Clinical efficacy of nitric oxide nasal spray (NONS) for the treatment of mild COVID-19 infection
et al., Journal of Infection, doi:10.1016/j.jinf.2021.05.009, May 2021
43rd treatment shown to reduce risk in
June 2022, now with p = 0.012 from 12 studies, recognized in 10 countries.
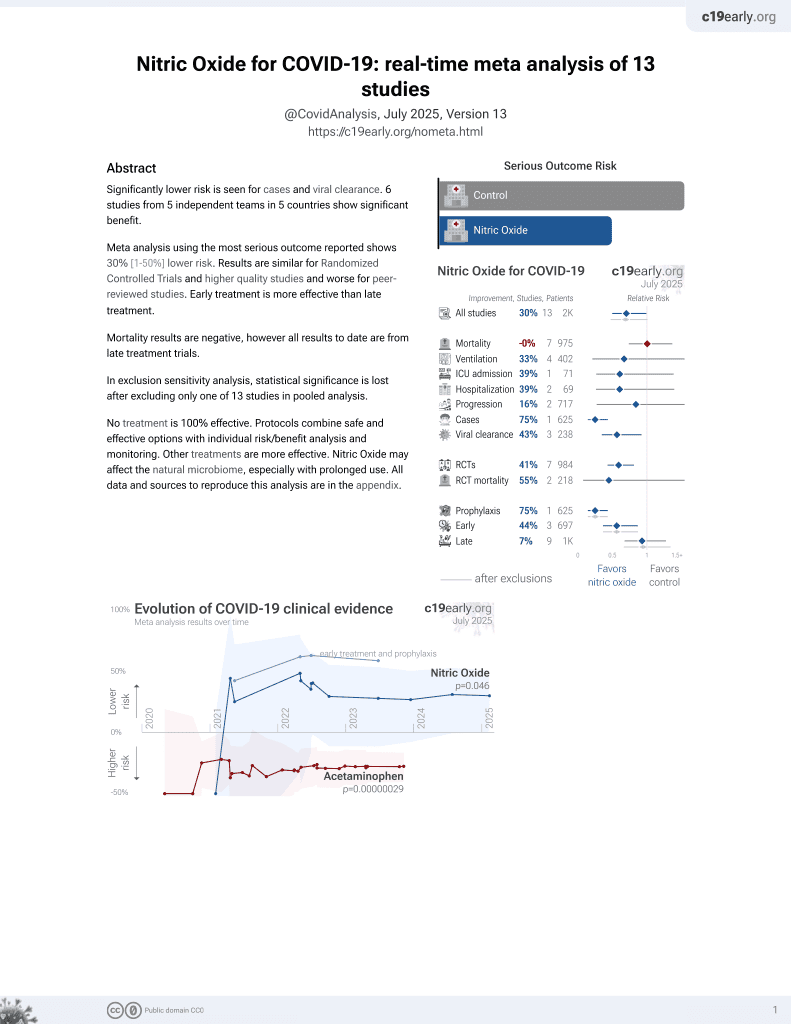
Lower risk for cases and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
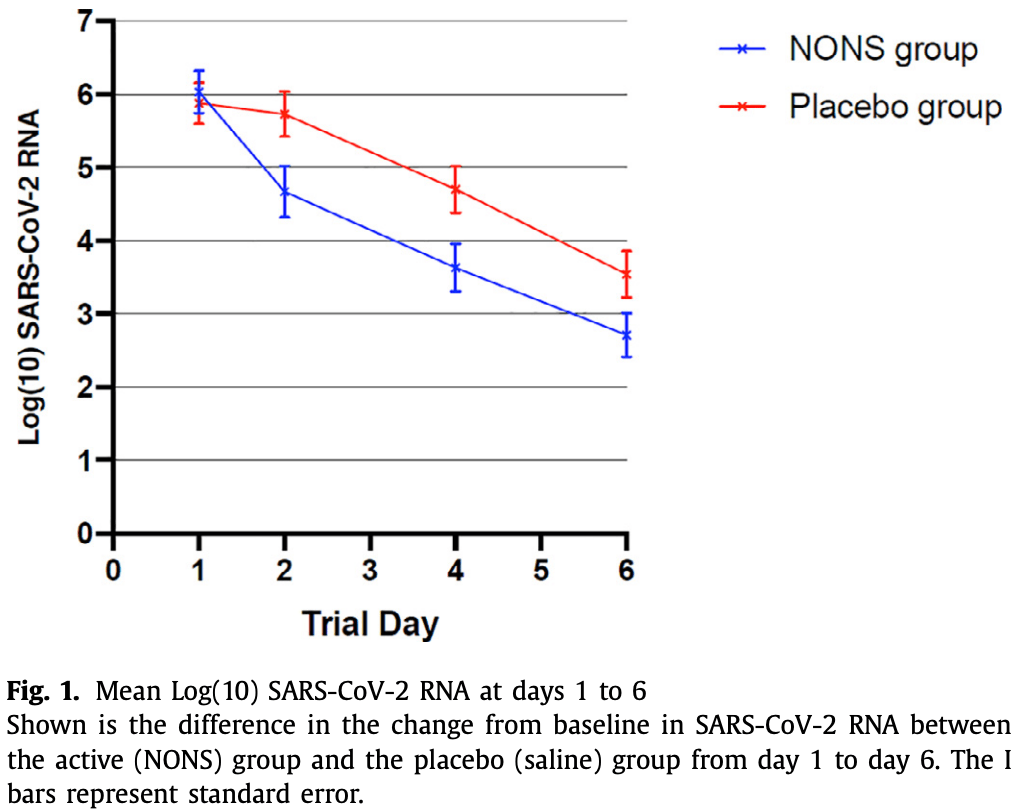
RCT with 40 nitric oxide and 40 placebo patients in the UK, showing faster viral clearance and greater improvement with treatment.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of no improvement, 42.0% lower, RR 0.58, p = 0.008, treatment 8 of 15 (53.3%), control 23 of 25 (92.0%), NNT 2.6.
|
|
viral load, 51.3% lower, relative load 0.49, p = 0.001, treatment 40, control 40, AUC relative mean change.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Winchester et al., 13 May 2021, Double Blind Randomized Controlled Trial, placebo-controlled, United Kingdom, peer-reviewed, 4 authors, study period 15 December, 2020 - 31 March, 2021.
Remdesivir treatment and transient bradycardia in patients with coronavirus diseases 2019 (COVID-19)
Journal of Infection, doi:10.1016/j.jinf.2021.05.025
Remdesivir treatment and transient bradycardia in patients with coronavirus diseases 2019 (COVID-19) Dear Editor,
Declaration of Competing Interest The authors report no potential conflicts of interest.
Declaration of Competing Interest The authors declare no conflict of interest. Andrade Pereira, Rayana de Castro da Paz and Regiane Tigulini de Souza Jordão insightful discussions on the best way to present the data of this manuscript.
Funding The authors received no specific funding for this work.
Supplementary materials Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.jinf.2021.05.012 .
Supplementary materials Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.jinf.2021.05.007 .
Conflict of interest statement No conflict of interest to declare.
Conflict of interest The authors declare that they have no conflict of interest.
Ethics PHE has legal permission, provided by Regulation 3 of The Health Service (Control of Patient Information) Regulations 2002, to process patient information for national surveillance of communicable diseases. Specific ethical approval was not required for this surveillance work.
Author's contributions HW, SE, IH, SR, KB and GA wrote the manuscript, with input from MR. GA, KB and MR contributed to conceptualization, funding acquisition and project administration. HW performed statistical analysis. EC, AL, CT, CC collated vaccine uptake statistics. EL, IH,
Author contributions All authors contributed to the study concept and design...
