Metformin Use Is Associated with Decreased Mortality in COVID-19 Patients with Diabetes: Evidence from Retrospective Studies and Biological Mechanism
et al., Journal of Clinical Medicine, doi:10.3390/jcm10163507, Aug 2021
Metformin for COVID-19
3rd treatment shown to reduce risk in
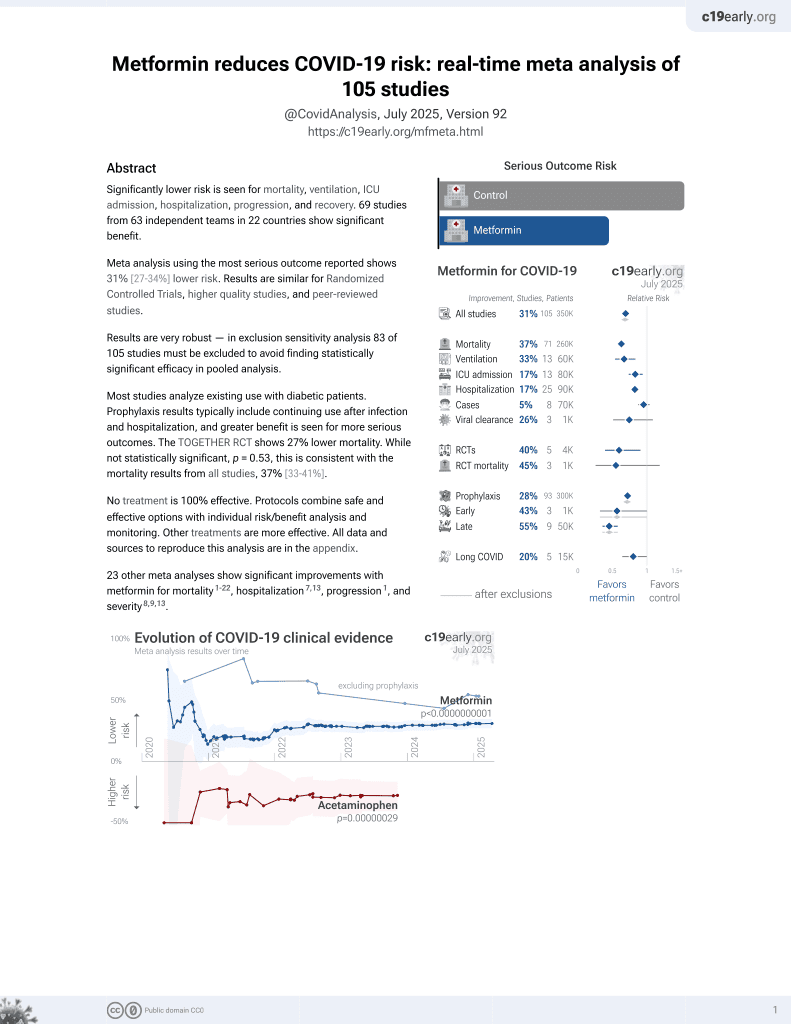
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
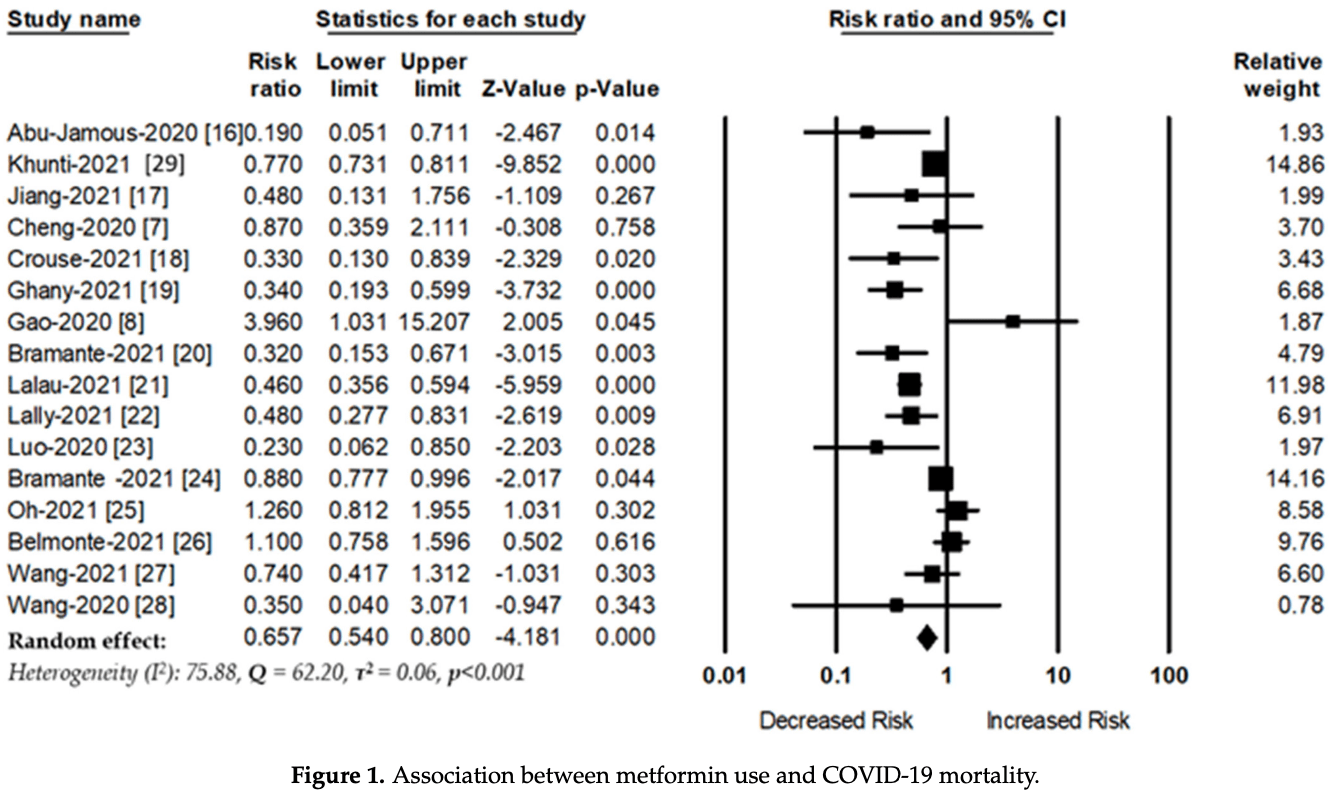
Meta analysis of 16 studies of diabetic patients showing significantly lower COVID-19 mortality with metformin use.
24 meta-analyses show significant improvements with metformin for mortality1-23,
hospitalization7,13,23 ,
progression1, and
severity8,9,13 .
Currently there are 110 metformin for COVID-19 studies, showing 36% lower mortality [32‑40%], 29% lower ventilation [12‑43%], 19% lower ICU admission [8‑28%], 17% lower hospitalization [11‑23%], and 5% fewer cases [-4‑13%].
|
risk of death, 34.3% lower, RR 0.66, p < 0.001.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Yang et al., The effect of metformin on mortality and severity in COVID-19 patients with diabetes mellitus, Diabetes Research and Clinical Practice, doi:10.1016/j.diabres.2021.108977.
2.
Lukito et al., The Effect of Metformin Consumption on Mortality in Hospitalized COVID-19 patients: a systematic review and meta-analysis, Diabetes & Metabolic Syndrome: Clinical Research & Reviews, doi:10.1016/j.dsx.2020.11.006.
3.
Kow et al., Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: A meta-analysis, Journal of Medical Virology, doi:10.1002/jmv.26498.
