Unlocking Insights: Navigating COVID-19 Challenges and Emulating Future Pandemic Resilience Strategies with Strengthening Natural Immunity
, S., Heliyon, doi:10.1016/j.heliyon.2024.e34691, Jul 2024
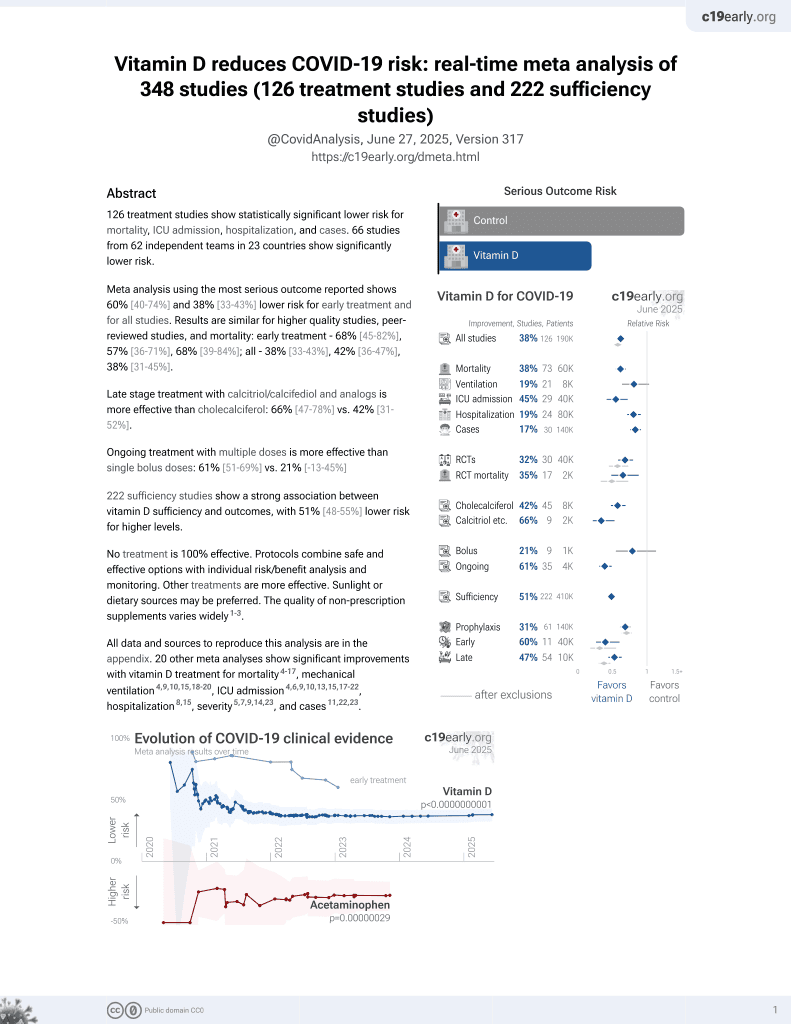
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Review showing reduced efficacy of interventions with new variants and suggesting that regulators and health organizations should consider approval and strategic use of cost-effective adjunct therapies such as vitamin D and ivermectin that are less variant dependent.
Review covers ivermectin and vitamin D.
1.
Jaurrieta-Largo et al., A Machine Learning Approach to Understanding the Genetic Role in COVID-19 Prognosis: The Influence of Gene Polymorphisms Related to Inflammation, Vitamin D, and ACE2, International Journal of Molecular Sciences, doi:10.3390/ijms26167975.
2.
Al-Khrasani et al., Do vitamins halt the COVID-19-evoked pro-inflammatory cytokines involved in the development of neuropathic pain?, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2025.118346.
3.
Kow et al., Vitamin D and COVID‐19: How much more evidence do we need?, Nutrition in Clinical Practice, doi:10.1002/ncp.11349.
4.
Bigman et al., A Comprehensive Scoping Review on Diet and Nutrition in Relation to Long COVID-19 Symptoms and Recovery, Nutrients, doi:10.3390/nu17111802.
5.
Hewison, M., COVID-19 and our understanding of vitamin D and immune function, The Journal of Steroid Biochemistry and Molecular Biology, doi:10.1016/j.jsbmb.2025.106710.
6.
Wimalawansa, S., Vitamin D Deficiency Meets Hill’s Criteria for Causation in SARS-CoV-2 Susceptibility, Complications, and Mortality: A Systematic Review, Nutrients, doi:10.3390/nu17030599.
7.
Sanduzzi Zamparelli et al., Immune-Boosting and Antiviral Effects of Antioxidants in COVID-19 Pneumonia: A Therapeutic Perspective, Life, doi:10.3390/life15010113.
8.
Fazli et al., Possible Link between Gut Microbiota, Diet, and COVID-19 Infection, Journal of Medical Bacteriology, 12:4, jmb.tums.ac.ir/index.php/jmb/article/view/525.
9.
Wojciulik et al., The impact of genetic polymorphism on course and severity of the SARS-CoV-2 infection and COVID-19 disease, Przeglad Epidemiologiczny, doi:10.32394/pe/194862.
10.
Wimalawansa (B), S., Unveiling the Interplay—Vitamin D and ACE-2 Molecular Interactions in Mitigating Complications and Deaths from SARS-CoV-2, Biology, doi:10.3390/biology13100831.
11.
Santa et al., Comparative analysis of COVID-19 responses in Japan and Africa: diet, phytochemicals, vitamin D, and gut microbiota in reducing mortality—A systematic review and meta-analysis, Frontiers in Nutrition, doi:10.3389/fnut.2024.1465324.
12.
Kaushal, A., Nutraceuticals and pharmacological to balance the transitional microbiome to extend immunity during COVID-19 and other viral infections, Journal of Translational Medicine, doi:10.1186/s12967-024-05587-9.
13.
Mu et al., Anti-inflammatory and Nutritional Interventions Against SARS-CoV-2: A Comprehensive Review, Journal of Agriculture and Food Research, doi:10.1016/j.jafr.2024.101422.
14.
Wimalawansa (C), S., Unlocking Insights: Navigating COVID-19 Challenges and Emulating Future Pandemic Resilience Strategies with Strengthening Natural Immunity, Heliyon, doi:10.1016/j.heliyon.2024.e34691.
15.
Imran et al., Therapeutic Role of Vitamin D in COVID-19 Patients, Clinical Nutrition Open Science, doi:10.1016/j.nutos.2024.07.004.
16.
Grant, W., Vitamin D and viral infections: Infectious diseases, autoimmune diseases, and cancers, Advances in Food and Nutrition Research, doi:10.1016/bs.afnr.2023.12.007.
17.
Polonowita et al., Molecular Quantum and Logic Process of Consciousness—Vitamin D Big-Data in COVID-19—A Case for Incorporating Machine Learning In Medicine, European Journal of Biomedical and Pharmaceutical sciences, doi:10.5281/zenodo.10435649.
18.
Gomaa et al., Pharmacological evaluation of vitamin D in COVID-19 and long COVID-19: recent studies confirm clinical validation and highlight metformin to improve VDR sensitivity and efficacy, Inflammopharmacology, doi:10.1007/s10787-023-01383-x.
19.
Gotelli et al., Understanding the immune-endocrine effects of vitamin D in SARS-CoV-2 infection: a role in protecting against neurodamage?, Neuroimmunomodulation, doi:10.1159/000533286.
20.
Cutolo et al., Involvement of the secosteroid vitamin D in autoimmune rheumatic diseases and COVID-19, Nature Reviews Rheumatology, doi:10.1038/s41584-023-00944-2.
21.
Schloss et al., Nutritional deficiencies that may predispose to long COVID, Inflammopharmacology, doi:10.1007/s10787-023-01183-3.
22.
Arora et al., Global Dietary and Herbal Supplement Use during COVID-19—A Scoping Review, Nutrients, doi:10.3390/nu15030771.
23.
Nicoll et al., COVID-19 Prevention: Vitamin D Is Still a Valid Remedy, Journal of Clinical Medicine, doi:10.3390/jcm11226818.
24.
Foshati et al., Antioxidants and clinical outcomes of patients with coronavirus disease 2019: A systematic review of observational and interventional studies, Food Science & Nutrition, doi:10.1002/fsn3.3034.
25.
Quesada-Gomez et al., Vitamin D Endocrine System and COVID-19: Treatment with Calcifediol, Nutrients, doi:10.3390/nu14132716.
26.
DiGuilio et al., Micronutrient Improvement of Epithelial Barrier Function in Various Disease States: A Case for Adjuvant Therapy, International Journal of Molecular Sciences, doi:10.3390/ijms23062995.
27.
Grant (B) et al., A Narrative Review of the Evidence for Variations in Serum 25-Hydroxyvitamin D Concentration Thresholds for Optimal Health, Nutrients, doi:10.3390/nu14030639.
28.
Shah Alam et al., The role of vitamin D in reducing SARS-CoV-2 infection: An update, International Immunopharmacology, doi:10.1016/j.intimp.2021.107686.
29.
Griffin et al., Perspective: Vitamin D supplementation prevents rickets and acute respiratory infections when given as daily maintenance but not as intermittent bolus: implications for COVID-19, Clinical Medicine, doi:10.7861/clinmed.2021-0035.
30.
Kohlmeier et al., When Mendelian randomisation fails, BMJ Nutrition, Prevention & Health, doi:10.1136/bmjnph-2021-000265.
31.
Brenner, H., Vitamin D Supplementation to Prevent COVID-19 Infections and Deaths—Accumulating Evidence from Epidemiological and Intervention Studies Calls for Immediate Action, Nutrients, doi:10.3390/nu13020411.
32.
Mercola et al., Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity, Nutrients 2020, 12:11, 3361, doi:10.3390/nu12113361.
33.
Basha et al., Is the shielding effect of cholecalciferol in SARS CoV-2 infection dependable? An evidence based unraveling, Clinical Epidemiology and Global Health, doi:10.1016/j.cegh.2020.10.005.
34.
Xu et al., The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19, Journal of Translational Medicine, doi:10.1186/s12967-020-02488-5.
35.
Alexander et al., Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance Against Progressive COVID-19, Nutrients, doi:10.3390/nu12082358.
36.
Andrade et al., Vitamin A and D deficiencies in the prognosis of respiratory tract infections: A systematic review with perspectives for COVID-19 and a critical analysis on supplementation, SciELO preprints, doi:10.1590/SciELOPreprints.839.
37.
Grant (C) et al., Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths, Nutrients, 12:4, 988, doi:10.3390/nu12040988.
38.
McCullough et al., Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience, The Journal of Steroid Biochemistry and Molecular Biology, doi:10.1016/j.jsbmb.2018.12.010.
39.
EFSA, Scientific Opinion on the substantiation of health claims related to vitamin D and normal function of the immune system and inflammatory response (ID 154, 159), maintenance of normal muscle function (ID 155) and maintenance of normal cardiovascular function (ID 159) pursuant to Article 13(1) of Regulation (E, EFSA Journal, doi:10.2903/j.efsa.2010.1468.
40.
EFSA (B), Scientific Opinion on the substantiation of a health claim related to vitamin D and contribution to the normal function of the immune system pursuant to Article 14 of Regulation (EC) No 1924/2006, EFSA Journal, doi:10.2903/j.efsa.2015.4096.
Wimalawansa et al., 17 Jul 2024, USA, peer-reviewed, 1 author, study period December 2020 - September 2021.
Contact: suniljw@hotmail.com, sunil.wimalawasa@rutgers.edu.
Unlocking Insights: Navigating COVID-19 Challenges and Emulating Future Pandemic Resilience Strategies with Strengthening Natural Immunity
Heliyon, doi:10.1016/j.heliyon.2024.e34691
The original COVID-19 vaccines, developed against SARS-CoV-2, initially mitigated hospitalizations. Bivalent vaccine boosters were used widely during 2022-23, but the outbreaks persisted. Despite this, hospitalizations, mortality, and outbreaks involving dominant mutants like Alpha and Delta increased during winters when the population's vitamin D levels were at their lowest. Notably, 75% of human immune cell/system functions, including post-vaccination adaptive immunity, rely on adequate circulatory vitamin D levels. Consequently, hypovitaminosis compromises innate and adaptive immune responses, heightening susceptibility to infections and complications. COVID-19 vaccines primarily target SARS-CoV-2 Spike proteins, thus offering limited protection through antibodies. mRNA vaccines, such as those for COVID-19, fail to generate secretory/mucosal immunity-like IgG responses, rendering them ineffective in halting viral spread. Additionally, mutations in the SARS-CoV-2 binding domain reduce immune recognition by vaccine-derived antibodies, leading to immune evasion by mutant viruses like Omicron variants. Meanwhile, the repeated administration of bivalent boosters intended to enhance efficacy resulted in the immunoparesis of recipients. As a result, relying solely on vaccines for outbreak prevention became less effective. Dominant variants exhibit increased affinity to angiotensin-converting enzyme receptor-2, enhancing infectivity but reducing virulence. Meanwhile, spike protein-related viral mutations do not impact the potency of widely available, repurposed early therapies, like vitamin D and ivermectin. With the re-emergence of COVID-19 and impending coronaviral pandemics, regulators and health organizations should proactively consider approval and strategic use of costeffective adjunct therapies mentioned above to counter the loss of vaccine efficacy against emerging variants and novel coronaviruses and eliminate serious adverse effects. Timely implementation of these strategies could reduce morbidity, mortality, and healthcare costs and provide a rational approach to address future epidemics and pandemics. This perspective critically reviews relevant literature, providing insights, justifications, and viewpoints into how the scientific community and health authorities can leverage this knowledge cost-effectively.
Conflicts of interest: The author declares no conflicts of interest. The author did not receive any funding or writing assistance. Ethics statement: This study does not have issues related to any ethical aspect.
