Risk of Metformin in Patients With Type 2 Diabetes With COVID-19: A Preliminary Retrospective Report
et al., Clinical and Translational Science, doi:10.1111/cts.12897, Oct 2020
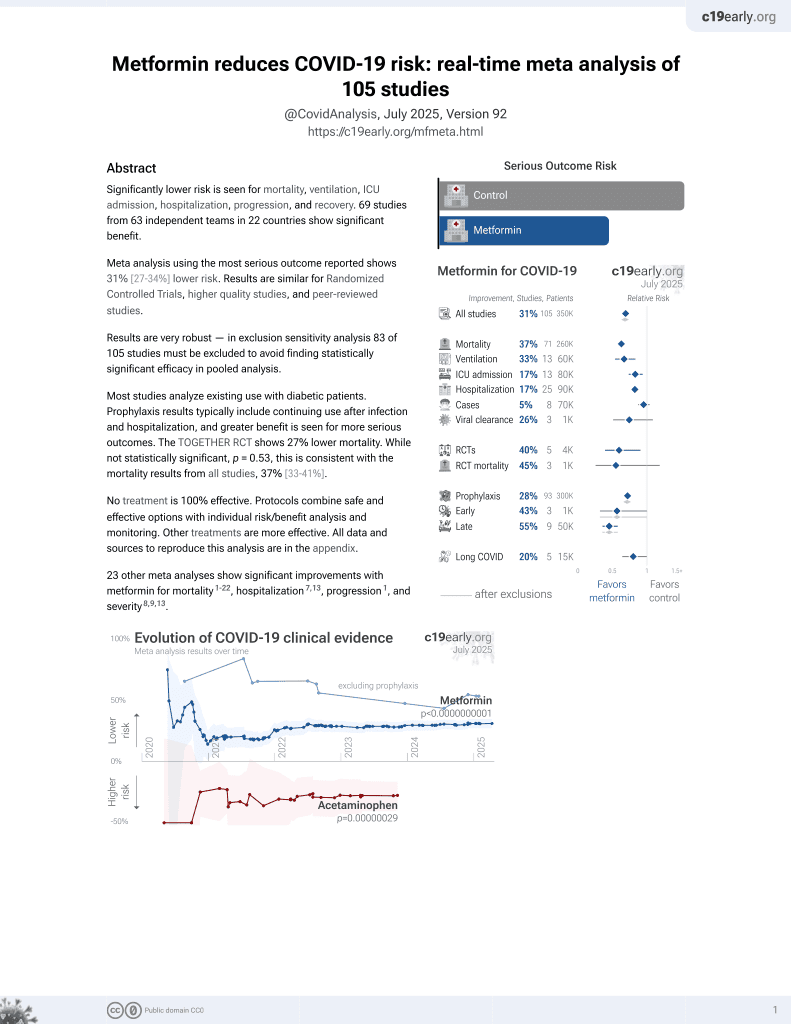
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 110 hospitalized COVID-19 patients with diabetes in China, showing increased risk of severity with metformin.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
risk of progression, 225.0% higher, RR 3.25, p = 0.045, treatment 16 of 56 (28.6%), control 4 of 54 (7.4%), odds ratio converted to relative risk, progression to life threatening complications.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Gao et al., 19 Oct 2020, retrospective, China, peer-reviewed, 7 authors, study period 31 January, 2020 - 20 March, 2020.
Risk of Metformin in Patients With Type 2 Diabetes With COVID‐19: A Preliminary Retrospective Report
Clinical and Translational Science, doi:10.1111/cts.12897
The current outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has spread across the world. No specific antiviral agents have been adequately evidenced for the treatment of coronavirus disease 2019 (COVID-19). Although metformin has been recommended as a host-directed therapy for COVID-19, there are some opposite views. The effects of metformin on the disease severity of patients with COVID-19 with diabetes during hospitalization remains unclear. This study aimed to determine the effect of metformin on disease severity. We enrolled 110 hospitalized patients with COVID-19 with diabetes prescribed either metformin or non-metformin hypoglycemic treatment for a case-control study. The primary outcome was the occurrence of life-threatening complications. There were no differences between the two groups in age, sex, comorbidities, and clinical severity at admission. Blood glucose and lactate dehydrogenase levels of the metformin group were higher than those of the non-metformin group at admission. Other laboratory parameters at admission and treatments after admission were not different between the two groups. Strikingly, the percentage of patients who experienced life-threatening complications was significantly higher in the metformin group (28.6% (16/56) vs. 7.4% (4/54), P = 0.004). Antidiabetic therapy with metformin was associated with a higher risk of disease progression in patients with COVID-19 with diabetes during hospitalization (adjusted odds ratio = 3.964, 95% confidence interval 1.034-15.194, P = 0.045). This retrospective analysis suggested a potential safety signal for metformin, the use of which was associated with a higher risk of severe COVID-19. We propose that metformin withdrawal in patients with COVID-19 be considered to prevent disease progression. The current outbreak of coronavirus disease 2019 (COVID-19) by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread quickly through human-to-human transmission, 1 and has been declared as "Public Health Emergency of International Concern" by the World Health Organization (WHO). As both SARS-CoV 2 and Middle East respiratory syndrome coronavirus (MERS-CoV), 3 the SARS-CoV-2 outbreak has caused a large number of human deaths
Conflict of Interest. All other authors declared no competing interests for this work.