4.
Hariyanto et al., Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection, Obesity Medicine, doi:10.1016/j.obmed.2020.100290.
5.
Ma et al., Is metformin use associated with low mortality in patients with type 2 diabetes mellitus hospitalized for COVID-19? a multivariable and propensity score-adjusted meta-analysis, PLOS ONE, doi:10.1371/journal.pone.0282210.
6.
Parveen et al., Association of Metformin with Mortality in COVID-19 Patients: A Systematic Review and Meta-Analysis, Annals of the National Academy of Medical Sciences (India), doi:10.1055/s-0042-1760353.
7.
Li et al., Metformin in Patients With COVID-19: A Systematic Review and Meta-Analysis, Frontiers in Medicine, doi:10.3389/fmed.2021.704666.
8.
Schlesinger et al., Risk phenotypes of diabetes and association with COVID-19 severity and death: an update of a living systematic review and meta-analysis, Diabetologia, doi:10.1007/s00125-023-05928-1.
9.
Petrelli et al., Metformin and Covid-19: a systematic review of systematic reviews with meta-analysis, Acta Biomedica Atenei Parmensis, doi:10.23750/abm.v94iS3.14405.
10.
Oscanoa et al., Metformin therapy and severity and mortality of SARS-CoV-2 infection: a meta-analysis, Clinical Diabetology, doi:10.5603/DK.a2021.0035.
11.
Kan et al., Mortality Risk of Antidiabetic Agents for Type 2 Diabetes With COVID-19: A Systematic Review and Meta-Analysis, Frontiers in Endocrinology, doi:10.3389/fendo.2021.708494.