Conflicts of interest The author declares no conflicts of interest. He did not receive any funding or writing assistance Funding: None J o u r n a l P r e -p r o o f
References
Acharya, Schrom, Mitchell, Viral Load Among Vaccinated and Unvaccinated, Asymptomatic and Symptomatic Persons Infected With the SARS-CoV-2 Delta Variant, Open Forum Infect Dis, doi:10.1093/ofid/ofac135
Ader, Bouscambert-Duchamp, Hites, Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00485-0212
Adhikari, Cheah, Seidlein, Trust is the common denominator for COVID-19 vaccine acceptance: A literature review, Vaccine X, doi:10.1016/j.jvacx.2022.100213
Ahmadi, Bazargan, Elahi, Esmaeilzadeh, Immune evasion of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2); molecular approaches, Mol Immunol, doi:10.1016/j.molimm.2022.11.020
Almadani, Alshammari, Vaccine adverse event reporting system (VAERS): Evaluation of 31 years of reports and pandemics' impact, Saudi Pharm J, doi:10.1016/j.jsps.2022.10.001
Amin, Drenos, No evidence that vitamin D is able to prevent or affect the severity of COVID-19 in individuals with European ancestry: a Mendelian randomisation study of open data, BMJ Nutr Prev Health, doi:10.1136/bmjnph-2020-000151134
Anderson, Sg, Risks elaborated vs. risks downplayed: The effect of risk comparisons in mainstream mediaduring COVID-19 on risk perceptions and anxiety levels, Frontiers: Sci Environ Commun, doi:10.3389/fcomm.2021.646001146
Antonelli, Pujol, Spector, Ourselin, Steves, Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2, Lancet, doi:10.1016/S0140-6736(22)00941-2172
Arashkia, Jalilvand, Mohajel, Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects, Rev Med Virol, doi:10.1002/rmv.2183
Aslam, Danziger-Isakov, Mehra, COVID-19 vaccination immune paresis in heart and lung transplantation, J Heart Lung Transplant, doi:10.1016/j.healun.2021.04.018166
Athas, Lc, Mogul, Gonzalez-Roman, Sri Lanka is 'bankrupt,' Prime Minister says
Auladell, Jia, Hensen, Recalling the Future: Immunological Memory Toward Unpredictable Influenza Viruses, Front Immunol, doi:10.3389/fimmu.2019.01400
Baden, Sahly, Essink, Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine, N Engl J Med, doi:10.1056/NEJMoa2035389192
Basu, Sinha, Ong, Basu, Balogun, Recent advancements in emerging technologies for healthcare management systems: A survey, Indian J Dermatol, doi:10.4103/ijd.IJD_421_20115
Bellavite, Ferraresi, Isidoro, Immune response and molecular mechanisms of cardiovascular adverse effects of Spike Proteins from SARS-CoV-2 and mRNA vaccines, Biomedicines, doi:10.3390/biomedicines11020451
Ben-Zuk, Dechtman, Henn, Potential prophylactic treatments for COVID-19, Viruses, doi:10.3390/v13071292135
Bergman, Lindh, Bjorkhem-Bergman, Lindh, Vitamin D and respiratory tract iInfections: A systematic review and meta-analysis of randomized controlled trials, PLoS One, doi:10.1371/journal.pone.0065835
Bergwerk, Gonen, Lustig, Covid-19 Breakthrough Infections in Vaccinated Health Care Workers, N Engl J Med, doi:10.1056/NEJMoa2109072
Biswas, Rahaman, Biswas, Haque, Association of sex, sge, and comorbidities with mortality in COVID-19 Patients: A systematic review and meta-analysis, Intervirology, doi:10.1159/000512592161
Bitterman, Martins, Cices, Nadendla, Comparison of trials using ivermectin for COVID-19 between regions with high and low prevalence of strongyloidiasis: A meta-analysis, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.3079Jo
Bleier, Ramanathan, Lane, COVID-19 Vaccines May Not Prevent Nasal SARS-CoV-2 Infection and Asymptomatic Transmission, Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery, doi:10.1177/0194599820982633
Boschi, Scheim, Bancod, SARS-CoV-2 Spike protein induces hemagglutination: implications for COVID-19 morbidities and therapeutics and for vaccine adverse effects, Int J Mol Sci, doi:10.3390/ijms232415480
Bouwman, Verhaegh, Holtzer, Van De Stolpe, Measurement of Cellular Immune Response to Viral Infection and Vaccination, Front Immunol, doi:10.3389/fimmu.2020.575074
Bramante, Buse, Liebovitz, Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallelgroup, phase 3 trial, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00299-2227
Bramante, Huling, Tignanelli, Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19, N Engl J Med, doi:10.1056/NEJMoa2201662226
Bryant, Lawrie, Dowswell, Ivermectin for prevention and treatment of COVID-19 infection: A systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines, Am J Ther, doi:10.1097/MJT.0000000000001402219
Burhan, Liu, Marwali, Characteristics and outcomes of patients with severe COVID-19 in Indonesia: Lessons from the first wave, PLoS One, doi:10.1371/journal.pone.0290964213
Cagigi, Lore, Immune responses induced by mRNA vaccination in mice, monkeys and humans, Vaccines, doi:10.3390/vaccines9010061
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res, doi:10.1016/j.antiviral.2020.104787239
Cannata-Andia, Diaz-Sottolano, Fernandez, A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: the COVID-VIT-D-a randomised multicentre international clinical trial, BMC Med, doi:10.1186/s12916-022-02290-8129
Cao, He, Gong, Wu, He, The effects of vitamin D on all-cause mortality in different diseases: an evidence-map and umbrella review of 116 randomized controlled trials, Front Nutr, doi:10.3389/fnut.2023.1132528238
Castillo, Costa, Barrios, Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2020.105751123
Cegolon, Negro, Mastrangelo, Filon, Ow et al., Breakthrough SARS-CoV-2 infections among patients with cancer following two and three doses of COVID-19 mRNA vaccines: a retrospective observational study from the COVID-19 and Cancer Consortium, Lancet Reg Health Am, doi:10.3390/v14122688169
Cereda, Bogliolo, Lobascio, Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy, Italy, Nutrition, doi:10.1016/j.nut.2020.111055133
Chalkias, Harper, Vrbicky, A bivalent Omicron-containing booster vaccine against Covid-19, N Engl J Med, doi:10.1056/NEJMoa2208343109
Chamie, Hibberd, Scheim, COVID-19 excess deaths in Peru's 25 States in 2020: Nationwide trends, confounding factors, and correlations with the extent of ivermectin treatment by State, Cureus, doi:10.7759/cureus.43168198
Chanda, Hines, Itoh, COVID-19 Vaccine Effectiveness Against Progression to In-Hospital Mortality in Zambia, 2021-2022, Open Forum Infect Dis, doi:10.1093/ofid/ofac469154
Charoenngam, Holick, Immunologic effects of vitamin D on human health and sisease, Nutrients, doi:10.3390/nu12072097
Chatzilena, Hyams, Challen, Relative vaccine effectiveness of mRNA COVID-19 boosters in people aged at least 75 years during the spring-summer (monovalent vaccine) and autumn-winter (bivalent vaccine) booster campaigns: a prospective test negative case-control study, United Kingdom, Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin, doi:10.2807/1560-7917.ES.2023.28.48.2300173110
Chavda, Sonak, Munshi, Dhamade, Pseudoscience and fraudulent products for COVID-19 management, Environmental science and pollution research international, doi:10.1007/s11356-022-21967-4148
Chenchula, Karunakaran, Sharma, Chavan, Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review, J Med Virol, doi:10.1002/jmv.27697JournalPre-proof111
Chi, Li, Huang, COVID-19 vaccine update: vaccine effectiveness, SARS-CoV-2 variants, boosters, adverse effects, and immune correlates of protection, J Biomed Sci, doi:10.1186/s12929-022-00853-8
Chowdhury, Bhattacharya, Gogoi, Veeranna, Kumar, An update on complications associated with SARS-CoV-2 infection and COVID-19 vaccination, Vaccines, doi:10.3390/vaccines10101639
Chung, Cho, General guideline in the endoscopy room to avoid air-borne infection during the COVID-19 pandemic, Clin Endosc, doi:10.5946/ce.2022.146
Colomin, Smh, Youngs, The impact of disinformation on democratic processes and human rights in the world, European Parliment
Connolly, Paik, Wimalawansa, Global epidemic of coronavirus-COVID-19: What can we do to minimize risks?, European J Biomed & Pharma Sci, doi:10.1016/j.jaci.2022.05.001187
Cosentino, Vernocchi, Martini, Early outpatient treatment of COVID-19: A retrospective analysis of 392 cases in Italy, J Clin Med, doi:10.3390/jcm11206138207
Daems, Maes, The race for COVID-19 vaccines: accelerating innovation, fair allocation and distribution, Vaccines, doi:10.3390/vaccines10091450
Das, Chisti, Haque, Evaluating association of vaccine response to low serum zinc and vitamin D levels in children of a birth cohort study in Dhaka, Vaccine, doi:10.1016/j.vaccine.2020.10.048186
Desai, Lavelle, Boursiquot, Wan, Long-term complications of COVID-19, Am J Physiol Cell Physiol, doi:10.1152/ajpcell.00375.2021
Dingemans, Van Der Veer, Gorgels, Investigating SARS-CoV-2 breakthrough infections per variant and vaccine type, Front Microbiol, doi:10.3389/fmicb.2022.1027271182
Duan, Luo, Zhao, Reporting and data sharing level for COVID-19 vaccine trials: A crosssectional study, EBioMedicine, doi:10.1016/j.ebiom.2022.103962
Duroseau, Kipshidze, Limaye, The impact of delayed access to COVID-19 vaccines in lowand lower-middle-income countries, Front Public Health, doi:10.3389/fpubh.2022.1087138
Fang, Liu, Li, Advances in COVID-19 mRNA vaccine development, Signal Transduct Target Ther, doi:10.1038/s41392-022-00950-y
Fda, COVID-19 bivalent vaccine boosters, FDA
Finsterer, Vaccine adverse Event reporting system could miss or misinterpret neurological side effects of COVID-19 vaccinations, Ann Neurol, doi:10.1002/ana.26369
Focosi, Maggi, Neutralising antibody escape of SARS-CoV-2 spike protein: Risk assessment for antibody-based Covid-19 therapeutics and vaccines, Rev Med Virol, doi:10.1002/rmv.2231
Forman, Shah, Jeurissen, Jit, Mossialos, COVID-19 vaccine challenges: What have we learned so far and what remains to be done?, Health Policy, doi:10.1016/j.healthpol.2021.03.013
Fraiman, Erviti, Jones, Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults, Vaccine, doi:10.1016/j.vaccine.2022.08.036
Fung, Baye, Baik, Mcdonald, Nirmatrelvir and Molnupiravir and post-COVID-19 condition in older patients, JAMA Intern Med, doi:10.1001/jamainternmed.2023.5099209
Glaser, Diexer, Klee, The contribution of SARS-CoV-2 to the burden of acute respiratory infections in winter season 2022/2023: Results from the DigiHero study, Int J Infect Dis, doi:10.1016/j.ijid.2024.107057
Grant, Vitamin D acceptance delayed by big pharma following the Disinformation Playbook
Grant, Wimalawansa, Wimalawansa, Prophylactic use of vitamin D to maintain a robust immune system against infections like SARS-CoV-2, Glob J Endocrinol Metab GJEM, doi:10.3390/nu15173842127
Guerin, Yahi, Azzaz, Chahinian, Sabatier et al., Structural Dynamics of the SARS-CoV-2 Spike Protein: A 2-Year Retrospective Analysis of SARS-CoV-2 Variants (from Alpha to Omicron) Reveals an Early Divergence between Conserved and Variable Epitopes, Molecules, doi:10.3390/molecules27123851157
Guo, Han, Zhang, SARS-CoV-2 Omicron variant: epidemiological features, biological characteristics, and clinical significance, Front Immunol, doi:10.3389/fimmu.2022.877101
Guzzo, Furtek, Porras, Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects, J Clin Pharmacol, doi:10.1177/009127002401382731221
Hagan, Mccormick, Lee, Outbreak of SARS-CoV-2 B.1.617.2 (delta) variant infections among incarcerated persons in a federal prison-Texas, July-August 2021, The Lancet-Infectious diseases
Hastie, Pell, Sattar, Vitamin D and COVID-19 infection and mortality in UK Biobank, Eur J Nutr, doi:10.1007/s00394-020-02372-4119
Havasi, Visan, Cainap, Cainap, Mihaila et al., Influenza A, Influenza B, and SARS-CoV-2 similarities and differences -A focus on diagnosis, Front Microbiol, doi:10.3389/fmicb.2022.908525
Hayward, Yu, Little, Ivermectin for COVID-19 in adults in the community (PRINCIPLE): An open, randomised, controlled, adaptive platform trial of short-and longer-term outcomes, J Infect, doi:10.1016/j.jinf.2024.106130223
Heinz, Stiasny, Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action, NPJ Vaccines, doi:10.1038/s41541-021-00369-6
Henrina, Lim, Pranata, COVID-19 and misinformation: how an infodemic fuelled the prominence of vitamin D, Br J Nutr, doi:10.1017/S0007114520002950JournalPre-proof153
Hess, Lancsar, Mariel, The path towards herd immunity: Predicting COVID-19 vaccination uptake through results from a stated choice study across six continents, Soc Sci Med, doi:10.1016/j.socscimed.2022.114800105
Hwang, Hwang, Park, Park, Son et al., Clinical severity according to the primary infection variant in patients with suspected SARS-CoV-2 reinfection in Korea, Epidemiol Health, doi:10.4178/epih.e2023007J
Ichhpujani, Parmar, Duggal, Kumar, COVID-19 vaccine-associated ocular adverse effects: An overview, Vaccines, doi:10.3390/vaccines10111879
Ioannou, Berry, Rajeevan, Effectiveness of Nirmatrelvir-Ritonavir against the development of post-COVID-19 conditions among U.S. Veterans : A target trial emulation, Ann Intern Med, doi:10.7326/M23-1394211
Israel, Shenhar, Green, Large-Scale Study of Antibody Titer Decay following BNT162b2 mRNA Vaccine or SARS-CoV-2 Infection, Vaccines, doi:10.3390/vaccines10010064156
Ittiwut, Mahasirimongkol, Srisont, Genetic basis of sudden death after COVID-19 vaccination in Thailand, Heart Rhythm, doi:10.1016/j.hrthm.2022.07.019
J O U R N A L P R E -P R O O F 90, Canning, Watson, Mccreedy, Olawepo, Ethics and effectiveness of US COVID-19 vaccine mandates and vaccination passports: A review, J Res Health Sci, doi:10.34172/jrhs.2022.81
J O U R N A L P R E, None
J O U R N A L P R E, None
Jain, Venkataraman, Wechsler, Peppas, Messenger RNA-based vaccines: Past, present, and future directions in the context of the COVID-19 pandemic, Adv Drug Deliv Rev, doi:10.1016/j.addr.2021.114000
Jiao, Huang, Sun, Yang, Research progress of post-acute sequelae after SARS-CoV-2 infection, Cell Death Dis, doi:10.1038/s41419-024-06642-5
Kaufman, Niles, Kroll, Bi, Holick, SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels, PLoS One, doi:10.1371/journal.pone.0239252117
Kindler, Thiel, To sense or not to sense viral RNA--essentials of coronavirus innate immune evasion, Curr Opin Microbiol, doi:10.1016/j.mib.2014.05.005
Komine-Aizawa, Haruyama, Deguchi, The vaccination status and adverse effects of COVID-19 vaccine among pregnant women in Japan in 2021, J Obstet Gynaecol Res, doi:10.1111/jog.15285
Konishi, Mutations in SARS-CoV-2 are on the increase against the acquired immunity, PLoS One, doi:10.1371/journal.pone.0271305
Kontopoulou, Nakas, Belai, Papazisis, Ewijk et al., Antibody titers after a third dose of the SARS-CoV-2 BNT162b2 vaccine in immunocompromised adults in Greece: Is a fourth dose necessary?, J Med Virol, doi:10.1002/jmv
Kory, Mj, The war on ivermectin: The medicine that saved millions and could have ended the pandemic
Koshak, Koshak, Mobeireek, Nigella sativa for the treatment of COVID-19: An openlabel randomized controlled clinical trial, Complement Ther Med, doi:10.1016/j.ctim.2021.102769205
Kumar, Rathi, Haq, Wimalawansa, Sharma et al., Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19, Virus Res, doi:10.1016/j.virusres.2020.198235120
Kumari, Lu, Li, A critical overview of current progress for COVID-19: development of vaccines, antiviral drugs, and therapeutic antibodies, J Biomed Sci, doi:10.1186/s12929-022-00852-9
Lam, Zhang, Man, Persistence in risk and effect of COVID-19 vaccination on longterm health consequences after SARS-CoV-2 infection, Nat Commun, doi:10.1038/s41467-024-45953-1
Lan, Lee, Chao, Chang, Lu et al., Efficacy of melatonin in the treatment of patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials, J Med Virol, doi:10.1002/jmv.27595206
Lau, Cheng, Leung, Author Correction: Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population, Nat Med, doi:10.1038/s41591-023-02648-2
Lee, Park, Kim, No correlation of neutralizing antibody titers against the Omicron variant after a booster dose of COVID-19 vaccines with subsequent breakthrough Omicron infections among healthcare workers, J Infect, doi:10.1016/j.jinf.2022.10.007112
Lenze, Mattar, Zorumski, Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: A randomized clinical trial, JAMA, doi:10.1001/jama.2020.22760202