References
Abu-Raddad, Chemaitelly, Butt, Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants, N Engl J Med, doi:10.1056/NEJMc2104974
Ahmed, Patel, Greenwood, Long-term clinical outcomes in survivors of severe acute respiratory syndrome (SARS) and middle east respiratory syndrome coronavirus (MERS) outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis
Anderson, Rouphael, Widge, Jackson, Roberts et al., Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults, N Engl J Med, doi:10.1056/NEJMoa2028436
Aveyard, Gao, Lindson, Hartmann-Boyce, Watkinson et al., Association between pre-existing respiratory disease and its treatment, and severe COVID-19: a population cohort study, Lancet Respir Med
Baek, Lee, Yoon, Duration of culturable SARS-CoV-2 within different specimens among mild and severe COVID-19 patients: a longitudinal study, J Infect, doi:10.1016/j.jinf.2021.04.025
Baker, Nelson, Overton, Lopman, Lash et al., Quantification of occupational and community risk factors for SARS-CoV-2 seropositivity among health care workers in a large U.S. health care system, Ann Intern Med
Barkas, Styla, Bechlioulis, Milionis, Liberopoulos, Sinus bradycardia associated with remdesivir treatment in COVID-19: a case report and literature review, J Cardiovasc Dev Dis
Bhimraj, Morgan, Shumaker, Lavergne, Baden et al., Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19, Infect Dis Soc Am
Bhoyar, Jain, Sehgal, High throughput detection and genetic epidemiology of SARS-CoV-2 using COVIDSeq next-generation sequencing, PLoS One
Breathnach Aodhán Seán, Andrew, Cotter, Patricia, Houston et al., Prior COVID-19 significantly reduces the risk of subsequent infection, but reinfections are seen after eight months, J Infect, doi:10.1016/j.jinf.2021.01.005
Bulilete, Lorente, Leiva, Carandell, Oliver et al., Panbio TM rapid antigen test for SARS-CoV-2 has acceptable accuracy in symptomatic patients in primary health care, J Infect
Candel, Barreiro, San Román, Recommendations for use of antigenic tests in the diagnosis of acute SARS-CoV-2 infection in the second pandemic wave: attitude in different clinical settings, Rev Esp Quimioter
Capetti, Stangalini, Borgonovo, Impressive boosting of anti-S1/S2 IgG production in COVID-19-experienced patients after the first shot of the BNT162b2 mRNA COVID-19 Vaccine, Clin Infect Dis, doi:10.1093/cid/ciab214
Caramello, Macciotta, De Salve, Clinical characteristics and management of COVID-19 patients accessing the emergency department in a hospital in Northern Italy in March and April 2020, Epidemiol Prev
Cdc, Stay home if you might have been exposed to COVID-19
Cerutti, Burdino, Milia, Allice, Gregori et al., Urgent need of rapid tests for SARS CoV-2 antigen detection: evaluation of the SD-Biosensor antigen test for SARS-CoV-2, J ClinVirol
Chen, Ye, Duo, Pang, Smith et al., First description of two new HIV-1 recombinant forms CRF82_cpx and CRF83_cpx among drug users in Northern Myanmar, Virulence
Chen, Zhou, Ye, Wang, Duo et al., Burmese injecting drug users in Yunnan play a pivotal role in the cross-border transmission of HIV-1 in the China-Myanmar border region, Virulence
Dagan, Barda, Kepten, BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting, N Engl J Med
Dan, Mateus, Kato, Hastie, Yu et al., Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection, Science, doi:10.1126/science.abf4063
Daniel, Nivet, Warner, Podolsky, Early evidence of the effect of SARS-CoV-2 vaccine at one medical center, N Engl J Med, doi:10.1056/NEJMc2102153
Diasorin, automated serology test for the detection of SARS CoV-2 IgG antibodies
Diez-Flecha, Rivero Rodríguez, Fernández-Villa, García, Ferreira De Jesús et al., Internal validity of a rapid test for COVID-19 antigens in a nursing home
Dinnes, Deeks, Adriano, Cochrane COVID-19 diagnostic test accuracy group. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection, Cochrane Database Syst Rev
Douglas, Sandmann, Allen, Celma, Beard et al., Impact of COVID-19 on national surveillance of norovirus in England and potential risk of increased disease activity in 2021, J Hospital Infect, doi:10.1016/j.jinf.2021.04.030
Dzieciatkowski, Szarpak, Filipiak, Jaguszewski, Ladny et al., COVID-19 challenge for modern medicine, Cardiol J, doi:10.5603/CJ.a2020.0055