12.
Poly et al., Metformin Use Is Associated with Decreased Mortality in COVID-19 Patients with Diabetes: Evidence from Retrospective Studies and Biological Mechanism, Journal of Clinical Medicine, doi:10.3390/jcm10163507.
13.
Song et al., The Effect of Antihyperglycemic Medications on COVID-19: A Meta-analysis and Systematic Review from Observational Studies, Therapeutic Innovation & Regulatory Science, doi:10.1007/s43441-024-00633-6.
14.
Ganesh et al., Does metformin affect outcomes in COVID‐19 patients with new or pre‐existing diabetes mellitus? A systematic review and meta‐analysis, British Journal of Clinical Pharmacology, doi:10.1111/bcp.15258.
15.
Nassar et al., Noninsulin‐based antihyperglycemic medications in patients with diabetes and COVID‐19: A systematic review and meta‐analysis, Journal of Diabetes, doi:10.1111/1753-0407.13359.
16.
Zhan et al., Effect of Antidiabetic Therapy on Clinical Outcomes of COVID-19 Patients With Type 2 Diabetes: A Systematic Review and Meta-Analysis, Annals of Pharmacotherapy, doi:10.1177/10600280221133577.
17.
Nguyen et al., Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: A meta-analysis, Metabolism, doi:10.1016/j.metabol.2022.155196.
18.
Han et al., Association Between Anti-diabetic Agents and Clinical Outcomes of COVID-19 in Patients with Diabetes: A Systematic Review and Meta-Analysis, Archives of Medical Research, doi:10.1016/j.arcmed.2021.08.002.
19.
Chen et al., The Association Between Antidiabetic Agents and Clinical Outcomes of COVID-19 Patients With Diabetes: A Bayesian Network Meta-Analysis, Frontiers in Endocrinology, doi:10.3389/fendo.2022.895458.
20.
Scheen, A., Metformin and COVID-19: From cellular mechanisms to reduced mortality, Diabetes & Metabolism, doi:10.1016/j.diabet.2020.07.006.
21.
Sun et al., Is Metformin Use Associated With a Decreased Mortality for COVID-19 Diabetic Patients? A Meta-Analysis, Journal of the Endocrine Society, doi:10.1210/jendso/bvab048.709.
Poly et al., 9 Aug 2021, Taiwan, peer-reviewed, 6 authors.
Contact: vkwang8888@yahoo.com.tw (corresponding author), d610108004@tmu.edu.tw, d610106004@tmu.edu.tw, jack@tmu.edu.tw, arbiter@tmu.edu.tw, 701056@tmu.edu.tw.
Metformin Use Is Associated with Decreased Mortality in COVID-19 Patients with Diabetes: Evidence from Retrospective Studies and Biological Mechanism
Journal of Clinical Medicine, doi:10.3390/jcm10163507
Background and Aims: The coronavirus disease 2019 (COVID-19) increases hyperinflammatory state, leading to acute lung damage, hyperglycemia, vascular endothelial damage, and a higher mortality rate. Metformin is a first-line treatment for type 2 diabetes and is known to have anti-inflammatory and immunosuppressive effects. Previous studies have shown that metformin use is associated with decreased risk of mortality among patients with COVID-19; however, the results are still inconclusive. This study investigated the association between metformin and the risk of mortality among diabetes patients with COVID-19. Methods: Data were collected from online databases such as PubMed, EMBASE, Scopus, and Web of Science, and reference from the most relevant articles. The search and collection of relevant articles was carried out between 1 February 2020, and 20 June 2021. Two independent reviewers extracted information from selected studies. The randomeffects model was used to estimate risk ratios (RRs), with a 95% confidence interval. Results: A total of 16 studies met all inclusion criteria. Diabetes patients given metformin had a significantly reduced risk of mortality (RR, 0.65; 95% CI: 0.54-0.80, p < 0.001, heterogeneity I 2 = 75.88, Q = 62.20, and τ 2 = 0.06, p < 0.001) compared with those who were not given metformin. Subgroup analyses showed that the beneficial effect of metformin was higher in the patients from North America (RR, 0.43; 95% CI: 0.26-0.72, p = 0.001, heterogeneity I 2 = 85.57, Q = 34.65, τ 2 = 0.31) than in patients from Europe (RR, 0.67; 95% CI: 0.47-0.94, p = 0.02, heterogeneity I 2 = 82.69, Q = 23.11, τ 2 = 0.10) and Asia (RR, 0.90; 95% CI: 0.43-1.86, p = 0.78, heterogeneity I 2 = 64.12, Q = 11.15, τ 2 = 0.40). Conclusions: This meta-analysis shows evidence that supports the theory that the use of metformin is associated with a decreased risk of mortality among diabetes patients with COVID-19. Randomized control trials with a higher number of participants are warranted to assess the effectiveness of metformin for reducing the mortality of COVID-19 patients.