Lewis, Murray, Adams, Absolute and Relative Vaccine Effectiveness of Primary and Booster Series of COVID-19 Vaccines (mRNA and Adenovirus Vector) Against COVID-19 Hospitalizations in the United States, December 2021-April 2022, Open Forum Infect Dis, doi:10.1093/ofid/ofac698177
Lind, Copin, Mccarthy, Use of Whole-Genome Sequencing to Estimate the Contribution of Immune Evasion and Waning Immunity on Decreasing COVID-19 Vaccine Effectiveness, J Infect Dis, doi:10.1093/infdis/jiac453164
Liu, Rocklov, The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus, J Travel Med, doi:10.1093/jtm/taab124
Ma, Zhu, Zhong, Serial negative response after standard and third (Booster) dose of COVID-19 inactivated vaccine is associated with low vitamin D levels in patients with solid cancers, Front Med, doi:10.3389/fmed.2022.898606178
Macfarlane, Tay, Hurlstone, Ecker, Refuting spurious COVID-19 treatment claims reduces demand and misinformation sharing, J Appl Res Mem Cogn, doi:10.1016/j.jarmac.2020.12.005140
Macintyre, Costantino, Heslop, The potential impact of a recent measles epidemic on COVID-19 in Samoa, BMC Infect Dis, doi:10.1186/s12879-020-05469-7175
Maeda, Saraiva, Hayashi, Seasonal variation in the serum 25-hydroxyvitamin D levels of young and elderly active and inactive adults in Sao Paulo, Brazil: The Sao PAulo Vitamin D Evaluation Study (SPADES), Dermatoendocrinol, doi:10.4161/derm.24476
Mahmud, Rahman, Alam, Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial, J Int Med Res, doi:10.1177/03000605211013550217
Marcolino, Meira, Guimaraes, Systematic review and meta-analysis of ivermectin for treatment of COVID-19: evidence beyond the hype, BMC Infect Dis, doi:10.1186/s12879-022-07589-8229
Marks, Califf, Bivalent Covid-19 Vaccines, N Engl J Med
Martinez, Lack of effectiveness of repurposed drugs for COVID-19 treatment, Front Immunol, doi:10.3389/fimmu.2021.635371139
Matsumoto, Shimizu, Yokoyama, Tsukahara, Yorifuji, Adverse reactions and attitudes toward the BNT162b2 COVID-19 vaccine in children 5 to 11 years of age in Japan, J Epidemiol, doi:10.2188/jea.JE20220265
Maziarz, Stencel, The failure of drug repurposing for COVID-19 as an effect of excessive hypothesis testing and weak mechanistic evidence, Hist Philos Life Sci, doi:10.1007/s40656-022-00532-9145
Meeus, Van Coile, Pottel, Efficacy and safety of in-hospital treatment of Covid-19 infection with low-dose hydroxychloroquine and azithromycin in hospitalized patients: A retrospective controlled cohort study, New Microbes New Infect, doi:10.1016/j.nmni.2023.101172240
Mocanu, Amariei, Salari, Hejazi, Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: An open label nonrandomized clinical trial, Phytother Res, doi:10.3390/plants11060740203
Mohammad, Mashayekhi, Seirafianpour, Mesgarha, Goodarzi, COVID-19 and COVID-19 vaccine-related dermatological reactions: An interesting case series with a narrative review of the potential critical and non-critical mucocutaneous adverse effects related to virus, therapy, and the vaccination, Clin Case Rep, doi:10.1002/ccr3.5775
Mosca, Colucci, Savoia, Vitamin D levels in the pre-and post-COVID-19 pandemic periods and related confinement at pediatric age, Nutrients, doi:10.3390/nu15092089
Murai, Fernandes, Sales, Effect of a single high dose of vitamin D3 on hospital length of stay in patients Wpwith moderate to severe COVID-19: A randomized clinical trial, JAMA. Mar, doi:10.1001/jama.2020.26848130
Mustafa, Shekhar, Concentration levels of serum 25-Hydroxyvitamin-D and vitamin D deficiency among children and adolescents of India: a descriptive cross-sectional study, BMC Pediatr, doi:10.1186/s12887-021-02803-z199
Naggie, Boulware, Lindsell, Effect of higher-dose ivermectin for 6 Days vs placebo on time to sustained recovery in outpatients with COVID-19: A randomized clinical trial, Clin Infect Dis, doi:10.1001/jama.2023.1650230
Naggie, Boulware, Lindsell, Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: A randomized clinical trial, JAMA, doi:10.1001/jama.2022.18590225
Najjar-Debbiny, Gronich, Weber, Effectiveness of Paxlovid in reducingsevere coronavirus gisease 2019 and mortality in high-risk patients, Clin Infect Dis, doi:10.1093/cid/ciac443210
Navarro, Camprubi, Requena-Mendez, Safety of high-dose ivermectin: a systematic review and meta-analysis, J Antimicrob Chemother, doi:10.1093/jac/dkz524220
Nuwarda, Ramzan, Weekes, Kayser, Vaccine hesitancy: Contemporary issues and historical background, Vaccines, doi:10.3390/vaccines10101595
Okumus, Demirturk, Cetinkaya, Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients, BMC Infect Dis, doi:10.1186/s12879-021-06104-9
Om, Spillane, Byrne, Neill, Harrington et al., Interventions in an ambulatory setting to prevent progression to severe disease in patients with COVID-19: A systematic review, Ann Pharmacother, doi:10.1177/10600280211028242233
Padhy, Mohanty, Das, Meher, Therapeutic potential of ivermectin as add on treatment in COVID 19: A systematic review and meta-analysis, J Pharm Pharm Sci, doi:10.18433/jpps31457125
Page, Mckenzie, Bossuyt, The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, BMJ, doi:10.1136/bmj.n71
Park, Lagniton, Liu, Xu, mRNA vaccines for COVID-19: what, why and how, International journal of biological sciences, doi:10.7150/ijbs.59233
Patel, Kelleman, West, Comparison of multisystem inflammatory syndrome in childrenrelated myocarditis, classic viral myocarditis, and COVID-19 vaccine-related myocarditis in children, J Am Heart Assoc, doi:10.1161/JAHA.121.024393
Pecina, Merry, Park, Td, Vitamin D Status and Severe COVID-19 Disease Outcomes in Hospitalized Patients, J Prim Care Community Health, doi:10.1177/21501327211041206132
Peng, Gao, Liu, Zheng, Liu, Interactive effect of booster vaccination and vitamin D status on antibody production of Omicron variant-infected adults: A real-world cohort study, Clin Respir J, doi:10.1111/crj.13694185
Pilz, Zittermann, Trummer, Vitamin D testing and treatment: a narrative review of current evidence, Endocr Connect, doi:10.1530/EC-18-0432138
Pioli, Plasma Cells, the Next Generation: Beyond Antibody Secretion, Front Immunol, doi:10.3389/fimmu.2019.02768
Polack, Thomas, Kitchin, Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine, N Engl J Med, doi:10.1056/NEJMoa2034577191
Polonowita, Wimalawansa, Molecular quantum and logic process of consciousness-Vitamin D big-data in COVID-19-A case for incorporating machine learning in medicine, Euro J Biomedical Pharma Sci
Polonowita, Wimalawansa, Sj, Impact of Withholding Cost-Effective Early Treatments, Such as Vitamin D, on COVID-19: An Analysis Using an Innovative Logical Paradigm, Wold J Adanced Pharma Life Sci
Popp, Reis, Schiesser, Ivermectin for preventing and treating COVID-19, Cochrane Database Syst Rev, doi:10.1002/14651858.CD015017.pub3228
Puhach, Adea, Hulo, Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2, Nat Med, doi:10.1038/s41591-022-01816-0
Pulvirenti, Mortari, Putotto, COVID-19 severity, cardiological outcome, and Immunogenicity of mRNA vaccine on adult patients with 22q11.2 DS, J Allergy Clin Immunol Pract, doi:10.1016/j.jaip.2022.10.010
Puspitarani, Sitaresmi, Ahmad, Adverse events following immunization of COVID-19 vaccine among children aged 6-11 years, Front Public Health, doi:10.3389/fpubh.2022.999354
Rago, Toth, Szalenko-Tokes, Results of a systematic review and meta-analysis of early studies on ivermectin in SARS-CoV-2 infection, Geroscience, doi:10.1007/s11357-023-00756-y218
Raisi-Estabragh, Mccracken, Bethell, Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK Biobank, Journal of public health, doi:10.1093/pubmed/fdaa095118
Rajan, Dashti, In-hospital mortality in SARS-CoV-2 stratified by serum 25hydroxy-vitamin D levels: A retrospective study, J Med Virol, doi:10.1002/jmv.27133JournalPre-proof131
Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of Ivermectin Is Associated With Lower Mortality in Hospitalized Patients With Coronavirus Disease 2019: The Ivermectin in COVID Nineteen Study, Chest, doi:10.1016/j.chest.2020.10.009124
Ravikirti, Pattadar, Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19: A double-blind randomized placebo Controlled ttrial in eastern India, J Pharm Pharm Sci, doi:10.18433/jpps32105216
Reed, Hendlin, Desikan, Mackinney, Berman et al., The disinformation playbook: how industry manipulates the science-policy process-and how to restore scientific integrity, J Public Health Policy, doi:10.1057/s41271-021-00318-6103
Reis, Silva, Silva, Effect of early treatment with Ivermectin among patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2115869224
Riemersma, Haddock, Wilson, Shedding of infectious SARS-CoV-2 despite vaccination, PLoS Pathog, doi:10.1371/journal.ppat.1010876174
Romer, Jamieson, Conspiratorial thinking as a precursor to opposition to COVID-19 vaccination in the US: a multi-year study from 2018 to 2021, Sci Rep, doi:10.1038/s41598-022-22014-5
Salomao, Queiroz, Mendes, COVID-19-related multisystem inflammatory syndrome in adult: the first death in Brazil, Rev Inst Med Trop Sao Paulo, doi:10.1590/S1678-9946202365050
Salter, Li, Trentino, Safety of Four COVID-19 Vaccines across Primary Doses 1, 2, 3 and Booster: A Prospective Cohort Study of Australian Community Pharmacy Vaccinations, doi:10.3390/vaccines10122017179
Samanovic, Oom, Cornelius, Vaccine-Acquired SARS-CoV-2 Immunity versus Infection-Acquired Immunity: A Comparison of Three COVID-19 Vaccines, Vaccines, doi:10.3390/vaccines10122152155
Santi Laurini, Montanaro, Broccoli, Bonaldo, Motola, Real-life safety profile of mRNA vaccines for COVID-19: An analysis of VAERS database, Vaccine, doi:10.1016/j.vaccine.2023.03.054
Schellack, Strydom, Pepper, Social media and COVID-19-perceptions and public deceptions of ivermectin, colchicine and hydroxychloroquine: Lessons for future pandemics, Antibiotics, doi:10.3390/antibiotics11040445142
Schlake, Thess, Fotin-Mleczek, Kallen, Developing mRNA-vaccine technologies, RNA Biol, doi:10.4161/rna.22269
Seneff, Nigh, Kyriakopoulos, Mccullough, Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs, Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, doi:10.1016/j.fct.2022.113008
Shahbaznejad, Davoudi, Eslami, Effects of ivermectin in patients With COVID-19: A multicenter, double-blind, randomized, controlled clinical trial, Clin Ther, doi:10.1016/j.clinthera.2021.04.007
Shahcheraghi, Ayatollahi, Aljabali, An overview of vaccine development for COVID-19, Ther Deliv, doi:10.4155/tde-2020-0129
Sharma, COVID-19 vaccines in 2023, Aust Prescr, doi:10.18773/austprescr.2023.020108
Sharun, Dhama, Patel, Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19, Ann Clin Microbiol Antimicrob, doi:10.1186/s12941-020-00368-w136
Shayak, Sharma, Gaur, Mishra, Impact of reproduction number on the multiwave spreading dynamics of COVID-19 with temporary immunity: A mathematical model, Int J Infect Dis, doi:10.1016/j.ijid.2021.01.018
Shin, Shahsavari, Lee, Laborada, Egeberg et al., Adverse Effects of the COVID-19 Vaccine in Patients With Psoriasis, Cutis, doi:10.12788/cutis.0700
Shrestha, Burke, Nowacki, Simon, Hagen et al., Effectiveness of the coronavirus disease 2019 (COVID-19) bivalent vaccine, MedRxiv, doi:10.1101/2022.12.17.22283625
Shrestha, Burke, Nowacki, Simon, Hagen et al., Effectiveness of the coronavirus disease 2019 bivalent vaccine, Open Forum Infect Dis, doi:10.1093/ofid/ofad209167
Shrestha, Burke, Nowacki, Terpeluk, Gordon, Necessity of coronavirus disease 2019 (COVID-19) vaccination in persons who have already had COVID-19, Clin Infect Dis, doi:10.1093/cid/ciac022184
Shrestha, Foster, Rawlinson, Tedla, Bull, Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: Implications for immune escape and transmission, Rev Med Virol, doi:10.1002/rmv.2381102
Shrestha, Shrestha, Burke, Nowacki, Terpeluk et al., Coronavirus Disease 2019 Vaccine Boosting in Previously Infected or Vaccinated Individuals, Clin Infect Dis, doi:10.1093/cid/ciac327168
Singanayagam, Hakki, Dunning, Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00648-4
Singh, Can Vitamins, as Epigenetic Modifiers, Enhance Immunity in COVID-19 Patients with Noncommunicable Disease?, Curr Nutr Rep, doi:10.1007/s13668-020-00330-4
Springer, Bauer, Medits, Bivalent COVID-19 mRNA booster vaccination (BA.1 or BA.4/BA.5) increases neutralization of matched Omicron variants, NPJ Vaccines, doi:10.1038/s41541-023-00708-9
Stati, Amerio, Nubile, Sancilio, Rossi et al., Concern about the effectiveness of mRNA vaccination technology and Its long-term safety: potential interference on miRNA machinery, Int J Mol Sci, doi:10.3390/ijms24021404Journ
Stowe, Miller, Andrews, Whitaker, Risk of myocarditis and pericarditis after a COVID-19 mRNA vaccine booster and after COVID-19 in those with and without prior SARS-CoV-2 infection: A selfcontrolled case series analysis in England, PLoS Med, doi:10.1371/journal.pmed.1004245
Sun, Wl, Doctors who put lives at risk with COVID misinformation rarely punished, Washington Post
Suryawanshi, Biswas, Herd immunity to fight against COVID-19: A narrative review, Cureus. Jan, doi:10.7759/cureus.33575160
Takashita, Yamayoshi, Simon, Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants, N Engl J Med, doi:10.1056/NEJMc2207519
Tamanaha, Zhang, Tanner, Profiling RT-LAMP tolerance of sequence variation for SARS-CoV-2 RNA detection, PLoS One, doi:10.1371/journal.pone.0259610181
Thimbleby, Technology and the future of healthcare, J Public Health Res, doi:10.4081/jphr.2013.e28113
Thompson, Burgess, Naleway, Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers -Eight U.S. Locations, doi:10.15585/mmwr.mm7013e3193
Thompson, Stenehjem, Grannis, Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings, N Engl J Med, doi:10.1056/NEJMoa2110362
Trougakos, Terpos, Alexopoulos, Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis, Trends Mol Med, doi:10.1016/j.molmed.2022.04.007
Tsiakalos, Ziakas, Polyzou, Schinas, Akinosoglou, Early fluvoxaminereduces the risk for clinical deterioration in symptomatic outpatients with COVID-19: A real-world, retrospective, before-after analysis, Microorganisms, doi:10.3390/microorganisms11082073201
Union, products for human use: Commission Directive
Uraki, Ito, Furusawa, Humoral immune evasion of the omicron subvariants BQ.1.1 and XBB, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00816-7183
Uversky, Redwan, Makis, Rubio-Casillas, IgG4 antibodies induced by repeated vaccination may generate immune tolerance to the SARS-CoV-2 Spike protein, Vaccines, doi:10.3390/vaccines11050991197
Van Der Pol, Aljofan, Blin, Drug repurposing of generic drugs: Challenges and the potential role for government, Applied health economics and health policy, doi:10.1007/s40258-023-00816-6
Varnaseri, Af, Jamshididan, Dargahi, Gheibi et al., Ivermectin as a potential addition to the limited anti-COVID-19 arsenal: A double-blinded clinical trial, Jundishapur J Health Sci, doi:10.5812/jjhs-146703222
Villasis-Keever, Lopez-Alarcon, Miranda-Novales, Efficacy and Safety of Vitamin D Supplementation to Prevent COVID-19 in Frontline Healthcare Workers. A Randomized Clinical Trial, Archives of medical research, doi:10.1016/j.arcmed.2022.04.003122
Vincent, Bergeron, Benjannet, Chloroquine is a potent inhibitor of SARS coronavirus infection and spread, Virol J, doi:10.1186/1743-422X-2-69237
Wan, Wang, Mathur, Molnupiravir and nirmatrelvir-ritonavir reduce mortality risk during post-acute COVID-19 phase, J Infect, doi:10.1016/j.jinf.2023.02.029208
Wang, Bowen, Valdez, Antibody Response to Omicron BA.4-BA.5 Bivalent Booster, N Engl J Med, doi:10.1056/NEJMc2213907176
Wang, Guo, Iketani, Antibody evasion by SARS-CoV-2 Omicron subvariants BA, BA.4 and BA.5, doi:10.1038/s41586-022-05053-w171
Wang, Iketani, Li, Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants, Cell. Jan, doi:10.1016/j.cell.2022.12.018
Weixel, CDC: More vaccinated adults hospitalized with omicron, but fewer admitted to ICU
Willis, Bloomfield, Berry, The impact of a vaccine mandate and the COVID-19 pandemic on influenza vaccination uptake in Western Australian health care students, Vaccine, doi:10.1016/j.vaccine.2022.08.040
Wimalawansa, Decoding the paradox: Understanding elevated hospitalization and reduced mortality in SARS-CoV-2 variants, Int J Frontiers in Sci and Technol Res, doi:10.53294/ijfstr.2024.6.2.0031
