Metformin therapy and severity and mortality of SARS-CoV-2 infection: a meta-analysis
et al., Clinical Diabetology, doi:10.5603/DK.a2021.0035, May 2021
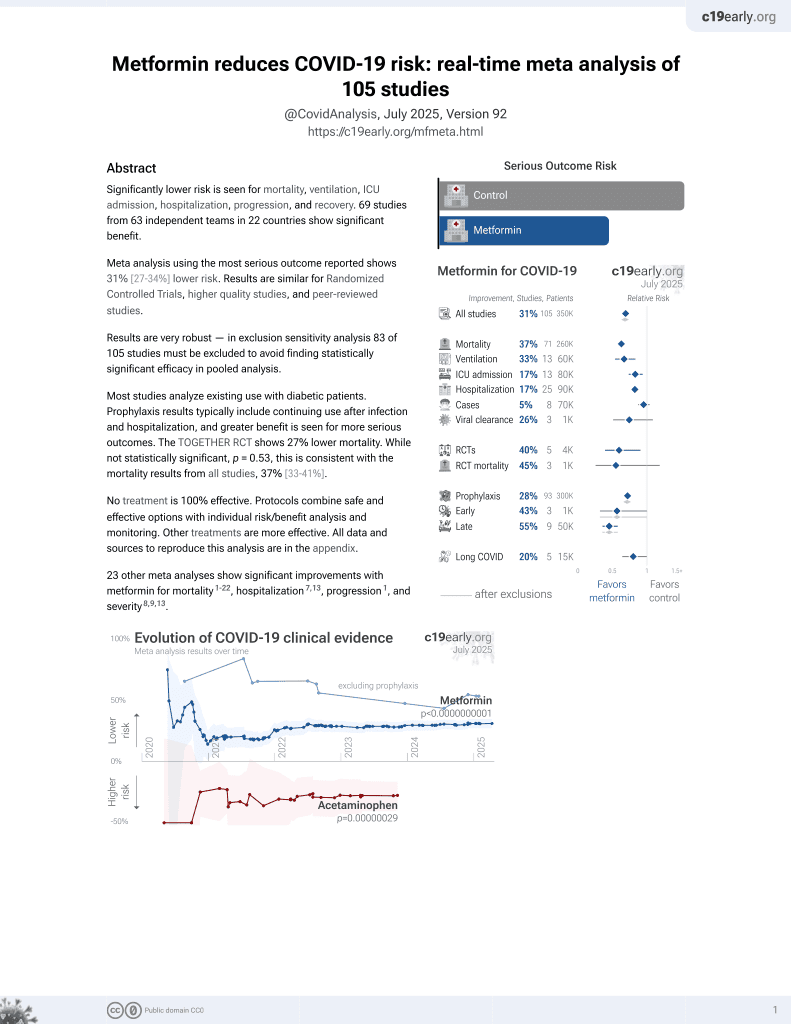
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
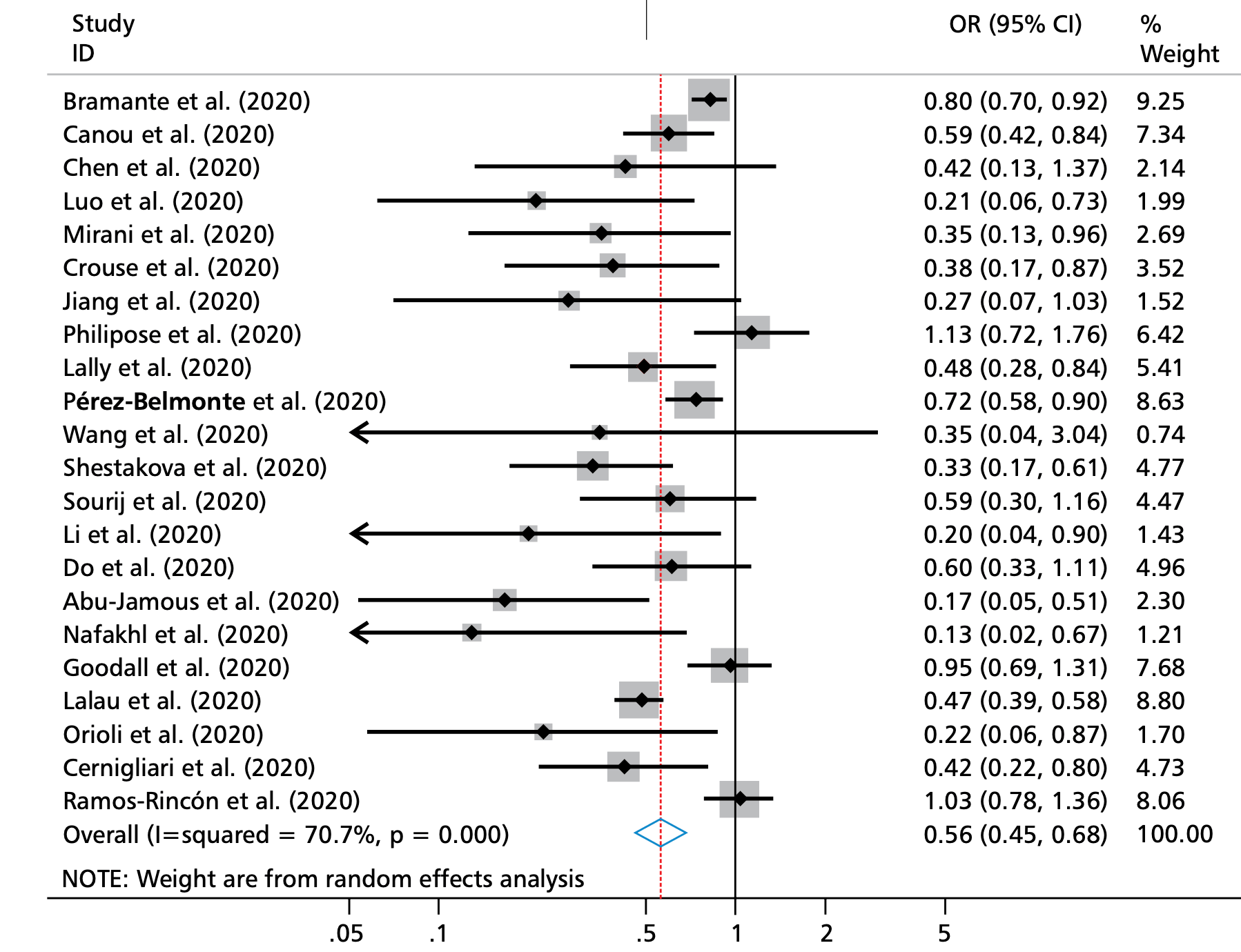
Meta analysis of 32 studies showing significantly lower COVID-19 mortality with metformin use.
24 meta-analyses show significant improvements with metformin for mortality1-23,
hospitalization7,13,23 ,
progression1, and
severity8,9,13 .
Currently there are 110 metformin for COVID-19 studies, showing 36% lower mortality [32‑40%], 29% lower ventilation [12‑43%], 19% lower ICU admission [8‑28%], 17% lower hospitalization [11‑23%], and 5% fewer cases [-4‑13%].
|
risk of death, 44.0% lower, OR 0.56, p < 0.001, RR approximated with OR.
|
|
risk of severe case, 15.0% lower, OR 0.85, p = 0.08, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Yang et al., The effect of metformin on mortality and severity in COVID-19 patients with diabetes mellitus, Diabetes Research and Clinical Practice, doi:10.1016/j.diabres.2021.108977.
2.
Lukito et al., The Effect of Metformin Consumption on Mortality in Hospitalized COVID-19 patients: a systematic review and meta-analysis, Diabetes & Metabolic Syndrome: Clinical Research & Reviews, doi:10.1016/j.dsx.2020.11.006.
3.
Kow et al., Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: A meta-analysis, Journal of Medical Virology, doi:10.1002/jmv.26498.
4.
Hariyanto et al., Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection, Obesity Medicine, doi:10.1016/j.obmed.2020.100290.
5.
Ma et al., Is metformin use associated with low mortality in patients with type 2 diabetes mellitus hospitalized for COVID-19? a multivariable and propensity score-adjusted meta-analysis, PLOS ONE, doi:10.1371/journal.pone.0282210.
6.
Parveen et al., Association of Metformin with Mortality in COVID-19 Patients: A Systematic Review and Meta-Analysis, Annals of the National Academy of Medical Sciences (India), doi:10.1055/s-0042-1760353.
7.
Li et al., Metformin in Patients With COVID-19: A Systematic Review and Meta-Analysis, Frontiers in Medicine, doi:10.3389/fmed.2021.704666.