Edridge, Seasonal coronavirus protective immunity is shortlasting, Nat Med, doi:10.1038/s41591-020-1083-1
Ella, Vadrevu, Jogdand, Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial, Lancet Infect Dis
Emary, Golubchik, Aley, Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial, Lancet
Fang, Perspectives series: host/pathogen interactions. mechanisms of nitric oxide-related antimicrobial activity, J Clin Invest
Faria, Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil, Science, doi:10.1126/science.abh2644
Feng, Takebe, Wei, He, Hsi et al., Geographic origin and evolutionary history of China's two predominant HIV-1 circulating recombinant forms, CRF07_BC and CRF08_BC, Sci Rep
Fernández-De-Las-Peñas, Palacios-Ceña, Gómez-Mayordomo, Cuadrado, Florencio, Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification, Int J Environ Res Public Health
Fernández-Vázquez, Reguero, Sánchez-Antolín, Martín-Sánchez, rapid antigen testing and the 5% pre-test probability
Galanis, Vraka, Fragkou, Bilali, Kaitelidou, Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis, J Hosp Infect
Galanti, Birger, Ud-Dean, Rates of asymptomatic respiratory virus infection across age groups, Epidemiol Infect, doi:10.1017/S0950268819000505
Garcia-Pachon, Grau-Delgado, Soler-Sempere, Zamora-Molina, Baeza-Martinez et al., Low prevalence of post-COVID-19 syndrome in patients with asthma, J Infect
Garg, Muthu, Sehgal, Ramachandran, Kaur et al., Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature, Mycopathologia, doi:10.1007/s11046-021-00528-2
Garrigues, Janvier, Kherabi, Bot, Hamon et al., Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19, J Infect
Garrigues, Janvier, Kherabi, Bot, Hamon et al., Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19, J Infect
Gordon, Tchesnokov, Woolner, Perry, Feng et al., Remdesivir is a direct antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency, J Biol Chem, doi:10.1016/j.jinf.2020.12.023
Gubitosa, Kakar, Gerula, Nossa, Finkel et al., Marked sinus bradycardia associated with remdesivir in COVID-19: a case and literature review, JACC Case Rep
Gómez-Ochoa, Franco, Rojas, Raguindin, Roa-Díaz et al., COVID-19 in health-care workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes, Am J Epidemiol
Han, An, Zhao, Duan, Yang et al., High prevalence of HIV-1 intersubtype B'/C recombinants among injecting drug users in Dehong, China, PLoS ONE, doi:10.1016/j.jinf.2021.02.012
Han, Choi, Chang, Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic of Korea, JAMA Pediatr
Harris, Whitaker, Andrews, Aiano, Amin-Chowdhury et al., Serological surveillance of SARS-CoV-2: six-month trends and antibody response in a cohort of public health workers, J Infect, doi:10.1016/j.jinf.2021.03.015
Hartley, Edwards, Aui, Varese, Stojanovic et al., Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence, Sci Immunol, doi:10.1126/sciimmunol.abf8891
Hedman, Ljótsson, Blom, El Alaoui, Kraepelien et al., Telephone versus internet administration of self-report measures of social anxiety, depressive symptoms, and insomnia: psychometric evaluation of a method to reduce the impact of missing data, J Med Internet Res
Hodcroft, Emergence in late 2020 of multiple lineages of SARS-CoV-2 Spike protein variants affecting amino acid position 677, doi:10.1101/2021.02.12.21251658v1
Holm Hansen Christian, Daniela, Gubbels, Madeleine, Kåre et al., Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study, Lancet
Huang, Huang, Wang, 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study, Lancet
Hussein, Elshazli, Attia, Nguyen, Aboueisha et al., Asthma and COVID-19; different entities, same outcome: a meta-analysis of 107,983 patients, J Asthma, doi:10.1080/02770903.2021.1881970
Jacob, Baker, Fridkin, Lopman, Steinberg et al., Risk factors associated with SARS-CoV-2 seropositivity among us health care personnel, JAMA Netw Open
Jangra, Ye, Rathnasinghe, SARS-CoV-2 spike E484K mutation reduces antibody neutralisation, Lancet Microbe, doi:10.1016/S2666-5247(21)00068-9
Kaminska, Szarpak, Kosior, Wieczorek, Szarpak et al., Impact of diabetes mellitus on in-hospital mortality in adult patients with COVID-19: a systematic review and meta-analysis, Acta Diabetol, doi:10.1007/s00592-021-01701-1