Wimalawansa, Effective and practical ways to overcome vitamin D deficiency, J Family Med Community Health
Wimalawansa, Fighting against COVID-19: Boosting the immunity with micronutrients, stress reduction, physical activity, and vitamin D, Nutrition and Food Journal (Sci Literature)
Wimalawansa, Maintaining optimum health requires longer-term stable vitamin D concentrations, Int J Regenr Med, doi:10.31487/j.RGM.2020.03.03162
Wimalawansa, Modes of preventing secondary community spread and future epidemics of COVID-19, J Immuno Biol, doi:10.37421/JIB.2020.5.147):1-5
Wimalawansa, Overcoming infections including COVID-19, by maintaining circulating 25(OH)D concentrations above 50 ng/mL, Pathology & Lab Medicine Int
Wimalawansa, Physiological basis for using vitamin D to improve health, Biomedicines, doi:10.3390/biomedicines11061542
Wimalawansa, Rapidly increasing serum 25(OH)D boosts the immune system, against infectionssepsis and COVID-19, Nutrients, doi:10.3390/nu14142997
Wimalawansa, What modelling and reproduction numbers are useful in predicting COVID-19 spread?, Can J Biomed Res & Tech
Wu, Shi, He, Protection of the receptor binding domain (RBD) dimer against SARS-CoV-2 and its variants, J Virol, doi:10.1128/jvi.01279-23
Xu, Li, Kirui, Effectiveness of COVID-19 Vaccines over 13 Months Covering the Period of the Emergence of the Omicron Variant in the Swedish Population, Vaccines, doi:10.3390/vaccines10122074234
Xu, Ren, Wu, Real-world safety of COVID-19 mRNA vaccines: A systematic review and meta-snalysis, Vaccines, doi:10.3390/vaccines11061118
Yang, Deroo, Disciplining physicians who spread medical misinformation, J Public Health Manag Pract, doi:10.1097/PHH.0000000000001616141
Yang, Rao, Structural biology of SARS-CoV-2 and implications for therapeutic development, Nat Rev Microbiol, doi:10.1038/s41579-021-00630-8
Yang, Shen, Hou, Is ivermectin effective in treating COVID-19?, Frontiers in pharmacology, doi:10.3389/fphar.2022.858693214
Yang, Tang, Zhang, Liu, Recent Advances in the Molecular Design and Delivery Technology of mRNA for Vaccination Against Infectious Diseases, Front Immunol, doi:10.3389/fimmu.2022.896958
Yarlagadda, Patel, Gupta, COVID-19 vaccine challenges in developing and developed countries, Cureus, doi:10.7759/cureus.23951
Yin, Fang, Wen, Effectiveness of COVID-19 vaccines against SARS-CoV-2 Omicron variants during two outbreaks from March to May 2022 in Quzhou, China, Hum Vaccin Immunother, doi:10.1080/21645515.2022.2163813200
Younus, Aa, An overview of COVID-19 vaccine safety and post-marketing surveillance systems, Innov Pharm, doi:10.24926/iip.v12i4.4294
Yu, Tsao, Ng, Cardiovascular assessment up to one year after COVID-19 vaccineassociated myocarditis, Circulation, doi:10.1161/CIRCULATIONAHA.123.064772
Yuan, Pan, Kong, 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter, J Biol Chem, doi:10.1074/jbc.M705495200
Zhang, Chen, Ip, Omicron sublineage recombinant XBB evades neutralising antibodies in recipients of BNT162b2 or CoronaVac vaccines, Lancet Microbe, doi:10.1016/S2666-5247(22)00335-4165
Zhang, Song, Ci, Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflamm Res, doi:10.1007/s00011-008-8007-8236
Zhang, Wang, Han, Intraduodenal Delivery of Exosome-Loaded SARS-CoV-2 RBD mRNA Induces a Neutralizing Antibody Response in Mice, Vaccines, doi:10.3390/vaccines11030673190
Zhang, Yang, Chen, Yue, Zhang et al., Why does COVID-19 continue to spread despite mass vaccination?, Front Public Health, doi:10.3389/fpubh.2022.938108
Zhou, Wang, Luo, Cui, Guan et al., Single-injection COVID-19 subunit vaccine elicits potent immune responses, Acta Biomater, doi:10.1016/j.actbio.2022.08.006189
DOI record:
{
"DOI": "10.1016/j.heliyon.2024.e34691",
"ISSN": [
"2405-8440"
],
"URL": "http://dx.doi.org/10.1016/j.heliyon.2024.e34691",
"alternative-id": [
"S2405844024107220"
],
"article-number": "e34691",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Unlocking Insights: Navigating COVID-19 Challenges and Emulating Future Pandemic Resilience Strategies with Strengthening Natural Immunity"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Heliyon"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.heliyon.2024.e34691"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 Published by Elsevier Ltd."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1096-8595",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wimalawansa",
"given": "Sunil J.",
"sequence": "first"
}
],
"container-title": "Heliyon",
"container-title-short": "Heliyon",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"cell.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
7,
18
]
],
"date-time": "2024-07-18T01:35:04Z",
"timestamp": 1721266504000
},
"deposited": {
"date-parts": [
[
2024,
7,
18
]
],
"date-time": "2024-07-18T01:36:34Z",
"timestamp": 1721266594000
},
"indexed": {
"date-parts": [
[
2024,
7,
18
]
],
"date-time": "2024-07-18T02:14:45Z",
"timestamp": 1721268885843
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
7
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
1
]
],
"date-time": "2024-07-01T00:00:00Z",
"timestamp": 1719792000000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
1
]
],
"date-time": "2024-07-01T00:00:00Z",
"timestamp": 1719792000000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 14,
"start": {
"date-parts": [
[
2024,
7,
15
]
],
"date-time": "2024-07-15T00:00:00Z",
"timestamp": 1721001600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2405844024107220?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2405844024107220?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "e34691",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
7
]
]
},
"published-print": {
"date-parts": [
[
2024,
7
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1038/s41541-023-00708-9",
"article-title": "Bivalent COVID-19 mRNA booster vaccination (BA.1 or BA.4/BA.5) increases neutralization of matched Omicron variants",
"author": "Springer",
"doi-asserted-by": "crossref",
"first-page": "110",
"issue": "1",
"journal-title": "NPJ Vaccines",
"key": "10.1016/j.heliyon.2024.e34691_bib1",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(21)00648-4",
"article-title": "Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study",
"author": "Singanayagam",
"doi-asserted-by": "crossref",
"first-page": "183",
"issue": "2",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib2",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1177/0194599820982633",
"article-title": "COVID-19 Vaccines May Not Prevent Nasal SARS-CoV-2 Infection and Asymptomatic Transmission",
"author": "Bleier",
"doi-asserted-by": "crossref",
"first-page": "305",
"issue": "2",
"journal-title": "Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery",
"key": "10.1016/j.heliyon.2024.e34691_bib3",
"volume": "164",
"year": "2021"
},
{
"article-title": "SARS-CoV-2 Omicron variant: epidemiological features, biological characteristics, and clinical significance",
"author": "Guo",
"journal-title": "Front Immunol",
"key": "10.1016/j.heliyon.2024.e34691_bib4",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3390/nu12072097",
"article-title": "Immunologic effects of vitamin D on human health and sisease",
"author": "Charoenngam",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "Nutrients",
"key": "10.1016/j.heliyon.2024.e34691_bib5",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.molimm.2022.11.020",
"article-title": "Immune evasion of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2); molecular approaches",
"author": "Ahmadi",
"doi-asserted-by": "crossref",
"first-page": "10",
"journal-title": "Mol Immunol",
"key": "10.1016/j.heliyon.2024.e34691_bib6",
"volume": "156",
"year": "2023"
},
{
"DOI": "10.1016/j.mib.2014.05.005",
"article-title": "To sense or not to sense viral RNA--essentials of coronavirus innate immune evasion",
"author": "Kindler",
"doi-asserted-by": "crossref",
"first-page": "69",
"journal-title": "Curr Opin Microbiol",
"key": "10.1016/j.heliyon.2024.e34691_bib7",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1074/jbc.M705495200",
"article-title": "1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter",
"author": "Yuan",
"doi-asserted-by": "crossref",
"first-page": "29821",
"issue": "41",
"journal-title": "J Biol Chem",
"key": "10.1016/j.heliyon.2024.e34691_bib8",
"volume": "282",
"year": "2007"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib9",
"unstructured": "FDA. Emergency use authorization for vaccines explained. FDA.gov. Accessed May 22nd, 2022. https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained"
},
{
"DOI": "10.1016/j.vaccine.2022.08.036",
"article-title": "Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults",
"author": "Fraiman",
"doi-asserted-by": "crossref",
"first-page": "5798",
"issue": "40",
"journal-title": "Vaccine",
"key": "10.1016/j.heliyon.2024.e34691_bib10",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.1186/s12879-021-06104-9",
"article-title": "Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients",
"author": "Okumus",
"doi-asserted-by": "crossref",
"first-page": "411",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib11",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.53294/ijfstr.2024.6.2.0031",
"article-title": "Decoding the paradox: Understanding elevated hospitalization and reduced mortality in SARS-CoV-2 variants",
"author": "Wimalawansa",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "2",
"journal-title": "Int J Frontiers in Sci and Technol Res",
"key": "10.1016/j.heliyon.2024.e34691_bib12",
"volume": "6",
"year": "2024"
},
{
"DOI": "10.1016/j.clinthera.2021.04.007",
"article-title": "Effects of ivermectin in patients With COVID-19: A multicenter, double-blind, randomized, controlled clinical trial",
"author": "Shahbaznejad",
"doi-asserted-by": "crossref",
"first-page": "1007",
"issue": "6",
"journal-title": "Clin Ther",
"key": "10.1016/j.heliyon.2024.e34691_bib13",
"volume": "43",
"year": "2021"
},
{
"article-title": "The PRISMA 2020 statement: an updated guideline for reporting systematic reviews",
"author": "Page",
"journal-title": "BMJ",
"key": "10.1016/j.heliyon.2024.e34691_bib14",
"volume": "372",
"year": "2021"
},
{
"DOI": "10.1038/s41541-021-00369-6",
"article-title": "Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action",
"author": "Heinz",
"doi-asserted-by": "crossref",
"first-page": "104",
"issue": "1",
"journal-title": "NPJ Vaccines",
"key": "10.1016/j.heliyon.2024.e34691_bib15",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/j.addr.2021.114000",
"article-title": "Messenger RNA-based vaccines: Past, present, and future directions in the context of the COVID-19 pandemic",
"author": "Jain",
"doi-asserted-by": "crossref",
"journal-title": "Adv Drug Deliv Rev",
"key": "10.1016/j.heliyon.2024.e34691_bib16",
"volume": "179",
"year": "2021"
},
{
"DOI": "10.1002/rmv.2183",
"article-title": "Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects",
"author": "Arashkia",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "Rev Med Virol",
"key": "10.1016/j.heliyon.2024.e34691_bib17",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.7150/ijbs.59233",
"article-title": "mRNA vaccines for COVID-19: what, why and how",
"author": "Park",
"doi-asserted-by": "crossref",
"first-page": "1446",
"issue": "6",
"journal-title": "International journal of biological sciences",
"key": "10.1016/j.heliyon.2024.e34691_bib18",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1038/s41579-021-00630-8",
"article-title": "Structural biology of SARS-CoV-2 and implications for therapeutic development",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "685",
"issue": "11",
"journal-title": "Nat Rev Microbiol",
"key": "10.1016/j.heliyon.2024.e34691_bib19",
"volume": "19",
"year": "2021"
},
{
"article-title": "Recent Advances in the Molecular Design and Delivery Technology of mRNA for Vaccination Against Infectious Diseases",
"author": "Yang",
"journal-title": "Front Immunol",
"key": "10.1016/j.heliyon.2024.e34691_bib20",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3390/ijms24021404",
"article-title": "Concern about the effectiveness of mRNA vaccination technology and Its long-term safety: potential interference on miRNA machinery",
"author": "Stati",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "Int J Mol Sci.",
"key": "10.1016/j.heliyon.2024.e34691_bib21",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.3389/fimmu.2020.575074",
"article-title": "Measurement of Cellular Immune Response to Viral Infection and Vaccination",
"author": "Bouwman",
"doi-asserted-by": "crossref",
"journal-title": "Front Immunol",
"key": "10.1016/j.heliyon.2024.e34691_bib22",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2019.01400",
"article-title": "Recalling the Future: Immunological Memory Toward Unpredictable Influenza Viruses",
"author": "Auladell",
"doi-asserted-by": "crossref",
"first-page": "1400",
"journal-title": "Front Immunol",
"key": "10.1016/j.heliyon.2024.e34691_bib23",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1016/j.molmed.2022.04.007",
"article-title": "Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis",
"author": "Trougakos",
"doi-asserted-by": "crossref",
"first-page": "542",
"issue": "7",
"journal-title": "Trends Mol Med",
"key": "10.1016/j.heliyon.2024.e34691_bib24",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1038/s41392-022-00950-y",
"article-title": "Advances in COVID-19 mRNA vaccine development",
"author": "Fang",
"doi-asserted-by": "crossref",
"first-page": "94",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "10.1016/j.heliyon.2024.e34691_bib25",
"volume": "7",
"year": "2022"
},
{
"article-title": "What modelling and reproduction numbers are useful in predicting COVID-19 spread?",
"author": "Wimalawansa",
"first-page": "1",
"issue": "3",
"journal-title": "Can J Biomed Res & Tech",
"key": "10.1016/j.heliyon.2024.e34691_bib26",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.3389/fmicb.2022.908525",
"article-title": "Influenza A, Influenza B, and SARS-CoV-2 similarities and differences - A focus on diagnosis",
"author": "Havasi",
"doi-asserted-by": "crossref",
"journal-title": "Front Microbiol",
"key": "10.1016/j.heliyon.2024.e34691_bib27",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.4161/rna.22269",
"article-title": "Developing mRNA-vaccine technologies",
"author": "Schlake",
"doi-asserted-by": "crossref",
"first-page": "1319",
"issue": "11",
"journal-title": "RNA Biol",
"key": "10.1016/j.heliyon.2024.e34691_bib28",
"volume": "9",
"year": "2012"
},
{
"DOI": "10.3390/biomedicines11020451",
"article-title": "Immune response and molecular mechanisms of cardiovascular adverse effects of Spike Proteins from SARS-CoV-2 and mRNA vaccines",
"author": "Bellavite",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "Biomedicines",
"key": "10.1016/j.heliyon.2024.e34691_bib29",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1016/j.fct.2022.113008",
"article-title": "Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs",
"author": "Seneff",
"doi-asserted-by": "crossref",
"journal-title": "Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association",
"key": "10.1016/j.heliyon.2024.e34691_bib30",
"volume": "164",
"year": "2022"
},
{
"DOI": "10.4155/tde-2020-0129",
"article-title": "An overview of vaccine development for COVID-19",
"author": "Shahcheraghi",
"doi-asserted-by": "crossref",
"first-page": "235",
"issue": "3",
"journal-title": "Ther Deliv",
"key": "10.1016/j.heliyon.2024.e34691_bib31",
"volume": "12",
"year": "2021"
},
{
"article-title": "Immune responses induced by mRNA vaccination in mice, monkeys and humans",
"author": "Cagigi",
"issue": "1",
"journal-title": "Vaccines (Basel)",
"key": "10.1016/j.heliyon.2024.e34691_bib32",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2110362",
"article-title": "Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings",
"author": "Thompson",
"doi-asserted-by": "crossref",
"first-page": "1355",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "10.1016/j.heliyon.2024.e34691_bib33",
"volume": "385",
"year": "2021"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib34",
"unstructured": "Office-GAO-21-319 GA. Operation Warp Speed: Accelerated COVID-19 vaccine development status and efforts to address manufacturing challenges. GAO."
