Serious bradycardia and remdesivir for coronavirus 2019 (COVID-19): a new safety concerns
et al., Clinical Microbiology and Infection, doi:10.1016/j.cmi.2021.02.013, Feb 2021
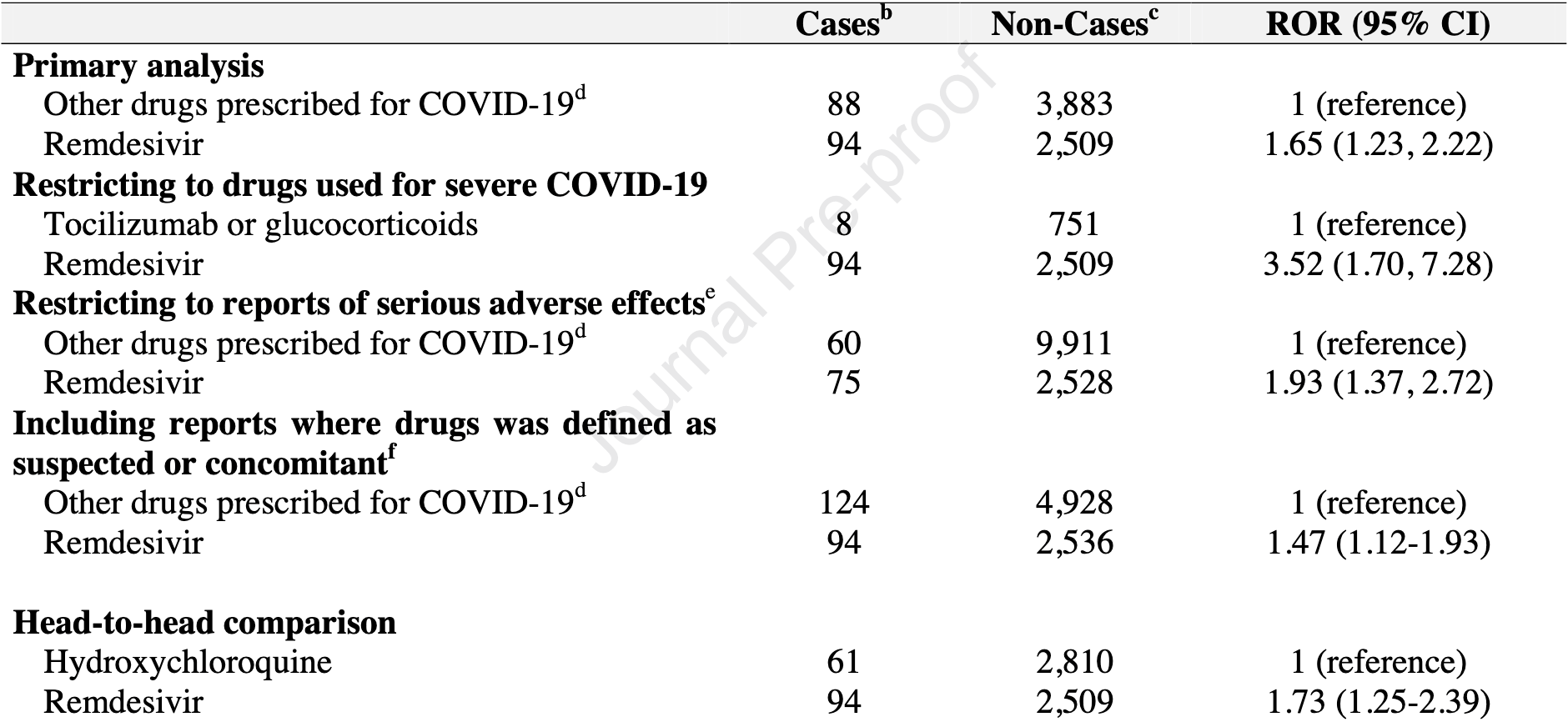
Comparison of bradycardia in COVID-19 patients treated with remdesivir compared to those treated with HCQ, lopinavir/ritonavir, tocilizumab or glucocorticoids, finding increased risk of bradycardia with remdesivir.