8.
Schlesinger et al., Risk phenotypes of diabetes and association with COVID-19 severity and death: an update of a living systematic review and meta-analysis, Diabetologia, doi:10.1007/s00125-023-05928-1.
9.
Petrelli et al., Metformin and Covid-19: a systematic review of systematic reviews with meta-analysis, Acta Biomedica Atenei Parmensis, doi:10.23750/abm.v94iS3.14405.
10.
Oscanoa et al., Metformin therapy and severity and mortality of SARS-CoV-2 infection: a meta-analysis, Clinical Diabetology, doi:10.5603/DK.a2021.0035.
11.
Kan et al., Mortality Risk of Antidiabetic Agents for Type 2 Diabetes With COVID-19: A Systematic Review and Meta-Analysis, Frontiers in Endocrinology, doi:10.3389/fendo.2021.708494.
12.
Poly et al., Metformin Use Is Associated with Decreased Mortality in COVID-19 Patients with Diabetes: Evidence from Retrospective Studies and Biological Mechanism, Journal of Clinical Medicine, doi:10.3390/jcm10163507.
13.
Song et al., The Effect of Antihyperglycemic Medications on COVID-19: A Meta-analysis and Systematic Review from Observational Studies, Therapeutic Innovation & Regulatory Science, doi:10.1007/s43441-024-00633-6.
14.
Ganesh et al., Does metformin affect outcomes in COVID‐19 patients with new or pre‐existing diabetes mellitus? A systematic review and meta‐analysis, British Journal of Clinical Pharmacology, doi:10.1111/bcp.15258.
15.
Nassar et al., Noninsulin‐based antihyperglycemic medications in patients with diabetes and COVID‐19: A systematic review and meta‐analysis, Journal of Diabetes, doi:10.1111/1753-0407.13359.
16.
Zhan et al., Effect of Antidiabetic Therapy on Clinical Outcomes of COVID-19 Patients With Type 2 Diabetes: A Systematic Review and Meta-Analysis, Annals of Pharmacotherapy, doi:10.1177/10600280221133577.
17.
Nguyen et al., Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: A meta-analysis, Metabolism, doi:10.1016/j.metabol.2022.155196.
18.
Han et al., Association Between Anti-diabetic Agents and Clinical Outcomes of COVID-19 in Patients with Diabetes: A Systematic Review and Meta-Analysis, Archives of Medical Research, doi:10.1016/j.arcmed.2021.08.002.
19.
Chen et al., The Association Between Antidiabetic Agents and Clinical Outcomes of COVID-19 Patients With Diabetes: A Bayesian Network Meta-Analysis, Frontiers in Endocrinology, doi:10.3389/fendo.2022.895458.
20.
Scheen, A., Metformin and COVID-19: From cellular mechanisms to reduced mortality, Diabetes & Metabolism, doi:10.1016/j.diabet.2020.07.006.
21.
Sun et al., Is Metformin Use Associated With a Decreased Mortality for COVID-19 Diabetic Patients? A Meta-Analysis, Journal of the Endocrine Society, doi:10.1210/jendso/bvab048.709.
Oscanoa et al., 24 May 2021, peer-reviewed, 5 authors.
Contact: tjoscanoae@gmail.com, toscanoae@usmp.pe.
Metformin therapy and severity and mortality of SARS-CoV-2 infection: a meta-analysis
Clinical Diabetology, doi:10.5603/dk.a2021.0035
background. It has been postulated that metformin could have anti-SARS-CoV-2 action. This raises the hypothesis that people who take metformin may have lower SARS-CoV-2 severity and/or mortality. Objectives. To conduct a meta-analysis of the association between the use of Metformin and risk of severity and mortality in SARS-CoV-2 infection. Methods. we searched PubMed, EMbASE, google scholar, the Cochrane Database of Systematic Reviews and preprint servers (medRxiv and Research Square) for studies published between December 2019 and January 2021. Data was extracted on study location, year of publication, design, number of participants, sex, age at baseline, body mass index, and exposure and outcome definition. Effect statistics were pooled using random effects models with 95% confidence intervals (CI). The quality of included studies was assessed with the newcastle-Ottawa Scale (nOS). Results. Thirty-two observational studies were included, combining to a total sample of 44306 participants. The mean nOS score of included studies was 7.9. Results suggested that metformin use was associated with a reduced risk of SARS-CoV-2 mortality (OR = 0.56, 95% CI: 0.46-0.68, P < 0.001; 22 studies) but not with disease severity (OR = 0.85, 95% CI: 0.71-1.02, P = 0.077; 15 studies). In the subgroup analysis, metformin reduces the risk of mortality (OR = 0.69, 95% CI: 0.55-0.88; P = 0.002) and severity (OR = 0.83, 95% CI: 0.70-0.97, P = 0.023) in patients aged 70 and above. Conclusions. The use of metformin was associated to lower risk of mortality from SARS-CoV-2 infection. This association does not imply causation and further research is required to clarify potential mechanisms.