},
{
"article-title": "Why does COVID-19 continue to spread despite mass vaccination?",
"author": "Zhang",
"journal-title": "Front Public Health",
"key": "10.1016/j.heliyon.2024.e34691_bib35",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.vaccine.2022.08.040",
"article-title": "The impact of a vaccine mandate and the COVID-19 pandemic on influenza vaccination uptake in Western Australian health care students",
"author": "Willis",
"doi-asserted-by": "crossref",
"first-page": "5651",
"issue": "39",
"journal-title": "Vaccine",
"key": "10.1016/j.heliyon.2024.e34691_bib36",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.1016/j.jvacx.2022.100213",
"article-title": "Trust is the common denominator for COVID-19 vaccine acceptance: A literature review",
"author": "Adhikari",
"doi-asserted-by": "crossref",
"journal-title": "Vaccine X",
"key": "10.1016/j.heliyon.2024.e34691_bib37",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.24926/iip.v12i4.4294",
"article-title": "An overview of COVID-19 vaccine safety and post-marketing surveillance systems",
"author": "Younus",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "Innov Pharm",
"key": "10.1016/j.heliyon.2024.e34691_bib38",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1007/s40258-023-00816-6",
"article-title": "Drug repurposing of generic drugs: Challenges and the potential role for government",
"author": "van der Pol",
"doi-asserted-by": "crossref",
"first-page": "831",
"issue": "6",
"journal-title": "Applied health economics and health policy",
"key": "10.1016/j.heliyon.2024.e34691_bib39",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1186/s12929-022-00852-9",
"article-title": "A critical overview of current progress for COVID-19: development of vaccines, antiviral drugs, and therapeutic antibodies",
"author": "Kumari",
"doi-asserted-by": "crossref",
"first-page": "68",
"issue": "1",
"journal-title": "J Biomed Sci",
"key": "10.1016/j.heliyon.2024.e34691_bib40",
"volume": "29",
"year": "2022"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib41",
"unstructured": "Union E. the European Parliament and of the Council on the Community code relating to medicinal products for human use: Commission Directive 2003/63/EC. European Union. Accessed November 12, 2022. https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32003L0063"
},
{
"DOI": "10.3389/fimmu.2019.02768",
"article-title": "Plasma Cells, the Next Generation: Beyond Antibody Secretion",
"author": "Pioli",
"doi-asserted-by": "crossref",
"first-page": "2768",
"journal-title": "Front Immunol",
"key": "10.1016/j.heliyon.2024.e34691_bib42",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1016/j.ebiom.2022.103962",
"article-title": "Reporting and data sharing level for COVID-19 vaccine trials: A cross-sectional study",
"author": "Duan",
"doi-asserted-by": "crossref",
"journal-title": "EBioMedicine",
"key": "10.1016/j.heliyon.2024.e34691_bib43",
"volume": "78",
"year": "2022"
},
{
"DOI": "10.3389/fpubh.2022.999354",
"article-title": "Adverse events following immunization of COVID-19 vaccine among children aged 6-11 years",
"author": "Puspitarani",
"doi-asserted-by": "crossref",
"journal-title": "Front Public Health",
"key": "10.1016/j.heliyon.2024.e34691_bib44",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1186/s12929-022-00853-8",
"article-title": "COVID-19 vaccine update: vaccine effectiveness, SARS-CoV-2 variants, boosters, adverse effects, and immune correlates of protection",
"author": "Chi",
"doi-asserted-by": "crossref",
"first-page": "82",
"issue": "1",
"journal-title": "J Biomed Sci",
"key": "10.1016/j.heliyon.2024.e34691_bib45",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.1371/journal.pmed.1004245",
"article-title": "Risk of myocarditis and pericarditis after a COVID-19 mRNA vaccine booster and after COVID-19 in those with and without prior SARS-CoV-2 infection: A self-controlled case series analysis in England",
"author": "Stowe",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "PLoS Med",
"key": "10.1016/j.heliyon.2024.e34691_bib46",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.2188/jea.JE20220265",
"article-title": "Adverse reactions and attitudes toward the BNT162b2 COVID-19 vaccine in children 5 to 11 years of age in Japan",
"author": "Matsumoto",
"doi-asserted-by": "crossref",
"first-page": "110",
"issue": "2",
"journal-title": "J Epidemiol",
"key": "10.1016/j.heliyon.2024.e34691_bib47",
"volume": "33",
"year": "2023"
},
{
"article-title": "An update on complications associated with SARS-CoV-2 infection and COVID-19 vaccination",
"author": "Chowdhury",
"issue": "10",
"journal-title": "Vaccines (Basel)",
"key": "10.1016/j.heliyon.2024.e34691_bib48",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.jsps.2022.10.001",
"article-title": "Vaccine adverse event reporting system (VAERS): Evaluation of 31 years of reports and pandemics' impact",
"author": "Almadani",
"doi-asserted-by": "crossref",
"first-page": "1725",
"issue": "12",
"journal-title": "Saudi Pharm J",
"key": "10.1016/j.heliyon.2024.e34691_bib49",
"volume": "30",
"year": "2022"
},
{
"DOI": "10.1016/j.vaccine.2023.03.054",
"article-title": "Real-life safety profile of mRNA vaccines for COVID-19: An analysis of VAERS database",
"author": "Santi",
"doi-asserted-by": "crossref",
"first-page": "2879",
"issue": "18",
"journal-title": "Vaccine",
"key": "10.1016/j.heliyon.2024.e34691_bib50",
"volume": "41",
"year": "2023"
},
{
"article-title": "Real-world safety of COVID-19 mRNA vaccines: A systematic review and meta-snalysis",
"author": "Xu",
"issue": "6",
"journal-title": "Vaccines (Basel)",
"key": "10.1016/j.heliyon.2024.e34691_bib51",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1038/s41419-024-06642-5",
"article-title": "Research progress of post-acute sequelae after SARS-CoV-2 infection",
"author": "Jiao",
"doi-asserted-by": "crossref",
"first-page": "257",
"issue": "4",
"journal-title": "Cell Death Dis.",
"key": "10.1016/j.heliyon.2024.e34691_bib52",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1038/s41467-024-45953-1",
"article-title": "Persistence in risk and effect of COVID-19 vaccination on long-term health consequences after SARS-CoV-2 infection",
"author": "Lam",
"doi-asserted-by": "crossref",
"first-page": "1716",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10.1016/j.heliyon.2024.e34691_bib53",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1016/j.hrthm.2022.07.019",
"article-title": "Genetic basis of sudden death after COVID-19 vaccination in Thailand",
"author": "Ittiwut",
"doi-asserted-by": "crossref",
"first-page": "1874",
"issue": "11",
"journal-title": "Heart Rhythm",
"key": "10.1016/j.heliyon.2024.e34691_bib54",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1590/s1678-9946202365050",
"article-title": "COVID-19-related multisystem inflammatory syndrome in adult: the first death in Brazil",
"author": "Salomao",
"doi-asserted-by": "crossref",
"journal-title": "Rev Inst Med Trop Sao Paulo.",
"key": "10.1016/j.heliyon.2024.e34691_bib55",
"volume": "65",
"year": "2023"
},
{
"DOI": "10.1161/JAHA.121.024393",
"article-title": "Comparison of multisystem inflammatory syndrome in children-related myocarditis, classic viral myocarditis, and COVID-19 vaccine-related myocarditis in children",
"author": "Patel",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "J Am Heart Assoc",
"key": "10.1016/j.heliyon.2024.e34691_bib56",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1111/jog.15285",
"article-title": "The vaccination status and adverse effects of COVID-19 vaccine among pregnant women in Japan in 2021",
"author": "Komine-Aizawa",
"doi-asserted-by": "crossref",
"first-page": "1561",
"issue": "7",
"journal-title": "J Obstet Gynaecol Res",
"key": "10.1016/j.heliyon.2024.e34691_bib57",
"volume": "48",
"year": "2022"
},
{
"DOI": "10.1161/CIRCULATIONAHA.123.064772",
"article-title": "Cardiovascular assessment up to one year after COVID-19 vaccine-associated myocarditis",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "436",
"issue": "5",
"journal-title": "Circulation",
"key": "10.1016/j.heliyon.2024.e34691_bib58",
"volume": "148",
"year": "2023"
},
{
"DOI": "10.3390/ijms232415480",
"article-title": "SARS-CoV-2 Spike protein induces hemagglutination: implications for COVID-19 morbidities and therapeutics and for vaccine adverse effects",
"author": "Boschi",
"doi-asserted-by": "crossref",
"issue": "24",
"journal-title": "Int J Mol Sci",
"key": "10.1016/j.heliyon.2024.e34691_bib59",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1016/j.jaip.2022.10.010",
"article-title": "COVID-19 severity, cardiological outcome, and Immunogenicity of mRNA vaccine on adult patients with 22q11.2 DS",
"author": "Pulvirenti",
"doi-asserted-by": "crossref",
"first-page": "292",
"issue": "1",
"journal-title": "J Allergy Clin Immunol Pract",
"key": "10.1016/j.heliyon.2024.e34691_bib60",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.12788/cutis.0700",
"article-title": "Adverse Effects of the COVID-19 Vaccine in Patients With Psoriasis",
"author": "Shin",
"doi-asserted-by": "crossref",
"first-page": "80",
"issue": "2",
"journal-title": "Cutis",
"key": "10.1016/j.heliyon.2024.e34691_bib61",
"volume": "111",
"year": "2023"
},
{
"article-title": "COVID-19 and COVID-19 vaccine-related dermatological reactions: An interesting case series with a narrative review of the potential critical and non-critical mucocutaneous adverse effects related to virus, therapy, and the vaccination",
"author": "Pour",
"issue": "4",
"journal-title": "Clin Case Rep",
"key": "10.1016/j.heliyon.2024.e34691_bib62",
"volume": "10",
"year": "2022"
},
{
"article-title": "COVID-19 vaccine-associated ocular adverse effects: An overview",
"author": "Ichhpujani",
"issue": "11",
"journal-title": "Vaccines (Basel)",
"key": "10.1016/j.heliyon.2024.e34691_bib63",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1002/ana.26369",
"article-title": "Vaccine adverse Event reporting system could miss or misinterpret neurological side effects of COVID-19 vaccinations",
"author": "Finsterer",
"doi-asserted-by": "crossref",
"first-page": "157",
"issue": "1",
"journal-title": "Ann Neurol",
"key": "10.1016/j.heliyon.2024.e34691_bib64",
"volume": "92",
"year": "2022"
},
{
"DOI": "10.1152/ajpcell.00375.2021",
"article-title": "Long-term complications of COVID-19",
"author": "Desai",
"doi-asserted-by": "crossref",
"first-page": "C1",
"issue": "1",
"journal-title": "Am J Physiol Cell Physiol",
"key": "10.1016/j.heliyon.2024.e34691_bib65",
"volume": "322",
"year": "2022"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib66",
"unstructured": "FDA. COVID-19 bivalent vaccine boosters. FDA. Accessed Dec 15, 2022. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-bivalent-vaccine-boosters"
},
{
"article-title": "Effectiveness of the coronavirus disease 2019 (COVID-19) bivalent vaccine",
"author": "Shrestha",
"journal-title": "Preprint: MedRxiv",
"key": "10.1016/j.heliyon.2024.e34691_bib67",
"year": "2023"
},
{
"article-title": "Vaccine hesitancy: Contemporary issues and historical background",
"author": "Nuwarda",
"issue": "10",
"journal-title": "Vaccines (Basel)",
"key": "10.1016/j.heliyon.2024.e34691_bib68",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1038/s41591-023-02219-5",
"article-title": "Author Correction: Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population",
"author": "Lau",
"doi-asserted-by": "crossref",
"journal-title": "Nat Med",
"key": "10.1016/j.heliyon.2024.e34691_bib69",
"year": "2023"
},
{
"DOI": "10.1038/s41598-022-22014-5",
"article-title": "Conspiratorial thinking as a precursor to opposition to COVID-19 vaccination in the US: a multi-year study from 2018 to 2021",
"author": "Romer",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Sci Rep",
"key": "10.1016/j.heliyon.2024.e34691_bib70",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2024.107057",
"article-title": "The contribution of SARS-CoV-2 to the burden of acute respiratory infections in winter season 2022/2023: Results from the DigiHero study",
"author": "Glaser",
"doi-asserted-by": "crossref",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib71",
"year": "2024"
},
{
"DOI": "10.4161/derm.24476",
"article-title": "Seasonal variation in the serum 25-hydroxyvitamin D levels of young and elderly active and inactive adults in Sao Paulo, Brazil: The Sao PAulo Vitamin D Evaluation Study (SPADES)",
"author": "Maeda",
"doi-asserted-by": "crossref",
"first-page": "211",
"issue": "1",
"journal-title": "Dermatoendocrinol",
"key": "10.1016/j.heliyon.2024.e34691_bib72",
"volume": "5",
"year": "2013"
},
{
"DOI": "10.3390/nu14142997",
"article-title": "Rapidly increasing serum 25(OH)D boosts the immune system, against infections-sepsis and COVID-19",
"author": "Wimalawansa",
"doi-asserted-by": "crossref",
"issue": "14",
"journal-title": "Nutrients",
"key": "10.1016/j.heliyon.2024.e34691_bib73",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3390/biomedicines11061542",
"article-title": "Physiological basis for using vitamin D to improve health",
"author": "Wimalawansa",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "Biomedicines",
"key": "10.1016/j.heliyon.2024.e34691_bib74",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.5946/ce.2022.146",
"article-title": "General guideline in the endoscopy room to avoid air-borne infection during the COVID-19 pandemic",
"author": "Chung",
"doi-asserted-by": "crossref",
"first-page": "688",
"issue": "5",
"journal-title": "Clin Endosc",
"key": "10.1016/j.heliyon.2024.e34691_bib75",
"volume": "55",
"year": "2022"
},
{
"article-title": "Modes of preventing secondary community spread and future epidemics of COVID-19",
"author": "Wimalawansa",
"first-page": "1",
"issue": "1",
"journal-title": "J Immuno Biol",
"key": "10.1016/j.heliyon.2024.e34691_bib76",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.2147/PLMI.S373617",
"article-title": "Overcoming infections including COVID-19, by maintaining circulating 25(OH)D concentrations above 50 ng/mL",
"author": "Wimalawansa",
"doi-asserted-by": "crossref",
"first-page": "37",
"journal-title": "Pathology & Lab Medicine Int.",
"key": "10.1016/j.heliyon.2024.e34691_bib77",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3390/nu15092089",
"article-title": "Vitamin D levels in the pre- and post-COVID-19 pandemic periods and related confinement at pediatric age",
"author": "Mosca",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "Nutrients",
"key": "10.1016/j.heliyon.2024.e34691_bib78",
"volume": "15",
"year": "2023"
},
{
"article-title": "Molecular quantum and logic process of consciousness—Vitamin D big-data in COVID-19—A case for incorporating machine learning in medicine",
"author": "Polonowita",
"first-page": "1",
"issue": "12",
"journal-title": "Euro J Biomedical Pharma Sci",
"key": "10.1016/j.heliyon.2024.e34691_bib79",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1093/ofid/ofac135",
"article-title": "Viral Load Among Vaccinated and Unvaccinated, Asymptomatic and Symptomatic Persons Infected With the SARS-CoV-2 Delta Variant",
"author": "Acharya",
"doi-asserted-by": "crossref",
"first-page": "ofac135",
"issue": "5",
"journal-title": "Open Forum Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib80",
"volume": "9",
"year": "2022"
},
{
"article-title": "Outbreak of SARS-CoV-2 B.1.617.2 (delta) variant infections among incarcerated persons in a federal prison—Texas, July–August 2021",
"author": "Hagan",
"first-page": "1349",
"journal-title": "The Lancet-Infectious diseases",
"key": "10.1016/j.heliyon.2024.e34691_bib81",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2022.12.018",
"article-title": "Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "279",
"issue": "2",
"journal-title": "Cell",
"key": "10.1016/j.heliyon.2024.e34691_bib82",
"volume": "186",
"year": "2023"
},
{
"DOI": "10.1002/rmv.2231",
"article-title": "Neutralising antibody escape of SARS-CoV-2 spike protein: Risk assessment for antibody-based Covid-19 therapeutics and vaccines",
"author": "Focosi",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "Rev Med Virol",
"key": "10.1016/j.heliyon.2024.e34691_bib83",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.1093/jtm/taab124",
"article-title": "The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus",
"author": "Liu",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "J Travel Med",
"key": "10.1016/j.heliyon.2024.e34691_bib84",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0271305",
"article-title": "Mutations in SARS-CoV-2 are on the increase against the acquired immunity",
"author": "Konishi",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "PLoS One",
"key": "10.1016/j.heliyon.2024.e34691_bib85",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1128/jvi.01279-23",
"article-title": "Protection of the receptor binding domain (RBD) dimer against SARS-CoV-2 and its variants",
"author": "Wu",
"doi-asserted-by": "crossref",
"journal-title": "J Virol",
"key": "10.1016/j.heliyon.2024.e34691_bib86",
"year": "2023"
},
{
"DOI": "10.1056/NEJMc2207519",
"article-title": "Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "468",
"issue": "5",
"journal-title": "N Engl J Med.",
"key": "10.1016/j.heliyon.2024.e34691_bib87",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2021.01.018",
"article-title": "Impact of reproduction number on the multiwave spreading dynamics of COVID-19 with temporary immunity: A mathematical model",
"author": "Shayak",
"doi-asserted-by": "crossref",
"first-page": "649",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib88",
"volume": "104",
"year": "2021"
},
{
"DOI": "10.1038/s41591-022-01816-0",
"article-title": "Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2",
"author": "Puhach",
"doi-asserted-by": "crossref",
"first-page": "1491",
"issue": "7",
"journal-title": "Nat Med",
"key": "10.1016/j.heliyon.2024.e34691_bib89",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.34172/jrhs.2022.81",
"article-title": "Ethics and effectiveness of US COVID-19 vaccine mandates and vaccination passports: A review",
"author": "Canning",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "J Res Health Sci.",
"key": "10.1016/j.heliyon.2024.e34691_bib90",
"volume": "22",
"year": "2022"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib91",