Touafchia et al., 27 Feb 2021, peer-reviewed, 6 authors.
Serious bradycardia and remdesivir for coronavirus 2019 (COVID-19): a new safety concerns
Clinical Microbiology and Infection, doi:10.1016/j.cmi.2021.02.013
Objectives: In recent clinical trials some cardiac arrhythmias were reported with use of remdesivir for COVID-19. To address this safety concern, we investigated whether use of remdesivir for COVID-19 is associated with an increased risk of bradycardia. Methods: Using VigiBase®, the World Health Organization Global Individual Case Safety Reports database, we compared the cases of bradycardia reported in COVID-19 patients exposed to remdesivir with those reported in COVID-19 patients exposed to hydroxychloroquine, lopinavir/ritonavir, tocilizumab or glucocorticoids. All reports of patients with COVID-19 registered up to the 23 September 2020 were included. We conducted disproportionality analyses allowing the estimation of reporting odds ratios (RORs) with 95% CI. Results: We found 302 cardiac effects including 94 bradycardia (31%) among the 2603 reports with remdesivir prescribed in COVID-19 patients. Most of the 94 reports were serious (75, 80%), and in 16 reports (17%) evolution was fatal. Compared with hydroxychloroquine, lopinavir/ritonavir, tocilizumab or glucocorticoids, the use of remdesivir was associated with an increased risk of reporting bradycardia (ROR 1.65; 95% CI 1.23e2.22). Consistent results were observed in other sensitivity analyses. Discussion: This post-marketing study in a real-world setting suggests that the use of remdesivir is significantly associated with an increased risk of reporting bradycardia and serious bradycardia when compared with the use of with hydroxychloroquine, lopinavir/ritonavir, tocilizumab or glucocorticoids. This result is in line with the pharmacodynamic properties of remdesivir.
Author contributions All authors conceived and designed the study. F.M. and A.T. acquired the data and did the statistical analyses. All authors analysed and interpreted the data. F.M. wrote the manuscript, and all authors critically revised the manuscript. F.M. supervised the study and is the guarantor. All authors approved the final version of the manuscript and are accountable for its accuracy.
References
Ahmad, Yin, Saffitz, Pockros, Lalezari et al., Cardiac dysfunction associated with a nucleotide polymerase inhibitor for treatment of hepatitis C, Hepatology, doi:10.1002/hep.27488
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of covid-19 e final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Coloma, Trifir O G, Patadia, Sturkenboom, Postmarketing safety surveillance: where does signal detection using electronic healthcare records fit into the big picture?, Drug Saf, doi:10.1007/s40264-013-0018-x
Fontaine, Pol, Pecriaux, Bagate, Sultanik, Bradyarrhythmias associated with sofosbuvir treatment, N Engl J Med, doi:10.1056/NEJMc1505967
Gagne, Finding meaningful patterns in adverse drug event reports, JAMA Intern Med, doi:10.1001/jamainternmed.2014.3270
Goldman, Bomze, Dankner, Hod, Meirson et al., Cardiovascular adverse events associated with hydroxychloroquine and chloroquine: a comprehensive pharmacovigilance analysis of pre-COVID-19 reports, Br J Clin Pharmacol, doi:10.1111/bcp.14546
Gordon, Tchesnokov, Woolner, Perry, Feng et al., Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency, J Biol Chem, doi:10.1074/jbc.RA120.013679
Michaud, Dow, Rihani, Deodhar, Arwood et al., Risk assessment of drug-induced long QT syndrome for some COVID-19 repurposed drugs, Clin Transl Sci, doi:10.1111/cts.12882
Montastruc, Thuriot, Durrieu, Hepatic disorders with the use of remdesivir for coronavirus 2019, Clin Gastroenterol Hepatol, doi:10.1016/j.cgh.2020.07.050
Mulangu, Dodd, Davey, Mbaya, Proschan et al., A randomized, controlled trial of Ebola virus disease therapeutics, N Engl J Med, doi:10.1056/NEJMoa1910993
