discomfort. Gargling with PVP–I may hasten virus clearance and reduce viral transmission via salivary droplets and aerosols in patients with asymptomatic-to-mild COVID-19. (Clinical trial registration numbers: jRCT1051200078 and dateof registration: 24/11/2020).</jats:p>",
"alternative-id": [
"24683"
],
"article-number": "20449",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "29 July 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "18 November 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "28 November 2022"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Matsuyama",
"given": "Akifumi",
"sequence": "first"
},
{
"affiliation": [],
"family": "Okura",
"given": "Hanayuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hashimoto",
"given": "Shyoji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tanaka",
"given": "Toshio",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
11,
28
]
],
"date-time": "2022-11-28T11:05:01Z",
"timestamp": 1669633501000
},
"deposited": {
"date-parts": [
[
2022,
11,
29
]
],
"date-time": "2022-11-29T02:20:17Z",
"timestamp": 1669688417000
},
"indexed": {
"date-parts": [
[
2022,
11,
29
]
],
"date-time": "2022-11-29T05:51:54Z",
"timestamp": 1669701114182
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
11,
28
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
28
]
],
"date-time": "2022-11-28T00:00:00Z",
"timestamp": 1669593600000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
28
]
],
"date-time": "2022-11-28T00:00:00Z",
"timestamp": 1669593600000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-022-24683-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-24683-8",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-24683-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2022,
11,
28
]
]
},
"published-online": {
"date-parts": [
[
2022,
11,
28
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1038/s41591-021-01296-8",
"author": "N Huang",
"doi-asserted-by": "publisher",
"first-page": "892",
"issue": "5",
"journal-title": "Nat. Med.",
"key": "24683_CR1",
"unstructured": "Huang, N. et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 27(5), 892–903 (2021).",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1038/s41368-020-0080-z",
"author": "R Xu",
"doi-asserted-by": "publisher",
"first-page": "11",
"issue": "1",
"journal-title": "Int. J. Oral Sci.",
"key": "24683_CR2",
"unstructured": "Xu, R. et al. Saliva: Potential diagnostic value and transmission of 2019-nCoV. Int. J. Oral Sci. 12(1), 11 (2020).",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.jhin.2021.04.004",
"author": "MV Mateos-Moreno",
"doi-asserted-by": "publisher",
"first-page": "30",
"journal-title": "J. Hosp. Infect.",
"key": "24683_CR3",
"unstructured": "Mateos-Moreno, M. V., Mira, A., Ausina-Márquez, V. & Ferrer, M. D. Oral antiseptics against coronavirus: In-vitro and clinical evidence. J. Hosp. Infect. 113, 30–43 (2021).",
"volume": "113",
"year": "2021"
},
{
"author": "H Kariwa",
"first-page": "105",
"issue": "3",
"journal-title": "Jpn. J. Vet. Res.",
"key": "24683_CR4",
"unstructured": "Kariwa, H., Fujii, N. & Takashima, I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions, and chemical reagents. Jpn. J. Vet. Res. 52(3), 105–112 (2004).",
"volume": "52",
"year": "2004"
},
{
"DOI": "10.1007/s40121-015-0091-9",
"author": "M Eggers",
"doi-asserted-by": "publisher",
"first-page": "491",
"issue": "4",
"journal-title": "Infect. Dis. Ther.",
"key": "24683_CR5",
"unstructured": "Eggers, M., Eickmann, M. & Zorn, J. Rapid and effective virucidal activity of povidone-iodine products against Middle East respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus Ankara (MVA). Infect. Dis. Ther. 4(4), 491–501 (2015).",
"volume": "4",
"year": "2015"
},
{
"DOI": "10.1007/s40121-020-00316-3",
"author": "DE Anderson",
"doi-asserted-by": "publisher",
"first-page": "669",
"issue": "3",
"journal-title": "Infect. Dis. Ther.",
"key": "24683_CR6",
"unstructured": "Anderson, D. E. et al. Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease. Infect. Dis. Ther. 9(3), 669–675 (2020).",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1111/odi.13526",
"author": "L Martínez Lamas",
"doi-asserted-by": "publisher",
"journal-title": "Oral Dis.",
"key": "24683_CR7",
"unstructured": "Martínez Lamas, L. et al. Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests. Oral Dis. https://doi.org/10.1111/odi.13526 (2020).",
"year": "2020"
},
{
"DOI": "10.1007/s15010-020-01563-9",
"author": "CJ Seneviratne",
"doi-asserted-by": "publisher",
"first-page": "305",
"issue": "2",
"journal-title": "Infection",
"key": "24683_CR8",