Katoh, Standley, MAFFT Multiple Sequence Alignment Software Version 7: improvements in performance and usability, Mol Biol Evol, doi:10.1093/molbev/mst010
Keehner, Horton, Pfeffer, SARS-CoV-2 infection after vaccination in health care workers in California, N Engl J Med, doi:10.1056/NEJMc2101927
Kow, Aldeyab, Hasan, Quality of adverse event reporting in clinical trials of remdesivir in patients with COVID-19, Eur J Clin Pharmacol
Krammer, Srivastava, Alshammary, Amoako, Awawda et al., Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine, N Engl J Med, doi:10.1056/NEJMc2101667
Krammer, Srivastava, Alshammary, Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine, N Engl J Med, doi:10.1056/NEJMc2101667
Lansbury, Lim, Baskaran, Lim, Co-infections in people with COVID-19: a systematic review and meta-analysis, J Infect, doi:10.1016/j.jinf.2020.05.046
Lauer, Grantz, Bi, The Incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application, Ann Int Med
Li, Gao, Zhang, Liu, Jia et al., A newly emerging HIV-1 circulating recombinant form (CRF110_BC) comprising subtype B and C among intravenous drug users in Yunnan, China, J Infect
Li, Sun, Jiang, Zeng, Li et al., The changes of positive selection within env Gene of HIV-1 B', CRF07_BC and CRF08_BC from China over time, Curr HIV Res
Linares, Pérez-Tanoira, Carrero, Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms, J ClinVirol
Liu, Vanblargan, Bloyet, Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization, Cell Host Microbe
Lumley, Donnell, Nethe, Oxford University Hospitals Staff Testing Group Antibody status and incidence of SARS-CoV-2 infection in health care workers, N Engl J Med, doi:10.1056/NEJMoa2034545
Lumley, Donnell, Stoesser, Matthews, Howarth et al., Oxford University Hospitals Staff Testing Group. antibody status and incidence of SARS-CoV-2 infection in health care workers, N Engl J Med, doi:10.1056/NEJMoa2034545
Mak, Cheng, Lau, Evaluation of rapid antigen test for detection of SARS-CoV-2 virus, J Clin Virol
Manisty, Otter, Treibel, Mcknight, Altmann et al., Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals, Lancet, doi:10.1016/S0140-6736(21)00501-8
Marks, Millat-Martinez, Ouchi, Transmission of COVID-19 in 282 clusters in Catalonia, Spain: A cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30985-3
Ministry, Health, Labour, Results of a survey on SARS-CoV-2 antibody in the general population in Japan as of December 2020
Moderbacher, Ramirez, Dan, Grifoni, Hastie et al., Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity, Cell
Moncada, Higgs, Endogenous nitric oxide: physiology, pathology and clinical relevance, Eu Jof Clin Invest
Montero-Escribano, Matías-Guiu, Gómez-Iglesias, Porta-Etessam, Pytel et al., Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: a case series of 60 patients from Madrid, Spain, Mult Scler Relat Disord, doi:10.1016/j.msard.2020.102185
Moreira, Watanabe, Camargo, Respiratory syncytial virus evaluation among asymptomatic and symptomatic subjects in a university hospital in Sao Paulo, Brazil, in the period of 2009-2013, Influenza Other Respir Viruses, doi:10.1111/irv.12518
Moreno-Pérez, Merino, Leon-Ramirez, Andres, Ramos et al., Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study, J Infect
Moreno-Pérez, Merino, Leon-Ramirez, Andres, Ramos et al., Post-acute COVID-19 syndrome. Incidence and risk factors: a mediterranean cohort study, J Infect
Munblit, Bobkova, Spiridonova, Risk factors for long-term consequences of COVID-19 in hospitalised adults in moscow using the ISARIC Global follow-up protocol: stop COVID cohort study, MedRxiv
Nabavi, Long COVID: how to define it and how to manage it, BMJ
Nguyen, Levine, Pollack, Engellenner, Thakore, Prevalence of SARS-CoV-2 infection among health care workers in a tertiary community hospital, JAMA Intern Med
Nguyen, Schmidt, Von Haeseler, Minh, IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies, Mol Biol Evol, doi:10.1093/molbev/msu300
Nonaka, Genomic Evidence of SARS-CoV-2 Reinfection Involving E484K Spike Mutation, Brazil, Emerg Infect Dis, doi:10.3201/eid2705.210191