Onakpoya, Rare adverse events in clinical trials: understanding the rule of three, BMJ Evid Based Med, doi:10.1136/ebmed-2017-110885
Pelleg, Belhassen, The mechanism of the negative chronotropic and dromotropic actions of adenosine 5'-triphosphate in the heart: an update, J Cardiovasc Pharmacol, doi:10.1097/FJC.0b013e3181e0f8b2
Regan, Morissette, Regan, Travis, Gerenser et al., Assessment of the clinical cardiac drug-drug interaction associated with the combination of hepatitis C virus nucleotide inhibitors and amiodarone in Guinea pigs and rhesus monkeys, Hepatology, doi:10.1002/hep.28752
Rihani, Smith, Bikmetov, Deodhar, Dow et al., Risk of adverse drug events following the virtual addition of covid-19 repurposed drugs to drug regimens of frail older adults with polypharmacy, J Clin Med, doi:10.3390/jcm9082591
Wang, Zhang, Du, Du, Zhao et al., Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet, doi:10.1016/S0140-6736(20)31022-9
DOI record:
{
"DOI": "10.1016/j.cmi.2021.02.013",
"ISSN": [
"1198-743X"
],
"URL": "http://dx.doi.org/10.1016/j.cmi.2021.02.013",
"alternative-id": [
"S1198743X2100094X"
],
"author": [
{
"affiliation": [],
"family": "Touafchia",
"given": "Anthony",
"sequence": "first"
},
{
"affiliation": [],
"family": "Bagheri",
"given": "Haleh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carrié",
"given": "Didier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Durrieu",
"given": "Geneviève",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sommet",
"given": "Agnès",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chouchana",
"given": "Laurent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montastruc",
"given": "François",
"sequence": "additional"
}
],
"container-title": "Clinical Microbiology and Infection",
"container-title-short": "Clinical Microbiology and Infection",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
2,
27
]
],
"date-time": "2021-02-27T10:01:52Z",
"timestamp": 1614420112000
},
"deposited": {
"date-parts": [
[
2022,
7,
10
]
],
"date-time": "2022-07-10T06:14:54Z",
"timestamp": 1657433694000
},
"funder": [
{
"DOI": "10.13039/100004423",
"doi-asserted-by": "publisher",
"name": "World Health Organization"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
14
]
],
"date-time": "2024-05-14T19:17:52Z",
"timestamp": 1715714272937
},
"is-referenced-by-count": 75,
"issue": "5",
"issued": {
"date-parts": [
[
2021,
5
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2021,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
1
]
],
"date-time": "2021-05-01T00:00:00Z",
"timestamp": 1619827200000
}
},
{
"URL": "http://www.elsevier.com/open-access/userlicense/1.0/",
"content-version": "vor",
"delay-in-days": 365,
"start": {
"date-parts": [
[
2022,
5,
1
]
],
"date-time": "2022-05-01T00:00:00Z",
"timestamp": 1651363200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1198743X2100094X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1198743X2100094X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "791.e5-791.e8",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
5
]
]
},
"published-print": {
"date-parts": [
[
2021,
5
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1074/jbc.RA120.013679",
"article-title": "Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "6785",
"journal-title": "J Biol Chem",
"key": "10.1016/j.cmi.2021.02.013_bib1",
"volume": "295",
"year": "2020"
},
{
"DOI": "10.1016/j.cgh.2020.07.050",
"article-title": "Hepatic disorders with the use of remdesivir for coronavirus 2019",
"author": "Montastruc",
"doi-asserted-by": "crossref",
"first-page": "2835",
"journal-title": "Clin Gastroenterol Hepatol",
"key": "10.1016/j.cmi.2021.02.013_bib2",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa1910993",
"article-title": "A randomized, controlled trial of Ebola virus disease therapeutics",
"author": "Mulangu",
"doi-asserted-by": "crossref",
"first-page": "2293",
"journal-title": "N Engl J Med",
"key": "10.1016/j.cmi.2021.02.013_bib3",