Conflict of interest None.
Appendix 1
MEDlInE search strategy We searched the NCBI and Medline databases for potentially eligible records. The search terms were as follows:
References
Abu-Jamous, Anisimovich, Baxter, Associations of comorbidities and medications with COVID-19 outcome: A retrospective analysis of real-world evidence data, doi:10.1101/2020.08.20.20174169
Akter, Mannan, Mehedi, Clinical manifestations along with biochemical and psychological outcomes of COVID-19 cases in diabetic individuals in Bangladesh, doi:10.1101/2020.09.24.20200790
Amin, Lux, 'callaghan, The journey of metformin from glycaemic control to mTOR inhibition and the suppression of tumour growth, Br J Clin Pharmacol, doi:10.1111/bcp.13780
Bharath, Agrawal, Mccambridge, Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation, Cell Metab, doi:10.1016/j.cmet.2020.04.015
Bo, Van Oekelen, Mouhieddine, A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward, J Hematol Oncol, doi:10.1186/s13045-020-00934-x
Bornstein, Rubino, Khunti, Practical recommendations for the management of diabetes in patients with COVID-19, The Lancet Diabetes & Endocrinology, doi:10.1016/s2213-8587(20)30152-2
Bramante, Ingraham, Murray, Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis, The Lancet Healthy Longevity, doi:10.1016/s2666-7568(20)30033-7
Cariou, Hadjadj, Wargny, Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study, Diabetologia, doi:10.1007/s00125-020-05180-x
Cernigliaro, Allotta, Scondotto, Can diabetes and its related hypoglycemic drug treatment be considered risk factors for health outcomes in COVID-19 patients? The results of a study in the population residing in Sicily Region (Southern Italy), Epidemiol Prev, doi:10.19191/EP20.5-6.S2.132
Chen, Gu, Guan, Metformin might inhibit virus through increasing insulin sensitivity, Chin Med J (Engl), doi:10.4103/0366-6999.223856
Chen, Guo, Li, Immunomodulatory and antiviral activity of metformin and its potential implications in treating coronavirus disease 2019 and lung injury, Front Immunol, doi:10.3389/fimmu.2020.02056
Chen, Jiang, Li, Association of metformin with mortality or ARDS in patients with COVID-19 and type 2 diabetes: A retrospective cohort study, Diabetes Res Clin Pract, doi:10.1016/j.diabres.2020.108619
Chen, Yang, Yang, Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication, Diabetes Care, doi:10.2337/dc20-0660
Cheng, He, Jung, Suppression of Kaposi's sarcomaassociated herpesvirus infection and replication by 5'-ampactivated protein kinase, J Virol, doi:10.1128/JVI.00624-16
Crouse, Grimes, Li, Metformin use is associated with reduced mortality in a diverse population with COVID-19 and diabetes, Front Endocrinol (Lausanne), doi:10.3389/fendo.2020.600439
Davies, Da, Fradkin, Management of hyperglycaemia in type 2 diabetes, doi:10.1007/s00125-018-4729-5
Dersimonian, Laird, Meta-analysis in clinical trials, Clinical Trials, doi:10.1016/0197-2456
Do, Kim, Park, Is there an association between metformin use and clinical outcomes in diabetes patients with COVID-19? Diabetes Metab, doi:10.1016/j.diabet.2020.10.006
Esam, A proposed mechanism for the possible therapeutic potential of Metformin in COVID-19, Diabetes Res Clin Pract, doi:10.1016/j.diabres.2020.108282
Gao, Liu, Zhong, Risk of metformin in patients with type 2 diabetes with COVID-19: a preliminary retrospective report, Clin Transl Sci, doi:10.1111/cts.12897
Gheblawi, Wang, Viveiros, Angiotensin-Converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2, Circ Res, doi:10.1161/CIRCRESAHA.120.317015
Glossmann, Lutz, Metformin and aging: a review, Gerontology, doi:10.1159/000502257
Goodall, Reed, Ardissino, Risk factors for severe disease in patients admitted with COVID-19 to a hospital in London, England: a retrospective cohort study, Epidemiol Infect, doi:10.1017/S0950268820002472