Omi, Takeda, Mori, SARS-CoV-2 qRT-PCR Ct value distribution in Japan and possible utility of rapid antigen testing kit, medRxiv
Ouyang, Shao, Ma, HIV-1 CRF_BC recombinants infection in China: molecular epidemic and characterizations, Curr HIV Res
Overbaugh, Understanding protection from SARS-CoV-2 by studying reinfection, Nat Med, doi:10.1038/s41591-020-1121-z
Pallotto, Suardi, Esperti, Tarquini, Grifoni et al., Increased CD4/CD8 ratio as a risk factor for critical illness in coronavirus disease 2019 (COVID-19): a retrospective multicentre study, Infect Dis
Pallotto, Suardi, Gabbuti, Esperti, Mecocci et al., Potential remdesivir-related transient bradycardia in patients with coronavirus disease 2019 (COVID-19), J Med Virol
Peeling, Olliaro, Boeras, Scaling up COVID-19 rapid antigen tests: promises and challenges, Lancet Infect Dis
Peluso, Kelly, Lu, Rapid implementation of a cohort for the study of post-acute sequelae of SARS-CoV-2 infection/COVID-19, MedRxiv
Perlis, Green, Santillana, Persistence of symptoms up to 10 months following acute COVID-19 illness, MedRxiv
Polack, Thomas, Kitchin, Absalon, Gurtman et al., Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine, N Engl J Med, doi:10.1056/NEJMoa2034577
Prendecki, Clarke, Brown, Cox, Gleeson et al., Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine, Lancet, doi:10.1016/S0140-6736(21)00502-X
Qin, Huai, Qingsi, Clinical characteristics of COVID-19 patients with complication of cardiac arrhythmia, J Infect
Quinti, Lougaris, Milito, Cinetto, Pecoraro et al., A possible role for B cells in COVID-19? lesson from patients with agammaglobulinemia, J Allergy Clin Immunol, doi:10.1016/j.jaci.2020.04.013
Rambaut, Holmes, O'toole, A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology, Nat Microbiol
Regev-Shoshani, Vimalanathan, Mcmullin, Road, Av-Gay et al., Gaseous nitric oxide reduces influenza infectivity in vitro, Nitric Oxide
Regev-Shoshani, Vimalanathan, Prema, Safety, bioavailability and mechanism of action of nitric oxide to control bovine respiratory disease complex in calves entering a feedlot, Res Vet Sci
Robilotti, Deresinski, Pinsky, Norovirus, None, Clin. Microbiol. Rev, doi:10.1128/CMR.00075-14
Rogan, Gladen, Estimating prevalence from the results of a screening test, Am J Epidemiol
Rosado, Scarsella, Pandolfi, Cascioli, Giorda et al., Serological surveillance of SARS-CoV-2: six-month trends and antibody response in a cohort of public health workers, Eur J Immunol, doi:10.1016/j.jinf.2021.03.015
Russell, Moldoveanu, Ogra, Mestecky, Mucosal immunity in COVID-19: a neglected but critical aspect of SARS-CoV-2 infection, Front Immunol, doi:10.3389/fimmu.2020.611337
Salathé, Althaus, Neher, Stringhini, Hodcroft et al., Reactogenicity, safety and antibody response, after one and two doses of mRNA-1273 in seronegative and seropositive healthcare workers, Swiss Med Wkly, doi:10.1016/j.jinf.2021.03.025
Salehi, Ahmadikia, Badali, Khodavaisy, Opportunistic fungal infections in the epidemic area of COVID-19: a clinical and diagnostic perspective from Iran, Mycopathologia, doi:10.1007/s11046-020-00472-7
Santos, Filho, Silva, Recurrent COVID-19 including evidence of reinfection and enhanced severity in thirty Brazilian healthcare workers, J Infect, doi:10.1016/j.jinf.2021.01.020
Schwarzkopf, Krawczyk, Knop, Klump, Heinold et al., Cellular immunity in COVID-19 Convalescents with PCR-confirmed infection but with undetectable SARS-CoV-2-specific IgG, Emerg Infect Dis
Scohy, Anantharajah, Bodéus, Kabamba-Mukadi, Verroken et al., Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis, J ClinVirol
Self, Tenforde, Stubblefield, Feldstein, Steingrub et al., Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network -13 academic medical centers, MMWR Morb Mortal Wkly Rep
Sharma, Grover, Bhargava, Samdani, Katarina, Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum, J Laryngol Otol, doi:10.1017/S0022215121000992
Sikkema, Pas, Nieuwenhuijse, O'toole, Verweij et al., COVID-19 in health-care workers in three hospitals in the south of the Netherlands: a cross-sectional study, Lancet Infect Dis
Skiada, Pavleas, Drogari-Apiranthitou, Epidemiology and diagnosis of mucormycosis: an update, J Fungi, doi:10.3390/jof6040265