Supplementary Materials: The following are available online at https://www.mdpi.com/article/10.3 390/jcm10163507/s1, Figure S1 : Searching strategy, Figure S2 : Metformin use and the risk of mortality of patients with COVID-19 (Studies included metformin users less than 1000 participants), Figure S3 : Metformin use and the risk of mortality of patients with COVID-19 (Studies included metformin users between 1000 and 10,000 participants), Figure S4 : Metformin use and the risk of mortality of patients with COVID-19 (Studies included metformin users more than 10,000 participants).
Conflicts of Interest: The authors declare no conflict of interest.
References
Abu-Jamous, Anisimovich, Baxter, Mackillop, Vizcaychipi et al., Associations of comorbidities and medications with COVID-19 outcome: A retrospective analysis of real-world evidence data, medRxiv, doi:10.1101/2020.08.20.20174169
Algire, Moiseeva, Deschênes-Simard, Amrein, Petruccelli et al., Metformin Reduces Endogenous Reactive Oxygen Species and Associated DNA Damage, Cancer Prev. Res, doi:10.1158/1940-6207.CAPR-11-0536
Andrzejewski, Siegel, St-Pierre, Metabolic profiles associated with metformin efficacy in cancer, Front. Endocrinol, doi:10.3389/fendo.2018.00372
Ansari, Mojtahedzadeh, Kajbaf, Najafi, Khajavi et al., How does blood glucose control with metformin influence intensive insulin protocols? Evidence for involvement of oxidative stress and inflammatory cytokines, Adv. Ther, doi:10.1007/s12325-008-0075-1
Borges, Fujihara, Malheiros, De Ávila, Formigari et al., Metformin arrests the progression of established kidney disease in the subtotal nephrectomy model of chronic kidney disease, Am. J. Physiol. Physiol, doi:10.1152/ajprenal.00539.2019
Bramante, Buse, Tamaritz, Palacio, Cohen et al., Outpatient metformin use is associated with reduced severity of COVID-19 disease in adults with overweight or obesity, J. Med. Virol, doi:10.1002/jmv.26873
Bramante, Ingraham, Murray, Marmor, Hovertsen et al., Metformin and risk of mortality in patients hospitalised with COVID-19: A retrospective cohort analysis, Lancet Health Longev, doi:10.1016/S2666-7568(20)30033-7
Cai, Ji, Treatment response between Asian and non-Asian patients with type 2 diabetes: Is there any similarity or difference?, Chin. Med. J, doi:10.1097/CM9.0000000000000012
Ceriello, De Nigris, Prattichizzo, Why is hyperglycaemia worsening COVID-19 and its prognosis?, Diabetes Obes. Metab, doi:10.1111/dom.14098
Ceriello, Hyperglycemia and the worse prognosis of COVID-19. Why a fast blood glucose control should be mandatory, Diabetes Res. Clin. Pract, doi:10.1016/j.diabres.2020.108186
Chen, Gu, Guan, Metformin Might Inhibit Virus through Increasing Insulin Sensitivity, Chin. Med. J, doi:10.4103/0366-6999.223856
Chen, Liu, Ye, Effects of metformin on blood and urine pro-inflammatory mediators in patients with type 2 diabetes, J. Inflamm, doi:10.1186/s12950-016-0142-3
Cheng, Liu, Li, Zhang, Lei et al., Metformin Is Associated with Higher Incidence of Acidosis, but Not Mortality, in Individuals with COVID-19 and Pre-existing Type 2 Diabetes, Cell Metab, doi:10.1016/j.cmet.2020.08.013
Christensen, Schiffer, Gustafsson, Krag, Nørregaard et al., Metformin attenuates renal medullary hypoxia in diabetic nephropathy through inhibition uncoupling protein-2, Diabetes/Metab. Res. Rev, doi:10.1002/dmrr.3091
Crouse, Grimes, Li, Might, Ovalle et al., Metformin Use Is Associated with Reduced Mortality in a Diverse Population with COVID-19 and Diabetes, Front. Endocrinol, doi:10.3389/fendo.2020.600439
Dalan, Metformin, neutrophils and COVID-19 infection, Diabetes Res. Clin. Pract, doi:10.1016/j.diabres.2020.108230
Desai, Roman, Rochelson, Gupta, Xue et al., Maternal metformin treatment decreases fetal inflammation in a rat model of obesity and metabolic syndrome, Am. J. Obstet. Gynecol, doi:10.1016/j.ajog.2013.05.001
Di Xiao, Zhang, Liu, Yin, Han et al., A Two-Stage Study Identifies Two Novel Polymorphisms in PRKAG2 Affecting Metformin Response in Chinese Type 2 Diabetes Patients, Pharm. Pers. Med, doi:10.2147/PGPM.S305020
El-Arabey, Abdalla, Metformin and COVID-19: A novel deal of an old drug, J. Med. Virol, doi:10.1002/jmv.25958
Eskens, Zuurbier, Van Haare, Vink, Van Teeffelen, Effects of two weeks of metformin treatment on whole-body glycocalyx barrier properties in db/db mice, Cardiovasc. Diabetol, doi:10.1186/1475-2840-12-175
Foretz, Guigas, Viollet, Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus, Nat. Rev. Endocrinol, doi:10.1038/s41574-019-0242-2