"unstructured": "Y T. Stop calling it a pandemic of the unvaccinated. https://www.theatlantic.com/ideas/archive/2021/09/persuade-unvaccinated-protect-unvaccinated/620091/. Accessed February 4th 2023, 2023. https://www.theatlantic.com/ideas/archive/2021/09/persuade-unvaccinated-protect-unvaccinated/620091/"
},
{
"DOI": "10.1056/NEJMoa2109072",
"article-title": "Covid-19 Breakthrough Infections in Vaccinated Health Care Workers",
"author": "Bergwerk",
"doi-asserted-by": "crossref",
"first-page": "1474",
"issue": "16",
"journal-title": "N Engl J Med",
"key": "10.1016/j.heliyon.2024.e34691_bib92",
"volume": "385",
"year": "2021"
},
{
"article-title": "The impact of delayed access to COVID-19 vaccines in low- and lower-middle-income countries",
"author": "Duroseau",
"journal-title": "Front Public Health",
"key": "10.1016/j.heliyon.2024.e34691_bib93",
"volume": "10",
"year": "2022"
},
{
"article-title": "The race for COVID-19 vaccines: accelerating innovation, fair allocation and distribution",
"author": "Daems",
"issue": "9",
"journal-title": "Vaccines (Basel)",
"key": "10.1016/j.heliyon.2024.e34691_bib94",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.healthpol.2021.03.013",
"article-title": "COVID-19 vaccine challenges: What have we learned so far and what remains to be done?",
"author": "Forman",
"doi-asserted-by": "crossref",
"first-page": "553",
"issue": "5",
"journal-title": "Health Policy",
"key": "10.1016/j.heliyon.2024.e34691_bib95",
"volume": "125",
"year": "2021"
},
{
"article-title": "COVID-19 vaccine challenges in developing and developed countries",
"author": "Yarlagadda",
"issue": "4",
"journal-title": "Cureus",
"key": "10.1016/j.heliyon.2024.e34691_bib96",
"volume": "14",
"year": "2022"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib97",
"unstructured": "Group-C19.com. Vitamin D for COVID-19: real-time analysis of all 300 studies: https://c19early.org/d. Accessed January 25, 2023, https://c19vitamind.com/"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib98",
"unstructured": "Annonymos. Vaccine monopolies make cost of vaccinating the world against COVID at least 5 times more expensive than it could be. Oxfam. 2023 March 10. https://www.oxfam.org/en/press-releases/vaccine-monopolies-make-cost-vaccinating-world-against-covid-least-5-times-more"
},
{
"article-title": "E E-P. It will be hard to find a farmer left’: Sri Lanka reels from rash fertiliser ban",
"journal-title": "Guardian",
"key": "10.1016/j.heliyon.2024.e34691_bib99",
"year": "2023"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib100",
"unstructured": "Athas I LC, Mogul R, Gonzalez-Roman D. Sri Lanka is ‘bankrupt,’ Prime Minister says. CNN, Sri Lanka. 2023, May 23. https://www.cnn.com/2022/07/05/asia/sri-lanka-bankrupt-fuel-crisis-intl-hnk/index.html"
},
{
"DOI": "10.1002/rmv.2381",
"article-title": "Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: Implications for immune escape and transmission",
"author": "Shrestha",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "Rev Med Virol",
"key": "10.1016/j.heliyon.2024.e34691_bib101",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.1057/s41271-021-00318-6",
"article-title": "The disinformation playbook: how industry manipulates the science-policy process-and how to restore scientific integrity",
"author": "Reed",
"doi-asserted-by": "crossref",
"first-page": "622",
"issue": "4",
"journal-title": "J Public Health Policy",
"key": "10.1016/j.heliyon.2024.e34691_bib102",
"volume": "42",
"year": "2021"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib103",
"unstructured": "Grant WB. Vitamin D acceptance delayed by big pharma following the Disinformation Playbook. Accessed March 22nd, 2022, http://orthomolecular.org/resources/omns/v14n22.shtml"
},
{
"DOI": "10.1016/j.socscimed.2022.114800",
"article-title": "The path towards herd immunity: Predicting COVID-19 vaccination uptake through results from a stated choice study across six continents",
"author": "Hess",
"doi-asserted-by": "crossref",
"journal-title": "Soc Sci Med",
"key": "10.1016/j.heliyon.2024.e34691_bib104",
"volume": "298",
"year": "2022"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib105",
"unstructured": "Spencer S. Widespread claims misrepresent effectiveness of COVID-19 vaccines. FactCheck.org. Accessed NOv 10, 2022."
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib106",
"unstructured": "FDA. Coronavirus (COVID-19) update: FDA authorizes Moderna, Pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose. FDA. January 5th, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use"
},
{
"DOI": "10.18773/austprescr.2023.020",
"article-title": "COVID-19 vaccines in 2023",
"author": "Sharma",
"doi-asserted-by": "crossref",
"first-page": "60",
"issue": "3",
"journal-title": "Aust Prescr",
"key": "10.1016/j.heliyon.2024.e34691_bib107",
"volume": "46",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2208343",
"article-title": "A bivalent Omicron-containing booster vaccine against Covid-19",
"author": "Chalkias",
"doi-asserted-by": "crossref",
"first-page": "1279",
"issue": "14",
"journal-title": "N Engl J Med",
"key": "10.1016/j.heliyon.2024.e34691_bib108",
"volume": "387",
"year": "2022"
},
{
"article-title": "Relative vaccine effectiveness of mRNA COVID-19 boosters in people aged at least 75 years during the spring-summer (monovalent vaccine) and autumn-winter (bivalent vaccine) booster campaigns: a prospective test negative case-control study, United Kingdom",
"author": "Chatzilena",
"journal-title": "Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin",
"key": "10.1016/j.heliyon.2024.e34691_bib109",
"year": "2022"
},
{
"DOI": "10.1002/jmv.27697",
"article-title": "Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review",
"author": "Chenchula",
"doi-asserted-by": "crossref",
"first-page": "2969",
"issue": "7",
"journal-title": "J Med Virol.",
"key": "10.1016/j.heliyon.2024.e34691_bib110",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2022.10.007",
"article-title": "No correlation of neutralizing antibody titers against the Omicron variant after a booster dose of COVID-19 vaccines with subsequent breakthrough Omicron infections among healthcare workers",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "e177",
"issue": "6",
"journal-title": "J Infect",
"key": "10.1016/j.heliyon.2024.e34691_bib111",
"volume": "85",
"year": "2022"
},
{
"DOI": "10.4081/jphr.2013.e28",
"article-title": "Technology and the future of healthcare",
"author": "Thimbleby",
"doi-asserted-by": "crossref",
"first-page": "e28",
"issue": "3",
"journal-title": "J Public Health Res",
"key": "10.1016/j.heliyon.2024.e34691_bib112",
"volume": "2",
"year": "2013"
},
{
"article-title": "Impact of Withholding Cost-Effective Early Treatments, Such as Vitamin D, on COVID-19: An Analysis Using an Innovative Logical Paradigm",
"author": "Polonowita",
"journal-title": "Wold J Adanced Pharma Life Sci.",
"key": "10.1016/j.heliyon.2024.e34691_bib113",
"year": "2023"
},
{
"DOI": "10.4103/ijd.IJD_421_20",
"article-title": "Artificial intelligence: How is it changing medical sciences and its future?",
"author": "Basu",
"doi-asserted-by": "crossref",
"first-page": "365",
"issue": "5",
"journal-title": "Indian J Dermatol",
"key": "10.1016/j.heliyon.2024.e34691_bib114",
"volume": "65",
"year": "2020"
},
{
"article-title": "Recent advancements in emerging technologies for healthcare management systems: A survey",
"author": "Junaid",
"issue": "10",
"journal-title": "Healthcare (Basel)",
"key": "10.1016/j.heliyon.2024.e34691_bib115",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0239252",
"article-title": "SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels",
"author": "Kaufman",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "PLoS One",
"key": "10.1016/j.heliyon.2024.e34691_bib116",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1093/pubmed/fdaa095",
"article-title": "Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK Biobank",
"author": "Raisi-Estabragh",
"doi-asserted-by": "crossref",
"first-page": "451",
"issue": "3",
"journal-title": "Journal of public health",
"key": "10.1016/j.heliyon.2024.e34691_bib117",
"volume": "42",
"year": "2020"
},
{
"DOI": "10.1007/s00394-020-02372-4",
"article-title": "Vitamin D and COVID-19 infection and mortality in UK Biobank",
"author": "Hastie",
"doi-asserted-by": "crossref",
"first-page": "545",
"issue": "1",
"journal-title": "Eur J Nutr",
"key": "10.1016/j.heliyon.2024.e34691_bib118",
"volume": "60",
"year": "2021"
},
{
"DOI": "10.1016/j.virusres.2020.198235",
"article-title": "Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19",
"author": "Kumar",
"doi-asserted-by": "crossref",
"journal-title": "Virus Res",
"key": "10.1016/j.heliyon.2024.e34691_bib119",
"volume": "292",
"year": "2021"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib120",
"unstructured": "Wimalawansa SJ, Polonowita, A. Boosting immunity with vitamin D for preventing complications and deaths from COVID-19. National Science Foundation, Sri Lanka.; 2021:171-198."
},
{
"DOI": "10.1016/j.arcmed.2022.04.003",
"article-title": "Efficacy and Safety of Vitamin D Supplementation to Prevent COVID-19 in Frontline Healthcare Workers. A Randomized Clinical Trial",
"author": "Villasis-Keever",
"doi-asserted-by": "crossref",
"first-page": "423",
"issue": "4",
"journal-title": "Archives of medical research",
"key": "10.1016/j.heliyon.2024.e34691_bib121",
"volume": "53",
"year": "2022"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"article-title": "Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study",
"author": "Entrenas Castillo",
"doi-asserted-by": "crossref",
"journal-title": "J Steroid Biochem Mol Biol.",
"key": "10.1016/j.heliyon.2024.e34691_bib122",
"volume": "203",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2020.10.009",
"article-title": "Use of Ivermectin Is Associated With Lower Mortality in Hospitalized Patients With Coronavirus Disease 2019: The Ivermectin in COVID Nineteen Study",
"author": "Rajter",
"doi-asserted-by": "crossref",
"first-page": "85",
"issue": "1",
"journal-title": "Chest",
"key": "10.1016/j.heliyon.2024.e34691_bib123",
"volume": "159",
"year": "2021"
},
{
"DOI": "10.18433/jpps31457",
"article-title": "Therapeutic potential of ivermectin as add on treatment in COVID 19: A systematic review and meta-analysis",
"author": "Padhy",
"doi-asserted-by": "crossref",
"first-page": "462",
"journal-title": "J Pharm Pharm Sci",
"key": "10.1016/j.heliyon.2024.e34691_bib124",
"volume": "23",
"year": "2020"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib125",
"unstructured": "Grant WB. Vitamin D acceptance delayed by big pharma following the Disinformation Playbook. Accessed March 22nd, 2022, http://orthomolecular.org/resources/omns/v14n22.shtml"
},
{
"article-title": "Infections and autoimmunity-The immune system and vitamin D: A Systematic Review",
"author": "Wimalawansa",
"first-page": "15",
"issue": "17",
"journal-title": "Nutrients",
"key": "10.1016/j.heliyon.2024.e34691_bib126",
"year": "2023"
},
{
"article-title": "Prophylactic use of vitamin D to maintain a robust immune system against infections like SARS-CoV-2",
"author": "Wimalawansa",
"first-page": "1",
"issue": "5",
"journal-title": "Glob J Endocrinol Metab GJEM 000571",
"key": "10.1016/j.heliyon.2024.e34691_bib127",
"volume": "3",
"year": "2023"
},
{
"DOI": "10.1186/s12916-022-02290-8",
"article-title": "A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: the COVID-VIT-D-a randomised multicentre international clinical trial",
"author": "Cannata-Andia",
"doi-asserted-by": "crossref",
"first-page": "83",
"issue": "1",
"journal-title": "BMC Med",
"key": "10.1016/j.heliyon.2024.e34691_bib128",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1001/jama.2020.26848",
"article-title": "Effect of a single high dose of vitamin D3 on hospital length of stay in patients Wpwith moderate to severe COVID-19: A randomized clinical trial",
"author": "Murai",
"doi-asserted-by": "crossref",
"first-page": "1053",
"issue": "11",
"journal-title": "JAMA",
"key": "10.1016/j.heliyon.2024.e34691_bib129",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1002/jmv.27133",
"article-title": "In-hospital mortality in SARS-CoV-2 stratified by serum 25-hydroxy-vitamin D levels: A retrospective study",
"author": "Al-Jarallah",
"doi-asserted-by": "crossref",
"first-page": "5880",
"issue": "10",
"journal-title": "J Med Virol",
"key": "10.1016/j.heliyon.2024.e34691_bib130",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1177/21501327211041206",
"article-title": "Vitamin D Status and Severe COVID-19 Disease Outcomes in Hospitalized Patients",
"author": "Pecina",
"doi-asserted-by": "crossref",
"journal-title": "J Prim Care Community Health",
"key": "10.1016/j.heliyon.2024.e34691_bib131",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.nut.2020.111055",
"article-title": "Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy, Italy",
"author": "Cereda",
"doi-asserted-by": "crossref",
"journal-title": "Nutrition",
"key": "10.1016/j.heliyon.2024.e34691_bib132",
"volume": "82",
"year": "2021"
},
{
"DOI": "10.1136/bmjnph-2020-000151",
"article-title": "No evidence that vitamin D is able to prevent or affect the severity of COVID-19 in individuals with European ancestry: a Mendelian randomisation study of open data",
"author": "Amin",
"doi-asserted-by": "crossref",
"first-page": "42",
"issue": "1",
"journal-title": "BMJ Nutr Prev Health",
"key": "10.1016/j.heliyon.2024.e34691_bib133",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.3390/v13071292",
"article-title": "Potential prophylactic treatments for COVID-19",
"author": "Ben-Zuk",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "Viruses",
"key": "10.1016/j.heliyon.2024.e34691_bib134",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1186/s12941-020-00368-w",
"article-title": "Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19",
"author": "Sharun",
"doi-asserted-by": "crossref",
"first-page": "23",
"issue": "1",
"journal-title": "Ann Clin Microbiol Antimicrob",
"key": "10.1016/j.heliyon.2024.e34691_bib135",
"volume": "19",
"year": "2020"
},
{
"article-title": "Fighting against COVID-19: Boosting the immunity with micronutrients, stress reduction, physical activity, and vitamin D",
"author": "Wimalawansa",
"first-page": "1",
"issue": "1",
"journal-title": "Nutrition and Food Science Journal (Sci Literature)",
"key": "10.1016/j.heliyon.2024.e34691_bib136",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1530/EC-18-0432",
"article-title": "Vitamin D testing and treatment: a narrative review of current evidence",
"author": "Pilz",
"doi-asserted-by": "crossref",
"first-page": "R27",
"issue": "2",
"journal-title": "Endocr Connect",
"key": "10.1016/j.heliyon.2024.e34691_bib137",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.3389/fimmu.2021.635371",
"article-title": "Lack of effectiveness of repurposed drugs for COVID-19 treatment",
"author": "Martinez",
"doi-asserted-by": "crossref",
"journal-title": "Front Immunol",
"key": "10.1016/j.heliyon.2024.e34691_bib138",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1037/h0101793",
"article-title": "Refuting spurious COVID-19 treatment claims reduces demand and misinformation sharing",
"author": "MacFarlane",
"doi-asserted-by": "crossref",
"first-page": "248",
"issue": "2",
"journal-title": "J Appl Res Mem Cogn",
"key": "10.1016/j.heliyon.2024.e34691_bib139",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1097/PHH.0000000000001616",
"article-title": "Disciplining physicians who spread medical misinformation",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "595",
"issue": "6",
"journal-title": "J Public Health Manag Pract",
"key": "10.1016/j.heliyon.2024.e34691_bib140",
"volume": "28",
"year": "2022"
},
{
"article-title": "Social media and COVID-19-perceptions and public deceptions of ivermectin, colchicine and hydroxychloroquine: Lessons for future pandemics",
"author": "Schellack",
"issue": "4",
"journal-title": "Antibiotics (Basel)",
"key": "10.1016/j.heliyon.2024.e34691_bib141",
"volume": "11",
"year": "2022"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib142",
"unstructured": "FDA. Why you should not use ivermectin to treat or prevent COVID-19. FDA. 2023 March 12. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib143",
"unstructured": "Sun LH WL, GodfreyH. Doctors who put lives at risk with COVID misinformation rarely punished. Washington Post. 2023, Aug 10. https://www.washingtonpost.com/health/2023/07/26/covid-misinformation-doctor-discipline/"
},
{
"DOI": "10.1007/s40656-022-00532-9",
"article-title": "The failure of drug repurposing for COVID-19 as an effect of excessive hypothesis testing and weak mechanistic evidence",
"author": "Maziarz",
"doi-asserted-by": "crossref",
"first-page": "47",
"issue": "4",
"journal-title": "Hist Philos Life Sci.",
"key": "10.1016/j.heliyon.2024.e34691_bib144",
"volume": "44",
"year": "2022"
},
{
"article-title": "Risks elaborated vs. risks downplayed: The effect of risk comparisons in mainstream mediaduring COVID-19 on risk perceptions and anxiety levels",
"author": "Anderson",
"journal-title": "Frontiers: Sci Environ Commun",
"key": "10.1016/j.heliyon.2024.e34691_bib145",
"volume": "6doi",
"year": "2021"
},
{
"article-title": "The impact of disinformation on democratic processes and human rights in the world",
"author": "Colomin",
"journal-title": "European Parliment",
"key": "10.1016/j.heliyon.2024.e34691_bib146",
"year": "2023"
},
{
"DOI": "10.1007/s11356-022-21967-4",
"article-title": "Pseudoscience and fraudulent products for COVID-19 management",
"author": "Chavda",
"doi-asserted-by": "crossref",
"first-page": "62887",
"issue": "42",
"journal-title": "Environmental science and pollution research international",
"key": "10.1016/j.heliyon.2024.e34691_bib147",
"volume": "29",
"year": "2022"
},
{
"article-title": "FLCCC treatmemetn protocols",
"journal-title": "FLCCC Alliance.",
"key": "10.1016/j.heliyon.2024.e34691_bib148",
"year": "2023"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib149",
"unstructured": "Anonymus. At home treatment guide. World Council of Heatlh. 2022, August 7. https://worldcouncilforhealth.org/wp-content/uploads/2021/09/WCH-At-Home-Treatment-Guide_30-Sept-2021.pdf"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib150",
"unstructured": "Kory P MJ. The war on ivermectin: The medicine that saved millions and could have ended the pandemic [https://rumble.com/v41agxg-2023-12-14-mikki-willis-pierre-kory-the-war-on-ivermectin.html]. vol 1. ICAN Press; 2023."