Suardi, Pallotto, Esperti, Tazzioli, Baragli et al., Risk factors for non-invasive/invasive-ventilatory support in patients with COVID-19 pneumonia: a retrospective study within a multidisciplinary approach, Int J Infect Dis, doi:10.1016/j.ijid.2020.09.012
Subbarao, Warrener, Hoschler, Perry, Shute et al., Robust Antibody Responses in 70-80 Year Olds following 1 or 2 Doses of Pfizer COVID-19 Vaccine, Eurosurveillance, doi:10.2807/1560-7917.ES.2021.26.12.2100329
Sunjaya, Allida, Tanna, Jenkins, Harris et al., Asthma and risk of infection, hospitalization, ICU admission and mortality from COVID-19: systematic review and meta-analysis, J Asthma, doi:10.1016/j.jinf.2021.03.015
Szarpak, Wisco, Boyer, How healthcare must respond to ventilatorassociated pneumonia (VAP) in invasively mechanically ventilated COVID-19 patients, Am J Emerg Med, doi:10.1016/j.ajem.2021.01.074
Tanaka, Yamamoto, Miyo, Mizoue, Maeda et al., Seroprevalence of antibodies against SARS-CoV-2 in a large national hospital and affiliated facility in Tokyo, Japan, J Infect
Tiraboschi, Sala, Effectiveness of BNT162b2 vaccine against the B.1.1.7 variant of SARS-CoV-2 among healthcare workers in Brescia, Italy, J Infect
Touafchia, Bagheri, Carrié, Durrieu, Sommet et al., Serious bradycardia and remdesivir for coronavirus 2019 (COVID-19): a new safety concerns, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.02.013
Tré, Hardy, Cupaiolo, Papleux, Reactogenicity, safety and antibody response, after one and two doses of mRNA-1273 in seronegative and seropositive healthcare workers, J Infect, doi:10.1016/j.jinf.2021.03.025
Tré-Hardy, Wilmet, Beukinga, Dogné, Douxfils et al., Validation of a chemiluminescent assay for specific SARS-CoV-2 antibody, Clin Chem Lab Med, doi:10.1515/cclm-2020-0594
Turcato, Zaboli, Pfeifer, Ciccariello, Sibilio et al., Clinical application of a rapid antigen test for the detection of SARS-CoV-2 infection in symptomatic and asymptomatic patients evaluated in the emergency department: a preliminary report, J Infect
Victoria, Sarah, Andre, Ana, Edward et al., SIREN Study Group. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN), Lancet
Voysey, Clemens, Madhi, Safety and efficacy of the ChA-dOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, Lancet
Voysey, Clemens, Madhi, Weckx, Folegatti et al., Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, Lancet, doi:10.1016/S0140-6736(20)32661-1
Walsh, Frenck, Falsey, Kitchin, Absalon et al., Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates, N Engl J Med, doi:10.1056/NEJMoa2027906
Walsh, Jordan, Clyne, SARS-CoV-2 detection, viral load and infectivity over the course of an infection, J Inf
Wang, Zhao, Li, Li, Zhang et al., Identification of a novel HIV-1 s-generation circulating recombinant form CRF109_0107 in China, J Infect
Weitzel, Legarraga, Iruretagoyena, Head-to-head comparison of four antigen-based rapid detection tests for the diagnosis of SARS-CoV-2 in respiratory samples, bioRxiv
Wise, Leon ; Ostrosky, Perelman, More than 50 long-term effects of COVID-19: a systematic review and meta-analysis, MedRxiv
Yonker, Neilan, Bartsch, Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses, J Pediatr
DOI record:
{
"DOI": "10.1016/j.jinf.2021.05.009",
"ISSN": [
"0163-4453"
],
"URL": "http://dx.doi.org/10.1016/j.jinf.2021.05.009",
"alternative-id": [
"S0163445321002516"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Clinical efficacy of nitric oxide nasal spray (NONS) for the treatment of mild COVID-19 infection"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jinf.2021.05.009"
},
{
"label": "Content Type",
"name": "content_type",
"value": "simple-article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The British Infection Association. Published by Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Winchester",
"given": "Stephen",
"sequence": "first"
},
{
"affiliation": [],
"family": "John",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jabbar",
"given": "Kashif",
"sequence": "additional"
},
{
"affiliation": [],
"family": "John",
"given": "Isaac",
"sequence": "additional"
}
],
"container-title": [
"Journal of Infection"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.jp",
"journalofinfection.com",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.fr",