Gao, Liu, Zhong, Liu, Zhou et al., Risk of Metformin in Patients with Type 2 Diabetes with COVID-19: A Preliminary Retrospective Report, Clin. Transl. Sci, doi:10.1111/cts.12897
Ghany, Palacio, Dawkins, Chen, Mccarter et al., Metformin is associated with lower hospitalizations, mortality and severe coronavirus infection among elderly medicare minority patients in 8 states in USA, Diabetes Metab. Syndr. Clin. Res. Rev, doi:10.1016/j.dsx.2021.02.022
Groen, Hamer, Snijders, Van Kranenburg, Frijns et al., Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes, J. Appl. Physiol, doi:10.1152/japplphysiol.00919.2013
Hariyanto, Kurniawan, Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection, Obes. Med, doi:10.1016/j.obmed.2020.100290
Hashimoto, Nomura, Shoda, Isobe, Kikuchi et al., Metformin increases urinary sodium excretion by reducing phosphorylation of the sodium-chloride cotransporter, Metabolism, doi:10.1016/j.metabol.2018.02.009
Hou, Song, Li, Zhang, Wang et al., Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2010.04.017
Islam, Iqbal, Walther, Atique, Dubey et al., Benzodiazepine Use and Risk of Dementia in the Elderly Population: A Systematic Review and Meta-Analysis, Neuroepidemiology, doi:10.1159/000454881
Isoda, Young, Zirlik, Macfarlane, Tsuboi et al., Metformin inhibits proinflammatory responses and nuclear factor-κB in human vascular wall cells, Arterioscler. Thromb. Vasc. Biol, doi:10.1161/01.ATV.0000201938.78044.75
Jiang, Chen, Liu, Yin, Yang et al., Association of metformin with mortality or ARDS in patients with COVID-19 and type 2 diabetes: A retrospective cohort study, Diabetes Res. Clin. Pr, doi:10.1016/j.diabres.2020.108619
Jiang, Ruan, -L.; Xue, Yang, Shi et al., Metformin reduces the senescence of renal tubular epithelial cells in diabetic nephropathy via the MBNL1/miR-130a-3p/STAT3 pathway, Oxidative Med. Cell. Longev, doi:10.1155/2020/8708236
Jing, Wu, Li, Yang, Li et al., Metformin improves obesity-associated inflammation by altering macrophages polarization, Mol. Cell. Endocrinol, doi:10.1016/j.mce.2017.09.025
Jung, Lee, Lee, Kim, Metformin prevents endoplasmic reticulum stress-induced apoptosis through AMPK-PI3K-c-Jun NH2 pathway, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2011.11.073
Kaneto, Kimura, Obata, Shimoda, Kaku, Multifaceted Mechanisms of Action of Metformin Which Have Been Unraveled One after Another in the Long History, Int. J. Mol. Sci, doi:10.3390/ijms22052596
Khunti, Knighton, Zaccardi, Bakhai, Barron et al., Prescription of glucose-lowering therapies and risk of COVID-19 mortality in people with type 2 diabetes: A nationwide observational study in England, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587(21)00050-4
Kow, Hasan, Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: A meta-analysis, J. Med. Virol, doi:10.1002/jmv.26498
Kumar, Arora, Sharma, Anikhindi, Bansal et al., Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis, Diabetes Metab. Syndr. Clin. Res. Rev, doi:10.1016/j.dsx.2020.04.044
Lalau, Al-Salameh, Hadjadj, Goronflot, Wiernsperger et al., Metformin use is associated with a reduced risk of mortality in patients with diabetes hospitalised for COVID-19, Diabetes Metab, doi:10.1016/j.diabet.2020.101216
Lally, Tsoukas, Halladay, O'neill, Gravenstein et al., Metformin is associated with decreased 30-day mortality among nursing home residents infected with SARS-CoV2, J. Am. Med Dir. Assoc, doi:10.1016/j.jamda.2020.10.031
Lippi, Wong, Henry, Hypertension and its severity or mortality in Coronavirus Disease 2019 (COVID-19): A pooled analysis, Pol. Arch. Intern. Med, doi:10.20452/pamw.15272
Lukito, Pranata, Henrina, Lim, Lawrensia et al., The Effect of Metformin Consumption on Mortality in Hospitalized COVID-19 patients: A systematic review and meta-analysis, Diabetes Metab. Syndr. Clin. Res. Rev, doi:10.1016/j.dsx.2020.11.006
Luo, Qiu, Liu, Liu, -L.; Zheng et al., Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis, Am. J. Trop. Med. Hyg, doi:10.4269/ajtmh.20-0375
Malhotra, Hepokoski, Mccowen, Shyy, Ace, Metformin, and COVID-19, iScience, doi:10.1016/j.isci.2020.101425
Moher, Liberati, Tetzlaff, Altman, The et al., Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement, PLoS Med, doi:10.1371/journal.pmed.1000097
Nesti, Natali, Metformin effects on the heart and the cardiovascular system: A review of experimental and clinical data, Nutr. Metab. Cardiovasc. Dis, doi:10.1016/j.numecd.2017.04.009