"unstructured": "Seneviratne, C. J. et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: Randomized control trial in Singapore. Infection 49(2), 305–311 (2021).",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1007/s40121-018-0200-7",
"author": "M Eggers",
"doi-asserted-by": "publisher",
"first-page": "249",
"issue": "2",
"journal-title": "Infect. Dis. Ther.",
"key": "24683_CR9",
"unstructured": "Eggers, M., Koburger-Janssen, T., Eickmann, M. & Zorn, J. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infect. Dis. Ther. 7(2), 249–259 (2018).",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1111/jopr.13209",
"author": "AS Bidra",
"doi-asserted-by": "publisher",
"first-page": "529",
"issue": "6",
"journal-title": "J. Prosthodont.",
"key": "24683_CR10",
"unstructured": "Bidra, A. S. et al. Rapid in-vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using povidone-iodine oral antiseptic rinse. J. Prosthodont. 29(6), 529–533 (2020).",
"volume": "29",
"year": "2020"
},
{
"DOI": "10.1016/j.joen.2020.03.008",
"author": "A Ather",
"doi-asserted-by": "publisher",
"first-page": "584",
"issue": "5",
"journal-title": "J. Endod.",
"key": "24683_CR11",
"unstructured": "Ather, A., Patel, B., Ruparel, N. B., Diogenes, A. & Hargreaves, K. M. Coronavirus Disease 19 (COVID-19): Implications for clinical dental care. J. Endod. 46(5), 584–595 (2020).",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.21203/rs.3.rs-1121993/v1",
"author": "B Killingley",
"doi-asserted-by": "publisher",
"journal-title": "Nat. Med.",
"key": "24683_CR12",
"unstructured": "Killingley, B. et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge. Nat. Med. https://doi.org/10.21203/rs.3.rs-1121993/v1 (2022).",
"year": "2022"
},
{
"DOI": "10.1159/000246034",
"author": "K Nobukuni",
"doi-asserted-by": "publisher",
"first-page": "69",
"issue": "Suppl 2",
"journal-title": "Dermatology",
"key": "24683_CR13",
"unstructured": "Nobukuni, K. et al. The influence of long-term treatment with povidone-iodine on thyroid function. Dermatology 195(Suppl 2), 69–72 (1997).",
"volume": "195",
"year": "1997"
},
{
"DOI": "10.1016/j.amjoto.2020.102880",
"author": "MM Khan",
"doi-asserted-by": "publisher",
"first-page": "102880",
"issue": "2",
"journal-title": "Am. J. Otolaryngol.",
"key": "24683_CR14",
"unstructured": "Khan, M. M. & Parab, S. R. Tolerability and usability of 0.5% PVP-I gargles and nasal drops in 6692 patients: Observational study. Am. J. Otolaryngol. 42(2), 102880 (2021).",
"volume": "42",
"year": "2021"
},
{
"key": "24683_CR15",
"unstructured": "ADA releases interim guidance on minimizing COVID-19 transmission risk when treating dental emergencies. https://www.ada.org/en/publications/ada-news/2020-archive/april/ada-releases-interim-guidance-on-minimizing-covid-19-transmission-risk-when-treating-emergencies (2022)."
},
{
"key": "24683_CR16",
"unstructured": "Risk Management Principles for Dentistry. Resource outlining how to apply evidence-informed infection control measures within a broader risk-based approach. https://www.ada.org.au/Covid-19-Portal/Files/pdf/COVID-19-Risk-Management-Guidance.aspx (2022)."
},
{
"author": "MJ Burton",
"first-page": "CD013626",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "24683_CR17",
"unstructured": "Burton, M. J. et al. Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID-19 infection. Cochrane Database Syst. Rev. 9, CD013626 (2020).",
"volume": "9",
"year": "2020"
},
{
"author": "MJ Burton",
"first-page": "CD013627",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "24683_CR18",
"unstructured": "Burton, M. J. et al. Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them. Cochrane Database Syst. Rev. 9, CD013627 (2020).",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2021.04.035",
"author": "RCS Seet",
"doi-asserted-by": "publisher",
"first-page": "314",
"journal-title": "Int. J. Infect. Dis.",
"key": "24683_CR19",
"unstructured": "Seet, R. C. S. et al. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial. Int. J. Infect. Dis. 106, 314–322 (2021).",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.1073/pnas.2104547118",
"author": "Q Yang",
"doi-asserted-by": "publisher",
"issue": "21",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "24683_CR20",
"unstructured": "Yang, Q. et al. Just 2% of SARS-CoV-2-positive individuals carry 90% of the virus circulating in communities. Proc. Natl. Acad. Sci. USA 118(21), e2104547118 (2021).",
"volume": "118",
"year": "2021"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-022-24683-8"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "A prospective, randomized, open-label trial of early versus late povidone-iodine gargling in patients with COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "12"
}