References
Azhar, Hui, Memish, Drosten, Zumla, The Middle East Respiratory Syndrome (MERS), Infect. Dis. Clin. North Am, doi:10.1016/j.idc.2019.08.001
Bornstein, Practical recommendations for the management of diabetes in patients with COVID-19, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587(20)30152-2
Chen, Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication, Diabetes Care, doi:10.2337/dc20-0660
Guan, Clinical characteristics of coronavirus disease 2019 in China, N. Engl. J. Med, doi:10.1056/NEJMoa2002032
Huang, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Hui, Zumla, Severe acute respiratory syndrome: historical, epidemiologic, and clinical features, Infect. Dis. Clin. North Am, doi:10.1016/j.idc.2019.07.001
Katulanda, Prevention and management of COVID-19 among patients with diabetes: an appraisal of the literature, Diabetologia, doi:10.1007/s00125-020-05164-x
Li, Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia, N. Engl. J. Med, doi:10.1056/NEJMoa2001316
Naicker, The novel coronavirus 2019 epidemic and kidneys, Kidney Int, doi:10.1016/j.kint.2020.03.001
Roncon, Zuin, Rigatelli, Zuliani, Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome, J. Clin. Virol, doi:10.1016/j.jcv.2020.104354
Shi, Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study, Diabetes Care, doi:10.2337/dc20-0598
Wang, O-GlcNAc transferase promotes influenza A virus-induced cytokine storm by targeting interferon regulatory factor-5, Sci. Adv, doi:10.1126/sciadv.aaz7086
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention, JAMA, doi:10.1001/jama.2020.2648
Zhang, AMP-activated protein kinase phosphorylation of angiotensin-converting enzyme 2 in endothelium mitigates pulmonary hypertension, Am. J. Respir. Crit. Care Med, doi:10.1164/rccm.201712-2570OC
Zhao, Analysis of the susceptibility to COVID-19 in pregnancy and recommendations on potential drug screening, Eur. J. Clin. Microbiol. Infect. Dis, doi:10.1007/s10096-020-03897-6
Zhou, A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature, doi:10.1038/s41586-020-2012-7
Zhou, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
Zhu, Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes, Cell Metab, doi:10.1016/j.cmet.2020.04.021
Zumla, Hui, Azhar, Memish, Maeurer, Reducing mortality from 2019-nCoV: host-directed therapies should be an option, Lancet, doi:10.1016/S0140-6736(20)30305-6
DOI record:
{
"DOI": "10.1111/cts.12897",
"ISSN": [
"1752-8054",
"1752-8062"
],
"URL": "http://dx.doi.org/10.1111/cts.12897",
"alternative-id": [
"10.1111/cts.12897"
],
"archive": [
"Portico"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-06-11"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2020-09-12"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2020-10-19"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Clinical Pharmacology Xiangya Hospital Central South University Changsha China"
},
{
"name": "Institute of Clinical Pharmacology Hunan Key Laboratory of Pharmacogenetics Central South University Changsha China"
},
{
"name": "Engineering Research Center of Applied Technology of Pharmacogenomics Ministry of Education Changsha China"
},
{
"name": "National Clinical Research Center for Geriatric Disorders Changsha China"
}
],
"family": "Gao",
"given": "Yongchao",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Shenzhen Center for Chronic Disease Control Shenzhen China"
}
],
"family": "Liu",
"given": "Tao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Pharmacology Xiangya Hospital Central South University Changsha China"
},
{
"name": "Institute of Clinical Pharmacology Hunan Key Laboratory of Pharmacogenetics Central South University Changsha China"
},
{
"name": "Engineering Research Center of Applied Technology of Pharmacogenomics Ministry of Education Changsha China"
},
{
"name": "National Clinical Research Center for Geriatric Disorders Changsha China"
}
],
"family": "Zhong",
"given": "Weijun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Pharmacology Xiangya Hospital Central South University Changsha China"
},
{
"name": "Institute of Clinical Pharmacology Hunan Key Laboratory of Pharmacogenetics Central South University Changsha China"
},
{
"name": "Engineering Research Center of Applied Technology of Pharmacogenomics Ministry of Education Changsha China"
},
{
"name": "National Clinical Research Center for Geriatric Disorders Changsha China"
}
],
"family": "Liu",
"given": "Rong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Pharmacology Xiangya Hospital Central South University Changsha China"
},
{
"name": "Institute of Clinical Pharmacology Hunan Key Laboratory of Pharmacogenetics Central South University Changsha China"
},
{
"name": "Engineering Research Center of Applied Technology of Pharmacogenomics Ministry of Education Changsha China"
},
{