Oh, Song, Metformin use and risk of COVID-19 among patients with type II diabetes mellitus: An NHIS-COVID-19 database cohort study, Acta Diabetol, doi:10.1007/s00592-020-01666-7
Ouslimani, Peynet, Bonnefont-Rousselot, Thérond, Legrand et al., Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells, Metabolism, doi:10.1016/j.metabol.2005.01.029
Poly, Islam, Yang, Lin, Jian et al., Obesity and Mortality Among Patients Diagnosed With COVID-19: A Systematic Review and Meta-Analysis, Front. Med, doi:10.3389/fmed.2021.620044
Pranata, Huang, Lim, Wahjoepramono, July, Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19-systematic review, meta-analysis, and meta-regression, J. Stroke Cerebrovasc. Dis, doi:10.1016/j.jstrokecerebrovasdis.2020.104949
Pérez-Belmonte, Torres-Peña, López-Carmona, Ayala-Gutiérrez, Fuentes-Jiménez et al., Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: A nationwide cohort study, BMC Med, doi:10.1186/s12916-020-01832-2
Scheen, Metformin and COVID-19: From cellular mechanisms to reduced mortality, Diabetes Metab, doi:10.1016/j.diabet.2020.07.006
Seifarth, Schehler, Schneider, Effectiveness of Metformin on Weight Loss in Non-Diabetic Individuals with Obesity, Exp. Clin. Endocrinol. Diabetes, doi:10.1055/s-0032-1327734
Sharma, Ray, Sadasivam, Metformin in COVID-19: A possible role beyond diabetes, Diabetes Res. Clin. Pr, doi:10.1016/j.diabres.2020.108183
Siri, Dastghaib, Zamani, Rahmani-Kukia, Geraylow et al., Unfolded Protein Response, and Neuropilin-1 Cross-Talk in SARS-CoV-2 Infection: What Can Be Learned from Other Coronaviruses, Int. J. Mol. Sci, doi:10.3390/ijms22115992
Siska, Rathmell, T cell metabolic fitness in antitumor immunity, Trends Immunol, doi:10.1016/j.it.2015.02.007
Sturmlechner, Durik, Sieben, Baker, Van Deursen, Cellular senescence in renal ageing and disease, Nat. Rev. Nephrol, doi:10.1038/nrneph.2016.183
Sukumar, Liu, Ji, Subramanian, Crompton et al., Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function, J. Clin. Investig, doi:10.1172/JCI69589
Targosz-Korecka, Malek-Zietek, Kloska, Rajfur, Stepien et al., Metformin attenuates adhesion between cancer and endothelial cells in chronic hyperglycemia by recovery of the endothelial glycocalyx barrier, Biochim. Biophys. Acta (BBA) Gen. Subj, doi:10.1016/j.bbagen.2020.129533
Wang, Cooper, Gokhale, Acosta-Mena, Dhalla et al., Association of Metformin with Susceptibility to COVID-19 in People with Type 2 Diabetes, J. Clin. Endocrinol. Metab, doi:10.1210/clinem/dgab067
Wang, Horby, Hayden, Gao, A novel coronavirus outbreak of global health concern, Lancet, doi:10.1016/S0140-6736(20)30185-9
Wang, Van Oekelen, Mouhieddine, Del, Valle et al., A tertiary center experience of multiple myeloma patients with COVID-19: Lessons learned and the path forward, J. Hematol. Oncol, doi:10.1186/s13045-020-00934-x
Wu, Lee, Su, Huang, Islam et al., Statin Use Is Associated with a Decreased Risk of Mortality among Patients with COVID-19, J. Clin. Med, doi:10.3390/jcm10071450
Xu, Liu, Xu, Xing, Ye, Metformin alleviates renal injury in diabetic rats by inducing Sirt1/FoxO1 autophagic signal axis, Clin. Exp. Pharmacol. Physiol, doi:10.1111/1440-1681.13226
Yamaoka-Tojo, Endothelial glycocalyx damage as a systemic inflammatory microvascular endotheliopathy in COVID-19, Biomed. J, doi:10.1016/j.bj.2020.08.007
Yao, Ye, Zhang, Cui, Huang et al., In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Clin. Infect. Dis, doi:10.1093/cid/ciaa237
Zangiabadian, Nejadghaderi, Zahmatkesh, Hajikhani, Mirsaeidi et al., The Efficacy and Potential Mechanisms of Metformin in the Treatment of COVID-19 in the Diabetics: A Systematic Review, Front. Endocrinol, doi:10.3389/fendo.2021.645194
Šestan, Marinović, Kavazovic, Cekinović, Wueest et al., Virus-Induced Interferon-γ Causes Insulin Resistance in Skeletal Muscle and Derails Glycemic Control in Obesity, Immunity, doi:10.1016/j.immuni.2018.05.005
DOI record:
{
"DOI": "10.3390/jcm10163507",
"ISSN": [
"2077-0383"
],
"URL": "http://dx.doi.org/10.3390/jcm10163507",