"clinicalkey.com.au",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
5,
13
]
],
"date-time": "2021-05-13T15:40:14Z",
"timestamp": 1620920414000
},
"deposited": {
"date-parts": [
[
2021,
8,
14
]
],
"date-time": "2021-08-14T17:33:57Z",
"timestamp": 1628962437000
},
"indexed": {
"date-parts": [
[
2022,
3,
7
]
],
"date-time": "2022-03-07T03:05:50Z",
"timestamp": 1646622350169
},
"is-referenced-by-count": 8,
"issn-type": [
{
"type": "print",
"value": "0163-4453"
}
],
"issue": "2",
"issued": {
"date-parts": [
[
2021,
8
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2021,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
8,
1
]
],
"date-time": "2021-08-01T00:00:00Z",
"timestamp": 1627776000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445321002516?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445321002516?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "237-279",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
8
]
]
},
"published-print": {
"date-parts": [
[
2021,
8
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.jinf.2021.04.025",
"article-title": "Duration of culturable SARS-CoV-2 within different specimens among mild and severe COVID-19 patients: a longitudinal study",
"author": "Baek",
"doi-asserted-by": "crossref",
"journal-title": "J Infect",
"key": "10.1016/j.jinf.2021.05.009_bib0001",
"year": "2021"
},
{
"key": "10.1016/j.jinf.2021.05.009_bib0002",
"series-title": "COVID-19 map - Johns Hopkins Coronavirus Resource Center",
"year": "2021"
},
{
"key": "10.1016/j.jinf.2021.05.009_bib0003",
"unstructured": "World Health Organization. Coronavirus disease (COVID-19): herd immunity, lockdowns and COVID-19.https://www.who.int/news-room/q-a-detail/herd-immunity-lockdowns-and-covid-19. December 31, 2020. Accessed February 27, 2021."
},
{
"DOI": "10.1111/j.1365-2362.1991.tb01383.x",
"article-title": "Endogenous nitric oxide: physiology, pathology and clinical relevance",
"author": "Moncada",
"doi-asserted-by": "crossref",
"first-page": "361",
"issue": "4",
"journal-title": "Eu Jof Clin Invest",
"key": "10.1016/j.jinf.2021.05.009_bib0004",
"volume": "21",
"year": "1991"
},
{
"DOI": "10.1172/JCI119473",
"article-title": "1997. Perspectives series: host/pathogen interactions. mechanisms of nitric oxide-related antimicrobial activity",
"author": "Fang",
"doi-asserted-by": "crossref",
"first-page": "2818",
"issue": "12",
"journal-title": "J Clin Invest",
"key": "10.1016/j.jinf.2021.05.009_bib0005",
"volume": "99",
"year": "1997"
},
{
"DOI": "10.1016/j.niox.2013.03.007",
"article-title": "Gaseous nitric oxide reduces influenza infectivity in vitro",
"author": "Regev-Shoshani",
"doi-asserted-by": "crossref",
"first-page": "48",
"journal-title": "Nitric Oxide",
"key": "10.1016/j.jinf.2021.05.009_bib0006",
"volume": "31",
"year": "2013"
},
{
"DOI": "10.1016/j.rvsc.2013.12.012",
"article-title": "2014. Safety, bioavailability and mechanism of action of nitric oxide to control bovine respiratory disease complex in calves entering a feedlot",
"author": "Regev-Shoshani",
"doi-asserted-by": "crossref",
"first-page": "328",
"issue": "2",
"journal-title": "Res Vet Sci",
"key": "10.1016/j.jinf.2021.05.009_bib0007",
"volume": "96",
"year": "2014"
},
{
"DOI": "10.1016/j.jinf.2020.06.067",
"article-title": "SARS-CoV-2 detection, viral load and infectivity over the course of an infection",
"author": "Walsh",
"doi-asserted-by": "crossref",
"first-page": "357",
"journal-title": "J Inf",
"key": "10.1016/j.jinf.2021.05.009_bib0008",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30985-3",
"doi-asserted-by": "crossref",
"key": "10.1016/j.jinf.2021.05.009_bib0009",
"unstructured": "Marks M, Millat-Martinez P, Ouchi D, et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: A cohort study. Lancet Infect Dis. 2021;Feb 2:S1473-3099(20)30985-3. doi: 10.1016/S1473-3099(20)30985-3. Epub ahead of print."
}
],
"reference-count": 9,
"references-count": 9,
"relation": {},
"score": 1,
"short-container-title": [
"Journal of Infection"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": [
"Clinical efficacy of nitric oxide nasal spray (NONS) for the treatment of mild COVID-19 infection"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "83"
}