Hariyanto, Kurniawan, Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COV-ID-19) infection, Obes Med, doi:10.1016/j.obmed.2020.100290
Higgins, Altman, Gøtzsche, The Cochrane Collaboration's tool for assessing risk of bias in randomised trials, BMJ, doi:10.1136/bmj.d5928
Hippisley-Cox, Young, Coupland, Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people, Heart, doi:10.1136/heartjnl-2020-317393
Izzi-Engbeaya, Distaso, Amin, Severe COVID-19 and diabetes -a retrospective cohort study from three london teaching hospitals. medRxiv, doi:10.1101/2020.08.07.20160275
Kim, You, Regulation of organelle function by metformin, IUBMB Life, doi:10.1002/iub.1633
Lalau, Al-Salameh, Hadjadj, Metformin use is associated with a reduced risk of mortality in patients with diabetes hospitalised for COVID-19, Diabetes Metab, doi:10.1016/j.diabet.2020.101216
Lalau, Lacroix, Measurement of metformin concentration in erythrocytes: clinical implications, Diabetes Obes Metab, doi:10.1046/j.1463-1326.2003.00241.x
Lally, Tsoukas, Halladay, Metformin is associated with decreased 30-day mortality among nursing home residents infected with SARS-CoV-2, J Am Med Dir Assoc, doi:10.1016/j.jamda.2020.10.031
Lasbleiz, Cariou, Darmon, Phenotypic characteristics and development of a hospitalization prediction risk score for outpatients with diabetes and COVID-19: the DIABCOVID study, J Clin Med, doi:10.3390/jcm9113726
Li, Han, Xiao, Metformin sensitizes EGFR-TKI-resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal, Clin Cancer Res, doi:10.1158/1078-0432.CCR-13-2613
Li, Qi, Li, Metformin use in diabetes prior to hospitalization: effects on mortality in covid-19, Endocr Pract, doi:10.4158/EP-2020-0466
Liang, Ding, Li, Association of preadmission metformin use and mortality in patients with sepsis and diabetes mellitus: a systematic review and meta-analysis of cohort studies, Crit Care, doi:10.1186/s13054-019-2346-4
Liu, Xi, Han, The association of diabetes and the prognosis of COVID-19 patients: A retrospective study, Diabetes Res Clin Pract, doi:10.1016/j.dia-bres.2020.108386
Lukito, Pranata, Henrina, The effect of metformin consumption on mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis, Diabetes Metab Syndr, doi:10.1016/j.dsx.2020.11.006
Luo, Qiu, Yi, Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis, Am J Trop Med Hyg, doi:10.4269/ajtmh.20-0375
Mik, Jeon, Kim, The clinical characteristics and outcomes of patients with moderate-to-severe coronavirus disease 2019 infection and diabetes in Daegu, South Korea, Diabetes Metab J, doi:10.4093/dmj.2020.0146
Mirani, Favacchio, Carrone, Impact of comorbidities and glycemia at admission and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes with COVID-19: a case series from an Academic Hospital in Lombardy, Italy. Diabetes Care, doi:10.2337/dc20-1340
Mishra, Dingli, Metformin inhibits IL-6 signaling by decreasing IL-6R expression on multiple myeloma cells, Leukemia, doi:10.1038/s41375-019-0470-4
Moher, Liberati, Tetzlaff, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, Int J Surg, doi:10.1016/j.ijsu.2010.02.007
Mueller, Mcnamara, Sinclair, Why does COVID-19 disproportionately affect older people?, Aging (Albany NY), doi:10.18632/aging.103344
Nafakhi, Alareedh, Al-Buthabhak, Predictors of adverse in-hospital outcome and recovery in patients with diabetes mellitus and COVID-19 pneumonia in Iraq, Diabetes Metab Syndr, doi:10.1016/j.dsx.2020.12.014
Orioli, Servais, Belkhir, Clinical characteristics and short-term prognosis of in-patients with diabetes and COVID-19: A retrospective study from an academic center in Belgium, Diabetes Metab Syndr, doi:10.1016/j.dsx.2020.12.020
Oscanoa, Romero-Ortuno, Carvajal, A pharmacological perspective of chloroquine in SARS-CoV-2 infection: An old drug for the fight against a new coronavirus, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.106078