"abstract": "<jats:p>Background and Aims: The coronavirus disease 2019 (COVID-19) increases hyperinflammatory state, leading to acute lung damage, hyperglycemia, vascular endothelial damage, and a higher mortality rate. Metformin is a first-line treatment for type 2 diabetes and is known to have anti-inflammatory and immunosuppressive effects. Previous studies have shown that metformin use is associated with decreased risk of mortality among patients with COVID-19; however, the results are still inconclusive. This study investigated the association between metformin and the risk of mortality among diabetes patients with COVID-19. Methods: Data were collected from online databases such as PubMed, EMBASE, Scopus, and Web of Science, and reference from the most relevant articles. The search and collection of relevant articles was carried out between 1 February 2020, and 20 June 2021. Two independent reviewers extracted information from selected studies. The random-effects model was used to estimate risk ratios (RRs), with a 95% confidence interval. Results: A total of 16 studies met all inclusion criteria. Diabetes patients given metformin had a significantly reduced risk of mortality (RR, 0.65; 95% CI: 0.54–0.80, p < 0.001, heterogeneity I2 = 75.88, Q = 62.20, and τ2 = 0.06, p < 0.001) compared with those who were not given metformin. Subgroup analyses showed that the beneficial effect of metformin was higher in the patients from North America (RR, 0.43; 95% CI: 0.26–0.72, p = 0.001, heterogeneity I2 = 85.57, Q = 34.65, τ2 = 0.31) than in patients from Europe (RR, 0.67; 95% CI: 0.47–0.94, p = 0.02, heterogeneity I2 = 82.69, Q = 23.11, τ2 = 0.10) and Asia (RR, 0.90; 95% CI: 0.43–1.86, p = 0.78, heterogeneity I2 = 64.12, Q = 11.15, τ2 = 0.40). Conclusions: This meta-analysis shows evidence that supports the theory that the use of metformin is associated with a decreased risk of mortality among diabetes patients with COVID-19. Randomized control trials with a higher number of participants are warranted to assess the effectiveness of metformin for reducing the mortality of COVID-19 patients.</jats:p>",
"alternative-id": [
"jcm10163507"
],
"author": [
{
"affiliation": [],
"family": "Poly",
"given": "Tahmina Nasrin",
"sequence": "first"
},
{
"affiliation": [],
"family": "Islam",
"given": "Md. Mohaimenul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Yu-Chuan (Jack)",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9624-9705",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lin",
"given": "Ming-Chin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hsu",
"given": "Min-Huei",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0094-8016",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wang",
"given": "Yao-Chin",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Medicine",
"container-title-short": "JCM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
8,
9
]
],
"date-time": "2021-08-09T13:03:53Z",
"timestamp": 1628514233000
},
"deposited": {
"date-parts": [
[
2021,
8,
10
]
],
"date-time": "2021-08-10T13:32:37Z",
"timestamp": 1628602357000
},
"indexed": {
"date-parts": [
[
2023,
9,
28
]
],
"date-time": "2023-09-28T13:52:48Z",
"timestamp": 1695909168274
},
"is-referenced-by-count": 10,
"issue": "16",
"issued": {
"date-parts": [
[
2021,
8,
9
]
]
},
"journal-issue": {
"issue": "16",
"published-online": {
"date-parts": [
[
2021,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
8,
9
]
],
"date-time": "2021-08-09T00:00:00Z",
"timestamp": 1628467200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2077-0383/10/16/3507/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "3507",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
8,
9
]
]
},
"published-online": {
"date-parts": [
[
2021,
8,
9
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30185-9",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1093/cid/ciaa237",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.3389/fmed.2021.620044",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.20452/pamw.15272",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1016/j.dsx.2020.04.044",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1016/j.jstrokecerebrovasdis.2020.104949",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1016/j.cmet.2020.08.013",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1111/cts.12897",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1038/s41574-019-0242-2",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1016/j.immuni.2018.05.005",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.3389/fendo.2018.00372",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1016/j.it.2015.02.007",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1371/journal.pmed.1000097",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1159/000454881",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.3390/jcm10071450",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1101/2020.08.20.20174