"volume": "381",
"year": "2019"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1569",
"journal-title": "Lancet",
"key": "10.1016/j.cmi.2021.02.013_bib4",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of covid-19 – final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "10.1016/j.cmi.2021.02.013_bib5",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1136/ebmed-2017-110885",
"article-title": "Rare adverse events in clinical trials: understanding the rule of three",
"author": "Onakpoya",
"doi-asserted-by": "crossref",
"first-page": "6",
"journal-title": "BMJ Evid Based Med",
"key": "10.1016/j.cmi.2021.02.013_bib6",
"volume": "23",
"year": "2018"
},
{
"article-title": "Cardiovascular adverse events associated with hydroxychloroquine and chloroquine: a comprehensive pharmacovigilance analysis of pre-COVID-19 reports",
"author": "Goldman",
"journal-title": "Br J Clin Pharmacol",
"key": "10.1016/j.cmi.2021.02.013_bib7",
"year": "2020"
},
{
"DOI": "10.1002/hep.27488",
"article-title": "Cardiac dysfunction associated with a nucleotide polymerase inhibitor for treatment of hepatitis C",
"author": "Ahmad",
"doi-asserted-by": "crossref",
"first-page": "409",
"journal-title": "Hepatology",
"key": "10.1016/j.cmi.2021.02.013_bib8",
"volume": "62",
"year": "2015"
},
{
"DOI": "10.1056/NEJMc1505967",
"article-title": "Bradyarrhythmias associated with sofosbuvir treatment",
"author": "Fontaine",
"doi-asserted-by": "crossref",
"first-page": "1886",
"journal-title": "N Engl J Med",
"key": "10.1016/j.cmi.2021.02.013_bib9",
"volume": "373",
"year": "2015"
},
{
"DOI": "10.1002/hep.28752",
"article-title": "Assessment of the clinical cardiac drug-drug interaction associated with the combination of hepatitis C virus nucleotide inhibitors and amiodarone in Guinea pigs and rhesus monkeys",
"author": "Regan",
"doi-asserted-by": "crossref",
"first-page": "1430",
"journal-title": "Hepatology",
"key": "10.1016/j.cmi.2021.02.013_bib10",
"volume": "64",
"year": "2016"
},
{
"DOI": "10.1097/FJC.0b013e3181e0f8b2",
"article-title": "The mechanism of the negative chronotropic and dromotropic actions of adenosine 5’-triphosphate in the heart: an update",
"author": "Pelleg",
"doi-asserted-by": "crossref",
"first-page": "106",
"journal-title": "J Cardiovasc Pharmacol",
"key": "10.1016/j.cmi.2021.02.013_bib11",
"volume": "56",
"year": "2010"
},
{
"DOI": "10.1007/s40264-013-0018-x",
"article-title": "Postmarketing safety surveillance: where does signal detection using electronic healthcare records fit into the big picture?",
"author": "Coloma",
"doi-asserted-by": "crossref",
"first-page": "183",
"journal-title": "Drug Saf",
"key": "10.1016/j.cmi.2021.02.013_bib12",
"volume": "36",
"year": "2013"
},
{
"DOI": "10.1001/jamainternmed.2014.3270",
"article-title": "Finding meaningful patterns in adverse drug event reports",
"author": "Gagne",
"doi-asserted-by": "crossref",
"first-page": "1934",
"journal-title": "JAMA Intern Med",
"key": "10.1016/j.cmi.2021.02.013_bib13",
"volume": "174",
"year": "2014"
},
{
"article-title": "Risk of adverse drug events following the virtual addition of covid-19 repurposed drugs to drug regimens of frail older adults with polypharmacy",
"author": "Al Rihani",
"first-page": "2591",
"journal-title": "J Clin Med",
"key": "10.1016/j.cmi.2021.02.013_bib14",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1111/cts.12882",
"article-title": "Risk assessment of drug-induced long QT syndrome for some COVID-19 repurposed drugs",
"author": "Michaud",
"doi-asserted-by": "crossref",
"first-page": "20",
"journal-title": "Clin Transl Sci",
"key": "10.1016/j.cmi.2021.02.013_bib15",
"volume": "14",
"year": "2020"
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1198743X2100094X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Serious bradycardia and remdesivir for coronavirus 2019 (COVID-19): a new safety concerns",
"type": "journal-article",
"volume": "27"
}