Oscanoa, Vidal, Kanters, Frequency of long QT in patients with sars-cov-2 infection treated with hydroxychloroquine: a meta-analysis, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.106212
Pan, Han, He, Metformin: one of the possible options to reduce the mortality of severe coronavirus disease 2019? Zhonghua Wei Zhong Bing Ji Jiu Yi Xue, doi:10.3760/cma.j.cn121430-20200514-00662
Philipose, Smati, Wong, Obesity, old age and frailty are the true risk factors for COVID-19 mortality and not chronic disease or ethnicity in Croydon, doi:10.1101/2020.08.12.20156257
Pijls, Jolani, Atherley, Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a metaanalysis of 59 studies, BMJ Open, doi:10.1136/bmjopen-2020-044640
Plattner, Bibb, Serine and threonine phosphorylation, doi:10.1016/b978-0-12-374947-5.00025-0
Pérez-Belmonte, Torres-Peña, Md, Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: a nationwide cohort study, BMC Med, doi:10.1186/s12916-020-01832-2
Ramos-Rincón, Pérez-Belmonte, Carrasco-Sánchez, Association between prior cardiometabolic therapy and in-hospital mortality in very old patients with type 2 diabetes mellitus hospitalized due to COVID-19. A nationwide observational study in Spain, doi:10.21203/rs.3.rs-133358/v1
Rangarajan, Bone, Zmijewska, Metformin reverses established lung fibrosis in a bleomycin model, Nat Med, doi:10.1038/s41591-018-0087-6
Rena, Hardie, Pearson, The mechanisms of action of metformin, Diabetologia, doi:10.1007/s00125-017-4342-z
Robert, Fendri, Hary, Kinetics of plasma and erythrocyte metformin after acute administration in healthy subjects, Diabetes Metab, doi:10.1016/s1262-3636(07)70037-x
Scheen, Metformin and COVID-19: From cellular mechanisms to reduced mortality, Diabetes Metab, doi:10.1016/j.diabet.2020.07.006
Schlesinger, Neuenschwander, Lang, Risk phenotypes of diabetes and association with COVID-19 severity and death -a living systematic review and meta-analysis, SSRN Electronic Journal, doi:10.2139/ssrn.3730026
Sharma, Ray, Sadasivam, Metformin in COVID-19: A possible role beyond diabetes, Diabetes Res Clin Pract, doi:10.1016/j.diabres.2020.108183
Shenoy, Coronavirus, Covid-19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality, Inflamm Res, doi:10.1007/s00011-020-01389-z
Shestakova, Vikulova, Isakov, Diabetes and COV-ID-19: analysis of the clinical outcomes according to the data of the russian diabetes registry, Probl Endokrinol (Mosk), doi:10.14341/probl12458
Shytaj, Procopio, Tarek, Glycolysis downregulation is a hallmark of HIV-1 latency and sensitizes infected cells to oxidative stress, bioRxiv, doi:10.1101/2020.12.30.424810
Soberanes, Misharin, Jairaman, Metformin targets mitochondrial electron transport to reduce air-pollution-induced thrombosis, Cell Metab, doi:10.1016/j.cmet.2018.09.019
Soto-Acosta, Bautista-Carbajal, Cervantes-Salazar, DENV up-regulates the HMG-CoA reductase activity through the impairment of AMPK phosphorylation: A potential antiviral target, PLoS Pathog, doi:10.1371/journal.ppat.1006257
Sourij, Aziz, Bräuer, COVID-19 fatality prediction in people with diabetes and prediabetes using a simple score upon hospital admission, Diabetes Obes Metab, doi:10.1111/dom.14256
Stang, Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in metaanalyses, Eur J Epidemiol, doi:10.1007/s10654-010-9491-z
Thomas, Gregg, Metformin; a review of its history and future: from lilac to longevity, Pediatr Diabetes, doi:10.1111/pedi.12473
Tsaknis, Siempos, Kopterides, Metformin attenuates ventilator-induced lung injury, Crit Care, doi:10.1186/cc11439
Uddin, Akhter, Kubra, Metformin in acute respiratory distress syndrome: An opinion, Exp Gerontol, doi:10.1016/j.exger.2020.111197
Vachharajani, Liu, Brown, SIRT1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome, J Leukoc Biol, doi:10.1189/jlb.3MA0114-034RR