169",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1016/j.diabres.2020.108619",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.3389/fendo.2020.600439",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1016/j.dsx.2021.02.022",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1002/jmv.26873",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1016/j.diabet.2020.101216",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1016/j.jamda.2020.10.031",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.4269/ajtmh.20-0375",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1016/S2666-7568(20)30033-7",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1007/s00592-020-01666-7",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1186/s12916-020-01832-2",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1210/clinem/dgab067",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1186/s13045-020-00934-x",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1016/S2213-8587(21)00050-4",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1097/CM9.0000000000000012",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.2147/PGPM.S305020",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1016/j.obmed.2020.100290",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1002/jmv.26498",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1016/j.diabet.2020.07.006",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1016/j.dsx.2020.11.006",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.3389/fendo.2021.645194",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.1172/JCI69589",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1016/j.bbrc.2011.11.073",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1161/01.ATV.0000201938.78044.75",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.3390/ijms22115992",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.3390/ijms22052596",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1152/japplphysiol.00919.2013",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1016/j.bj.2020.08.007",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1186/1475-2840-12-175",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1016/j.bbagen.2020.129533",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1016/j.diabres.2020.108186",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1111/dom.14098",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.4103/0366-6999.223856",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1016/j.diabres.2020.108183",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1016/j.mce.2017.09.025",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.1016/j.ajog.2013.05.001",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.1002/jmv.25958",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.1055/s-0032-1327734",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.1007/s12325-008-0075-1",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"DOI": "10.1186/s12950-016-0142-3",
"doi-asserted-by": "publisher",
"key": "ref55"
},
{
"DOI": "10.1158/1940-6207.CAPR-11-0536",
"doi-asserted-by": "publisher",
"key": "ref56"
},
{
"DOI": "10.1016/j.metabol.2005.01.029",
"doi-asserted-by": "publisher",
"key": "ref57"
},
{
"DOI": "10.1016/j.bbrc.2010.04.017",
"doi-asserted-by": "publisher",
"key": "ref58"
},
{
"DOI": "10.1016/j.isci.2020.101425",
"doi-asserted-by": "publisher",
"key": "ref59"
},
{
"DOI": "10.1016/j.numecd.2017.04.009",
"doi-asserted-by": "publisher",
"key": "ref60"
},
{
"DOI": "10.1152/ajprenal.00539.2019",
"doi-asserted-by": "publisher",
"key": "ref61"
},
{
"DOI": "10.1038/nrneph.2016.183",
"doi-asserted-by": "publisher",
"key": "ref62"
},
{
"DOI": "10.1002/dmrr.3091",
"doi-asserted-by": "publisher",
"key": "ref63"
},
{
"DOI": "10.1155/2020/8708236",
"doi-asserted-by": "publisher",
"key": "ref64"
},
{
"DOI": "10.1016/j.diabres.2020.108230",
"doi-asserted-by": "publisher",
"key": "ref65"
},
{
"DOI": "10.1016/j.metabol.2018.02.009",
"doi-asserted-by": "publisher",
"key": "ref66"
},
{
"DOI": "10.1111/1440-1681.13226",
"doi-asserted-by": "publisher",
"key": "ref67"
}
],
"reference-count": 67,
"references-count": 67,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2077-0383/10/16/3507"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Metformin Use Is Associated with Decreased Mortality in COVID-19 Patients with Diabetes: Evidence from Retrospective Studies and Biological Mechanism",
"type": "journal-article",
"volume": "10"
}