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib151",
"unstructured": "A. A. Why cheap, older drugs that might treat COVID never get out of the lab. https://kffhealthnews.org/news/article/repurposing-cheap-older-drugs-as-covid-treatment-camostat/"
},
{
"DOI": "10.1017/S0007114520002950",
"article-title": "COVID-19 and misinformation: how an infodemic fuelled the prominence of vitamin D",
"author": "Henrina",
"doi-asserted-by": "crossref",
"first-page": "359",
"issue": "3",
"journal-title": "Br J Nutr",
"key": "10.1016/j.heliyon.2024.e34691_bib152",
"volume": "125",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofac469",
"article-title": "COVID-19 Vaccine Effectiveness Against Progression to In-Hospital Mortality in Zambia, 2021-2022",
"author": "Chanda",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "Open Forum Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib153",
"volume": "9",
"year": "2022"
},
{
"article-title": "Vaccine-Acquired SARS-CoV-2 Immunity versus Infection-Acquired Immunity: A Comparison of Three COVID-19 Vaccines",
"author": "Samanovic",
"issue": "12",
"journal-title": "Vaccines (Basel)",
"key": "10.1016/j.heliyon.2024.e34691_bib154",
"volume": "10",
"year": "2022"
},
{
"article-title": "Large-Scale Study of Antibody Titer Decay following BNT162b2 mRNA Vaccine or SARS-CoV-2 Infection",
"author": "Israel",
"issue": "1",
"journal-title": "Vaccines (Basel)",
"key": "10.1016/j.heliyon.2024.e34691_bib155",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.3390/molecules27123851",
"article-title": "Structural Dynamics of the SARS-CoV-2 Spike Protein: A 2-Year Retrospective Analysis of SARS-CoV-2 Variants (from Alpha to Omicron) Reveals an Early Divergence between Conserved and Variable Epitopes",
"author": "Guerin",
"doi-asserted-by": "crossref",
"issue": "12",
"journal-title": "Molecules",
"key": "10.1016/j.heliyon.2024.e34691_bib156",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1002/jmv.27954",
"article-title": "Antibody titers after a third dose of the SARS-CoV-2 BNT162b2 vaccine in immunocompromised adults in Greece: Is a fourth dose necessary?",
"author": "Kontopoulou",
"doi-asserted-by": "crossref",
"first-page": "5056",
"issue": "10",
"journal-title": "J Med Virol",
"key": "10.1016/j.heliyon.2024.e34691_bib157",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1016/j.vaccine.2022.09.080",
"article-title": "COVID-19 outbreak in an elderly care home: Very low vaccine effectiveness and late impact of booster vaccination campaign",
"author": "van Ewijk",
"doi-asserted-by": "crossref",
"first-page": "6664",
"issue": "46",
"journal-title": "Vaccine",
"key": "10.1016/j.heliyon.2024.e34691_bib158",
"volume": "40",
"year": "2022"
},
{
"article-title": "Herd immunity to fight against COVID-19: A narrative review",
"author": "Suryawanshi",
"issue": "1",
"journal-title": "Cureus",
"key": "10.1016/j.heliyon.2024.e34691_bib159",
"volume": "15",
"year": "2023"
},
{
"article-title": "Association of sex, sge, and comorbidities with mortality in COVID-19 Patients: A systematic review and meta-analysis",
"author": "Biswas",
"first-page": "1",
"journal-title": "Intervirology",
"key": "10.1016/j.heliyon.2024.e34691_bib160",
"year": "2020"
},
{
"article-title": "Maintaining optimum health requires longer-term stable vitamin D concentrations",
"author": "Wimalawansa",
"first-page": "1",
"issue": "3",
"journal-title": "Int J Regenr Med",
"key": "10.1016/j.heliyon.2024.e34691_bib161",
"volume": "3",
"year": "2020"
},
{
"article-title": "Effective and practical ways to overcome vitamin D deficiency",
"author": "Wimalawansa",
"first-page": "1",
"issue": "1",
"journal-title": "J Family Med Community Health",
"key": "10.1016/j.heliyon.2024.e34691_bib162",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1093/infdis/jiac453",
"article-title": "Use of Whole-Genome Sequencing to Estimate the Contribution of Immune Evasion and Waning Immunity on Decreasing COVID-19 Vaccine Effectiveness",
"author": "Lind",
"doi-asserted-by": "crossref",
"first-page": "663",
"issue": "5",
"journal-title": "J Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib163",
"volume": "227",
"year": "2023"
},
{
"DOI": "10.1016/S2666-5247(22)00335-4",
"article-title": "Omicron sublineage recombinant XBB evades neutralising antibodies in recipients of BNT162b2 or CoronaVac vaccines",
"author": "Zhang",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "Lancet Microbe",
"key": "10.1016/j.heliyon.2024.e34691_bib164",
"volume": "4",
"year": "2023"
},
{
"DOI": "10.1016/j.healun.2021.04.018",
"article-title": "COVID-19 vaccination immune paresis in heart and lung transplantation",
"author": "Aslam",
"doi-asserted-by": "crossref",
"first-page": "763",
"issue": "8",
"journal-title": "J Heart Lung Transplant",
"key": "10.1016/j.heliyon.2024.e34691_bib165",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofad209",
"article-title": "Effectiveness of the coronavirus disease 2019 bivalent vaccine",
"author": "Shrestha",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "Open Forum Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib166",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac327",
"article-title": "Coronavirus Disease 2019 Vaccine Boosting in Previously Infected or Vaccinated Individuals",
"author": "Shrestha",
"doi-asserted-by": "crossref",
"first-page": "2169",
"issue": "12",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib167",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.3390/v14122688",
"article-title": "Primary SARS-CoV-2 Infections, Re-infections and Vaccine Effectiveness during the Omicron Transmission Period in Healthcare Workers of Trieste and Gorizia (Northeast Italy), 1 December 2021-31 May 2022",
"author": "Cegolon",
"doi-asserted-by": "crossref",
"issue": "12",
"journal-title": "Viruses",
"key": "10.1016/j.heliyon.2024.e34691_bib168",
"volume": "14",
"year": "2022"
},
{
"article-title": "Breakthrough SARS-CoV-2 infections among patients with cancer following two and three doses of COVID-19 mRNA vaccines: a retrospective observational study from the COVID-19 and Cancer Consortium",
"author": "Choueiri",
"journal-title": "Lancet Reg Health Am",
"key": "10.1016/j.heliyon.2024.e34691_bib169",
"volume": "19",
"year": "2023"
},
{
"DOI": "10.1038/s41586-022-05053-w",
"article-title": "Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "603",
"issue": "7923",
"journal-title": "Nature",
"key": "10.1016/j.heliyon.2024.e34691_bib170",
"volume": "608",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00941-2",
"article-title": "Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2",
"author": "Antonelli",
"doi-asserted-by": "crossref",
"first-page": "2263",
"issue": "10343",
"journal-title": "Lancet",
"key": "10.1016/j.heliyon.2024.e34691_bib171",
"volume": "399",
"year": "2022"
},
{
"article-title": "Clinical severity according to the primary infection variant in patients with suspected SARS-CoV-2 reinfection in Korea",
"author": "Hwang",
"journal-title": "Epidemiol Health",
"key": "10.1016/j.heliyon.2024.e34691_bib172",
"volume": "45",
"year": "2023"
},
{
"DOI": "10.1371/journal.ppat.1010876",
"article-title": "Shedding of infectious SARS-CoV-2 despite vaccination",
"author": "Riemersma",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "PLoS Pathog",
"key": "10.1016/j.heliyon.2024.e34691_bib173",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1186/s12879-020-05469-7",
"article-title": "The potential impact of a recent measles epidemic on COVID-19 in Samoa",
"author": "MacIntyre",
"doi-asserted-by": "crossref",
"first-page": "735",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib174",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2213907",
"article-title": "Antibody Response to Omicron BA.4-BA.5 Bivalent Booster",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "567",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "10.1016/j.heliyon.2024.e34691_bib175",
"volume": "388",
"year": "2023"
},
{
"DOI": "10.1093/ofid/ofac698",
"article-title": "Absolute and Relative Vaccine Effectiveness of Primary and Booster Series of COVID-19 Vaccines (mRNA and Adenovirus Vector) Against COVID-19 Hospitalizations in the United States, December 2021-April 2022",
"author": "Lewis",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Open Forum Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib176",
"volume": "10",
"year": "2023"
},
{
"article-title": "Serial negative response after standard and third (Booster) dose of COVID-19 inactivated vaccine is associated with low vitamin D levels in patients with solid cancers",
"author": "Ma",
"journal-title": "Front Med (Lausanne)",
"key": "10.1016/j.heliyon.2024.e34691_bib177",
"volume": "9",
"year": "2022"
},
{
"article-title": "Safety of Four COVID-19 Vaccines across Primary Doses 1, 2, 3 and Booster: A Prospective Cohort Study of Australian Community Pharmacy Vaccinations",
"author": "Salter",
"issue": "12",
"journal-title": "Vaccines (Basel)",
"key": "10.1016/j.heliyon.2024.e34691_bib178",
"volume": "10",
"year": "2022"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib179",
"unstructured": "CDC U. Effectiveness of bivalent mRNA vaccines in preventing symptomatic SARS-CoV-2 infection—increasing community access to testing program, United States: CDC-MMWR Report, Sept.–Nov. 2022. CDC. Accessed January 6, 2023. https://www.cdc.gov/mmwr/volumes/71/wr/mm7148e1.htm?s_cid=mm7148e1_w#T2_down"
},
{
"DOI": "10.1371/journal.pone.0259610",
"article-title": "Profiling RT-LAMP tolerance of sequence variation for SARS-CoV-2 RNA detection",
"author": "Tamanaha",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "PLoS One",
"key": "10.1016/j.heliyon.2024.e34691_bib180",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.3389/fmicb.2022.1027271",
"article-title": "Investigating SARS-CoV-2 breakthrough infections per variant and vaccine type",
"author": "Dingemans",
"doi-asserted-by": "crossref",
"journal-title": "Front Microbiol",
"key": "10.1016/j.heliyon.2024.e34691_bib181",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00816-7",
"article-title": "Humoral immune evasion of the omicron subvariants BQ.1.1 and XBB",
"author": "Uraki",
"doi-asserted-by": "crossref",
"first-page": "30",
"issue": "1",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib182",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac022",
"article-title": "Necessity of coronavirus disease 2019 (COVID-19) vaccination in persons who have already had COVID-19",
"author": "Shrestha",
"doi-asserted-by": "crossref",
"first-page": "e662",
"issue": "1",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib183",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1111/crj.13694",
"article-title": "Interactive effect of booster vaccination and vitamin D status on antibody production of Omicron variant-infected adults: A real-world cohort study",
"author": "Peng",
"doi-asserted-by": "crossref",
"first-page": "1067",
"issue": "10",
"journal-title": "Clin Respir J",
"key": "10.1016/j.heliyon.2024.e34691_bib184",
"volume": "17",
"year": "2023"
},
{
"DOI": "10.1016/j.vaccine.2020.10.048",
"article-title": "Evaluating association of vaccine response to low serum zinc and vitamin D levels in children of a birth cohort study in Dhaka",
"author": "Das",
"doi-asserted-by": "crossref",
"first-page": "59",
"issue": "1",
"journal-title": "Vaccine",
"key": "10.1016/j.heliyon.2024.e34691_bib185",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.1016/j.jaci.2022.05.001",
"article-title": "SARS-CoV-2 vaccination in the immunocompromised host",
"author": "Connolly",
"doi-asserted-by": "crossref",
"first-page": "56",
"issue": "1",
"journal-title": "J Allergy Clin Immunol",
"key": "10.1016/j.heliyon.2024.e34691_bib186",
"volume": "150",
"year": "2022"
},
{
"article-title": "Global epidemic of coronavirus—COVID-19: What can we do to minimize risks?",
"author": "Wimalawansa",
"first-page": "432",
"issue": "3",
"journal-title": "European J Biomed & Pharma Sci",
"key": "10.1016/j.heliyon.2024.e34691_bib187",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/j.actbio.2022.08.006",
"article-title": "Single-injection COVID-19 subunit vaccine elicits potent immune responses",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "491",
"journal-title": "Acta Biomater",
"key": "10.1016/j.heliyon.2024.e34691_bib188",
"volume": "151",
"year": "2022"
},
{
"article-title": "Intraduodenal Delivery of Exosome-Loaded SARS-CoV-2 RBD mRNA Induces a Neutralizing Antibody Response in Mice",
"author": "Zhang",
"issue": "3",
"journal-title": "Vaccines (Basel)",
"key": "10.1016/j.heliyon.2024.e34691_bib189",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2034577",
"article-title": "Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine",
"author": "Polack",
"doi-asserted-by": "crossref",
"first-page": "2603",
"issue": "27",
"journal-title": "N Engl J Med",
"key": "10.1016/j.heliyon.2024.e34691_bib190",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2035389",
"article-title": "Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine",
"author": "Baden",
"doi-asserted-by": "crossref",
"first-page": "403",
"issue": "5",
"journal-title": "N Engl J Med",
"key": "10.1016/j.heliyon.2024.e34691_bib191",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.15585/mmwr.mm7013e3",
"author": "Thompson",
"doi-asserted-by": "crossref",
"first-page": "495",
"issue": "13",
"journal-title": "MMWR Morbidity and mortality weekly report",
"key": "10.1016/j.heliyon.2024.e34691_bib192",
"volume": "70",
"year": "2021"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib193",
"unstructured": "Anonymus. Coronavirus disease (COVID-19): Herd immunity, lockdowns and COVID-19. WHO-Questins and answers. Accessed 20 February 2024, https://www.who.int/news-room/questions-and-answers/item/herd-immunity-lockdowns-and-covid-19"
},
{
"DOI": "10.1007/s13668-020-00330-4",
"article-title": "Can Vitamins, as Epigenetic Modifiers, Enhance Immunity in COVID-19 Patients with Non-communicable Disease?",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "202",
"issue": "3",
"journal-title": "Curr Nutr Rep",
"key": "10.1016/j.heliyon.2024.e34691_bib194",
"volume": "9",
"year": "2020"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib195",
"unstructured": "Weixel N. CDC: More vaccinated adults hospitalized with omicron, but fewer admitted to ICU. https://thehill.com/policy/healthcare/592847-cdc-more-vaccinated-adults-hospitalized-with-omicron-but-fewer-admitted-to/"
},
{
"article-title": "IgG4 antibodies induced by repeated vaccination may generate immune tolerance to the SARS-CoV-2 Spike protein",
"author": "Uversky",
"issue": "5",
"journal-title": "Vaccines (Basel)",
"key": "10.1016/j.heliyon.2024.e34691_bib196",
"volume": "11",
"year": "2023"
},
{
"article-title": "COVID-19 excess deaths in Peru's 25 States in 2020: Nationwide trends, confounding factors, and correlations with the extent of ivermectin treatment by State",