Valle-Casuso, Angin, Volant, Cellular metabolism is a major determinant of HIV-1 reservoir seeding in CD4 t cells and offers an opportunity to tackle infection, Cell Metab, doi:10.1016/j.cmet.2018.11.015
Wu, Cen, Feng, Metformin activates the protective effects of the AMPK pathway in acute lung injury caused by paraquat poisoning, Oxid Med Cell Longev, doi:10.1155/2019/1709718
Wu, Tian, Huang, Metformin alleviated endotoxemiainduced acute lung injury via restoring AMPK-dependent suppression of mTOR, Chem Biol Interact, doi:10.1016/j.cbi.2018.05.018
Xiang, Wong, So, Exploring drugs and vaccines associated with altered risks and severity of COVID-19: a UK Biobank cohort study of all ATC level-4 drug categories. medRxiv, doi:10.1101/2020.12.05.20244426
Xie, Wang, Dai, Activation of AMPK restricts coxsackievirus B3 replication by inhibiting lipid accumulation, J Mol Cell Cardiol, doi:10.1016/j.yjmcc.2015.05.021
Xu, Du, Zheng, Effect of metformin on serum interleukin-6 levels in polycystic ovary syndrome: a systematic review, BMC Womens Health, doi:10.1186/1472-6874-14-93
Xu, Liu, Li, Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID-19 and pre-existing type 2 diabetes, Cell Metab, doi:10.1016/j.cmet.2020.08.013
Yan, Valdes, Vijay, Role of drugs used for chronic disease management on susceptibility and severity of COVID-19: a large case-control study. medRxiv, doi:10.1101/2020.04.24.20077875
Zhang, Kong, Xia, Impaired fasting glucose and diabetes are related to higher risks of complications and mortality among patients with coronavirus disease, Front Endocrinol (Lausanne), doi:10.3389/fendo.2020.00525
Zhang, Li, Ma, Metformin activates AMPK through the lysosomal pathway, Cell Metab, doi:10.1016/j.cmet.2016.09.003
Zhang, Shang, Hui, The alleviative effects of metformin for lipopolysaccharide-induced acute lung injury rat model and its underlying mechanism, Saudi Pharm J, doi:10.1016/j.jsps.2017.05.001
Zhang, Zheng, Yan, Angiotensin-converting enzyme 2 regulates autophagy in acute lung injury through AMPK/ mTOR signaling, Arch Biochem Biophys, doi:10.1016/j.abb.2019.07.026
Zmijewski, Zhao, Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury, Am J Respir Crit Care Med, doi:10.1164/rccm.200710-1602OC
DOI record:
{
"DOI": "10.5603/dk.a2021.0035",
"ISSN": [
"2450-8187",
"2450-7458"
],
"URL": "http://dx.doi.org/10.5603/DK.a2021.0035",
"author": [
{
"affiliation": [],
"family": "Oscanoa",
"given": "Teodoro J.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Amado",
"given": "José",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vidal",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Savarino",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romero-Ortuno",
"given": "Roman",
"sequence": "additional"
}
],
"container-title": "Clinical Diabetology",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
5,
25
]
],
"date-time": "2021-05-25T10:36:00Z",
"timestamp": 1621938960000
},
"deposited": {
"date-parts": [
[
2021,
5,
25
]
],
"date-time": "2021-05-25T10:36:03Z",
"timestamp": 1621938963000
},
"indexed": {
"date-parts": [
[
2022,
3,
29
]
],
"date-time": "2022-03-29T01:53:46Z",
"timestamp": 1648518826218
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2021,
5,
24
]
]
},
"link": [
{
"URL": "https://journals.viamedica.pl/clinical_diabetology/article/viewFile/75056/62969",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "3595",
"original-title": [],
"prefix": "10.5603",
"published": {
"date-parts": [
[
2021,
5,
24
]
]
},
"published-online": {
"date-parts": [
[
2021,
5,
24
]
]
},
"publisher": "VM Media SP. zo.o VM Group SK",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.viamedica.pl/clinical_diabetology/article/view/75056"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Endocrinology, Diabetes and Metabolism",
"Internal Medicine"
],
"subtitle": [],
"title": "Metformin therapy and severity and mortality of SARS-CoV-2 infection: a meta-analysis",
"type": "journal-article"
}