"name": "National Clinical Research Center for Geriatric Disorders Changsha China"
}
],
"family": "Zhou",
"given": "Honghao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Pharmacology Xiangya Hospital Central South University Changsha China"
},
{
"name": "Institute of Clinical Pharmacology Hunan Key Laboratory of Pharmacogenetics Central South University Changsha China"
},
{
"name": "Engineering Research Center of Applied Technology of Pharmacogenomics Ministry of Education Changsha China"
},
{
"name": "National Clinical Research Center for Geriatric Disorders Changsha China"
}
],
"family": "Huang",
"given": "Weihua",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Pharmacology Xiangya Hospital Central South University Changsha China"
},
{
"name": "Institute of Clinical Pharmacology Hunan Key Laboratory of Pharmacogenetics Central South University Changsha China"
},
{
"name": "Engineering Research Center of Applied Technology of Pharmacogenomics Ministry of Education Changsha China"
},
{
"name": "National Clinical Research Center for Geriatric Disorders Changsha China"
}
],
"family": "Zhang",
"given": "Wei",
"sequence": "additional"
}
],
"container-title": [
"Clinical and Translational Science"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2020,
9,
21
]
],
"date-time": "2020-09-21T19:23:09Z",
"timestamp": 1600716189000
},
"deposited": {
"date-parts": [
[
2020,
12,
6
]
],
"date-time": "2020-12-06T18:23:41Z",
"timestamp": 1607279021000
},
"funder": [
{
"DOI": "10.13039/501100001809",
"award": [
"81874329",
"81573511",
"81472802"
],
"doi-asserted-by": "publisher",
"name": "National Natural Science Foundation of China"
},
{
"DOI": "10.13039/501100002822",
"doi-asserted-by": "publisher",
"name": "Central South University"
}
],
"indexed": {
"date-parts": [
[
2022,
2,
10
]
],
"date-time": "2022-02-10T09:05:48Z",
"timestamp": 1644483948451
},
"is-referenced-by-count": 24,
"issn-type": [
{
"type": "print",
"value": "1752-8054"
},
{
"type": "electronic",
"value": "1752-8062"
}
],
"issue": "6",
"issued": {
"date-parts": [
[
2020,
10,
19
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2020,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
19
]
],
"date-time": "2020-10-19T00:00:00Z",
"timestamp": 1603065600000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
19
]
],
"date-time": "2020-10-19T00:00:00Z",
"timestamp": 1603065600000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/cts.12897",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/cts.12897",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/cts.12897",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "1055-1059",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2020,
10,
19
]
]
},
"published-online": {
"date-parts": [
[
2020,
10,
19
]
]
},
"published-print": {
"date-parts": [
[
2020,
11
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1056/NEJMoa2001316",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_1_1"
},
{
"DOI": "10.1016/j.idc.2019.07.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"DOI": "10.1016/j.idc.2019.08.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"key": "e_1_2_10_4_1",
"unstructured": "World Health Organization (WHO).Coronavirus disease 2019 (COVID19): situation report—117. (WHO Geneva) <https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200516‐covid‐19‐sitrep‐117.pdf?sfvrsn=8f562cc_2> (2020). Accessed May 16 2020."
},
{
"DOI": "10.2337/dc20-0660",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_1"
},
{
"DOI": "10.1016/j.jcv.2020.104354",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.2337/dc20-0598",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.1016/j.cmet.2020.04.021",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1016/S0140-6736(20)30305-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1007/s10096-020-03897-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.1007/s00125-020-05164-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.1016/S2213-8587(20)30152-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"DOI": "10.1164/rccm.201712-2570OC",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_14_1"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_1"
},
{
"DOI": "10.1016/j.kint.2020.03.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.1126/sciadv.aaz7086",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_1"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"score": 1,
"short-container-title": [
"Clin Transl Sci"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Pharmacology, Toxicology and Pharmaceutics",
"General Biochemistry, Genetics and Molecular Biology",
"General Medicine",
"General Neuroscience"
],
"subtitle": [],
"title": [
"Risk of Metformin in Patients With Type 2 Diabetes With COVID‐19: A Preliminary Retrospective Report"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "13"
}