"author": "Chamie",
"issue": "8",
"journal-title": "Cureus",
"key": "10.1016/j.heliyon.2024.e34691_bib197",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1186/s12887-021-02803-z",
"article-title": "Concentration levels of serum 25-Hydroxyvitamin-D and vitamin D deficiency among children and adolescents of India: a descriptive cross-sectional study",
"author": "Mustafa",
"doi-asserted-by": "crossref",
"first-page": "334",
"issue": "1",
"journal-title": "BMC Pediatr",
"key": "10.1016/j.heliyon.2024.e34691_bib198",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1080/21645515.2022.2163813",
"article-title": "Effectiveness of COVID-19 vaccines against SARS-CoV-2 Omicron variants during two outbreaks from March to May 2022 in Quzhou, China",
"author": "Yin",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Hum Vaccin Immunother",
"key": "10.1016/j.heliyon.2024.e34691_bib199",
"volume": "19",
"year": "2023"
},
{
"DOI": "10.3390/microorganisms11082073",
"article-title": "Early fluvoxaminereduces the risk for clinical deterioration in symptomatic outpatients with COVID-19: A real-world, retrospective, before-after analysis",
"author": "Tsiakalos",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "Microorganisms",
"key": "10.1016/j.heliyon.2024.e34691_bib200",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1001/jama.2020.22760",
"article-title": "Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: A randomized clinical trial",
"author": "Lenze",
"doi-asserted-by": "crossref",
"first-page": "2292",
"issue": "22",
"journal-title": "JAMA",
"key": "10.1016/j.heliyon.2024.e34691_bib201",
"volume": "324",
"year": "2020"
},
{
"article-title": "Elderberries-A source of bioactive compounds with antiviral action",
"author": "Mocanu",
"issue": "6",
"journal-title": "Plants (Basel)",
"key": "10.1016/j.heliyon.2024.e34691_bib202",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1002/ptr.7004",
"article-title": "Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: An open label nonrandomized clinical trial",
"author": "Saber-Moghaddam",
"doi-asserted-by": "crossref",
"first-page": "2616",
"issue": "5",
"journal-title": "Phytother Res",
"key": "10.1016/j.heliyon.2024.e34691_bib203",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.ctim.2021.102769",
"article-title": "Nigella sativa for the treatment of COVID-19: An open-label randomized controlled clinical trial",
"author": "Koshak",
"doi-asserted-by": "crossref",
"journal-title": "Complement Ther Med",
"key": "10.1016/j.heliyon.2024.e34691_bib204",
"volume": "61",
"year": "2021"
},
{
"DOI": "10.1002/jmv.27595",
"article-title": "Efficacy of melatonin in the treatment of patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials",
"author": "Lan",
"doi-asserted-by": "crossref",
"first-page": "2102",
"issue": "5",
"journal-title": "J Med Virol",
"key": "10.1016/j.heliyon.2024.e34691_bib205",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.3390/jcm11206138",
"article-title": "Early outpatient treatment of COVID-19: A retrospective analysis of 392 cases in Italy",
"author": "Cosentino",
"doi-asserted-by": "crossref",
"issue": "20",
"journal-title": "J Clin Med",
"key": "10.1016/j.heliyon.2024.e34691_bib206",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2023.02.029",
"article-title": "Molnupiravir and nirmatrelvir-ritonavir reduce mortality risk during post-acute COVID-19 phase",
"author": "Wan",
"doi-asserted-by": "crossref",
"first-page": "622",
"issue": "6",
"journal-title": "J Infect",
"key": "10.1016/j.heliyon.2024.e34691_bib207",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.1001/jamainternmed.2023.5099",
"article-title": "Nirmatrelvir and Molnupiravir and post-COVID-19 condition in older patients",
"author": "Fung",
"doi-asserted-by": "crossref",
"first-page": "1404",
"issue": "12",
"journal-title": "JAMA Intern Med",
"key": "10.1016/j.heliyon.2024.e34691_bib208",
"volume": "183",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac443",
"article-title": "Effectiveness of Paxlovid in reducingsevere coronavirus gisease 2019 and mortality in high-risk patients",
"author": "Najjar-Debbiny",
"doi-asserted-by": "crossref",
"first-page": "e342",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib209",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.7326/M23-1394",
"article-title": "Effectiveness of Nirmatrelvir-Ritonavir against the development of post-COVID-19 conditions among U.S. Veterans : A target trial emulation",
"author": "Ioannou",
"doi-asserted-by": "crossref",
"first-page": "1486",
"issue": "11",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.heliyon.2024.e34691_bib210",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(21)00485-0",
"article-title": "Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial",
"author": "Ader",
"doi-asserted-by": "crossref",
"first-page": "209",
"issue": "2",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib211",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0290964",
"article-title": "Characteristics and outcomes of patients with severe COVID-19 in Indonesia: Lessons from the first wave",
"author": "Burhan",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "PLoS One",
"key": "10.1016/j.heliyon.2024.e34691_bib212",
"volume": "18",
"year": "2023"
},
{
"article-title": "Is ivermectin effective in treating COVID-19?",
"author": "Yang",
"journal-title": "Frontiers in pharmacology",
"key": "10.1016/j.heliyon.2024.e34691_bib213",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2022.3079",
"article-title": "Comparison of trials using ivermectin for COVID-19 between regions with high and low prevalence of strongyloidiasis: A meta-analysis",
"author": "Bitterman",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.heliyon.2024.e34691_bib214",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.18433/jpps32105",
"article-title": "Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19: A double-blind randomized placebo Controlled ttrial in eastern India",
"author": "Ravikirti",
"doi-asserted-by": "crossref",
"first-page": "343",
"journal-title": "J Pharm Pharm Sci",
"key": "10.1016/j.heliyon.2024.e34691_bib215",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.1177/03000605211013550",
"article-title": "Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial",
"author": "Mahmud",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "J Int Med Res",
"key": "10.1016/j.heliyon.2024.e34691_bib216",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1007/s11357-023-00756-y",
"article-title": "Results of a systematic review and meta-analysis of early studies on ivermectin in SARS-CoV-2 infection",
"author": "Rago",
"doi-asserted-by": "crossref",
"first-page": "2179",
"issue": "4",
"journal-title": "Geroscience",
"key": "10.1016/j.heliyon.2024.e34691_bib217",
"volume": "45",
"year": "2023"
},
{
"DOI": "10.1097/MJT.0000000000001402",
"article-title": "Ivermectin for prevention and treatment of COVID-19 infection: A systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines",
"author": "Bryant",
"doi-asserted-by": "crossref",
"first-page": "e434",
"issue": "4",
"journal-title": "Am J Ther",
"key": "10.1016/j.heliyon.2024.e34691_bib218",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1093/jac/dkz524",
"article-title": "Safety of high-dose ivermectin: a systematic review and meta-analysis",
"author": "Navarro",
"doi-asserted-by": "crossref",
"first-page": "827",
"issue": "4",
"journal-title": "J Antimicrob Chemother",
"key": "10.1016/j.heliyon.2024.e34691_bib219",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1177/009127002237994",
"article-title": "Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects",
"author": "Guzzo",
"doi-asserted-by": "crossref",
"first-page": "1122",
"issue": "10",
"journal-title": "J Clin Pharmacol",
"key": "10.1016/j.heliyon.2024.e34691_bib220",
"volume": "42",
"year": "2002"
},
{
"DOI": "10.5812/jjhs-146703",
"article-title": "Ivermectin as a potential addition to the limited anti-COVID-19 arsenal: A double-blinded clinical trial",
"author": "Varnaseri",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "Jundishapur J Health Sci",
"key": "10.1016/j.heliyon.2024.e34691_bib221",
"volume": "16",
"year": "2024"
},
{
"DOI": "10.1016/j.jinf.2024.106130",
"article-title": "Ivermectin for COVID-19 in adults in the community (PRINCIPLE): An open, randomised, controlled, adaptive platform trial of short- and longer-term outcomes",
"author": "Hayward",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "J Infect",
"key": "10.1016/j.heliyon.2024.e34691_bib222",
"volume": "88",
"year": "2024"
},
{
"DOI": "10.1056/NEJMoa2115869",
"article-title": "Effect of early treatment with Ivermectin among patients with Covid-19",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "1721",
"issue": "18",
"journal-title": "N Engl J Med",
"key": "10.1016/j.heliyon.2024.e34691_bib223",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jama.2022.18590",
"article-title": "Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: A randomized clinical trial",
"author": "Naggie",
"doi-asserted-by": "crossref",
"first-page": "1595",
"issue": "16",
"journal-title": "JAMA",
"key": "10.1016/j.heliyon.2024.e34691_bib224",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2201662",
"article-title": "Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19",
"author": "Bramante",
"doi-asserted-by": "crossref",
"first-page": "599",
"issue": "7",
"journal-title": "N Engl J Med",
"key": "10.1016/j.heliyon.2024.e34691_bib225",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(23)00299-2",
"article-title": "Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial",
"author": "Bramante",
"doi-asserted-by": "crossref",
"first-page": "1119",
"issue": "10",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib226",
"volume": "23",
"year": "2023"
},
{
"article-title": "Ivermectin for preventing and treating COVID-19",
"author": "Popp",
"issue": "6",
"journal-title": "Cochrane Database Syst Rev",
"key": "10.1016/j.heliyon.2024.e34691_bib227",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1186/s12879-022-07589-8",
"article-title": "Systematic review and meta-analysis of ivermectin for treatment of COVID-19: evidence beyond the hype",
"author": "Marcolino",
"doi-asserted-by": "crossref",
"first-page": "639",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib228",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1001/jama.2023.1650",
"article-title": "Effect of higher-dose ivermectin for 6 Days vs placebo on time to sustained recovery in outpatients with COVID-19: A randomized clinical trial",
"author": "Naggie",
"doi-asserted-by": "crossref",
"first-page": "888",
"issue": "11",
"journal-title": "JAMA",
"key": "10.1016/j.heliyon.2024.e34691_bib229",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.1001/jama.2021.3071",
"article-title": "Effect of ivermectin on time to resolution of symptoms Among adults with mild COVID-19: A randomized clinical trial",
"author": "Lopez-Medina",
"doi-asserted-by": "crossref",
"first-page": "1426",
"issue": "14",
"journal-title": "JAMA",
"key": "10.1016/j.heliyon.2024.e34691_bib230",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciab591",
"article-title": "Ivermectin for the treatment of coronavirus disease 2019: A systematic review and Meta-analysis of randomized controlled trials",
"author": "Roman",
"doi-asserted-by": "crossref",
"first-page": "1022",
"issue": "6",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.heliyon.2024.e34691_bib231",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.1177/10600280211028242",
"article-title": "Interventions in an ambulatory setting to prevent progression to severe disease in patients with COVID-19: A systematic review",
"author": "E",
"doi-asserted-by": "crossref",
"first-page": "309",
"issue": "3",
"journal-title": "Ann Pharmacother",
"key": "10.1016/j.heliyon.2024.e34691_bib232",
"volume": "56",
"year": "2022"
},
{
"article-title": "Effectiveness of COVID-19 Vaccines over 13 Months Covering the Period of the Emergence of the Omicron Variant in the Swedish Population",
"author": "Xu",
"issue": "12",
"journal-title": "Vaccines (Basel)",
"key": "10.1016/j.heliyon.2024.e34691_bib233",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0065835",
"article-title": "Vitamin D and respiratory tract iInfections: A systematic review and meta-analysis of randomized controlled trials",
"author": "Bergman",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "PLoS One",
"key": "10.1016/j.heliyon.2024.e34691_bib234",
"volume": "8",
"year": "2013"
},
{
"DOI": "10.1007/s00011-008-8007-8",
"article-title": "Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "524",
"issue": "11",
"journal-title": "Inflamm Res",
"key": "10.1016/j.heliyon.2024.e34691_bib235",
"volume": "57",
"year": "2008"
},
{
"DOI": "10.1186/1743-422X-2-69",
"article-title": "Chloroquine is a potent inhibitor of SARS coronavirus infection and spread",
"author": "Vincent",
"doi-asserted-by": "crossref",
"first-page": "69",
"journal-title": "Virol J",
"key": "10.1016/j.heliyon.2024.e34691_bib236",
"volume": "2",
"year": "2005"
},
{
"DOI": "10.3389/fnut.2023.1132528",
"article-title": "The effects of vitamin D on all-cause mortality in different diseases: an evidence-map and umbrella review of 116 randomized controlled trials",
"author": "Cao",
"doi-asserted-by": "crossref",
"journal-title": "Front Nutr",
"key": "10.1016/j.heliyon.2024.e34691_bib237",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "10.1016/j.heliyon.2024.e34691_bib238",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1016/j.nmni.2023.101172",
"article-title": "Efficacy and safety of in-hospital treatment of Covid-19 infection with low-dose hydroxychloroquine and azithromycin in hospitalized patients: A retrospective controlled cohort study",
"author": "Meeus",
"doi-asserted-by": "crossref",
"journal-title": "New Microbes New Infect",
"key": "10.1016/j.heliyon.2024.e34691_bib239",
"volume": "55",
"year": "2023"
},
{
"key": "10.1016/j.heliyon.2024.e34691_bib240",
"unstructured": "Anonymus. COVID-19 Treatment Guidelines-Vitamin D. NIH. Accessed March 17, 2024, https://www.covid19treatmentguidelines.nih.gov/therapies/supplements/vitamin-d/"
},
{
"DOI": "10.1056/NEJMc2301323",
"article-title": "Bivalent Covid-19 Vaccines",
"author": "Marks",
"doi-asserted-by": "crossref",
"first-page": "1151",
"issue": "12",
"journal-title": "N Engl J Med",
"key": "10.1016/j.heliyon.2024.e34691_bib241",
"volume": "388",
"year": "2023"
}
],
"reference-count": 241,
"references-count": 241,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2405844024107220"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Unlocking Insights: Navigating COVID-19 Challenges and Emulating Future Pandemic Resilience Strategies with Strengthening Natural Immunity",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}
wimalawansa