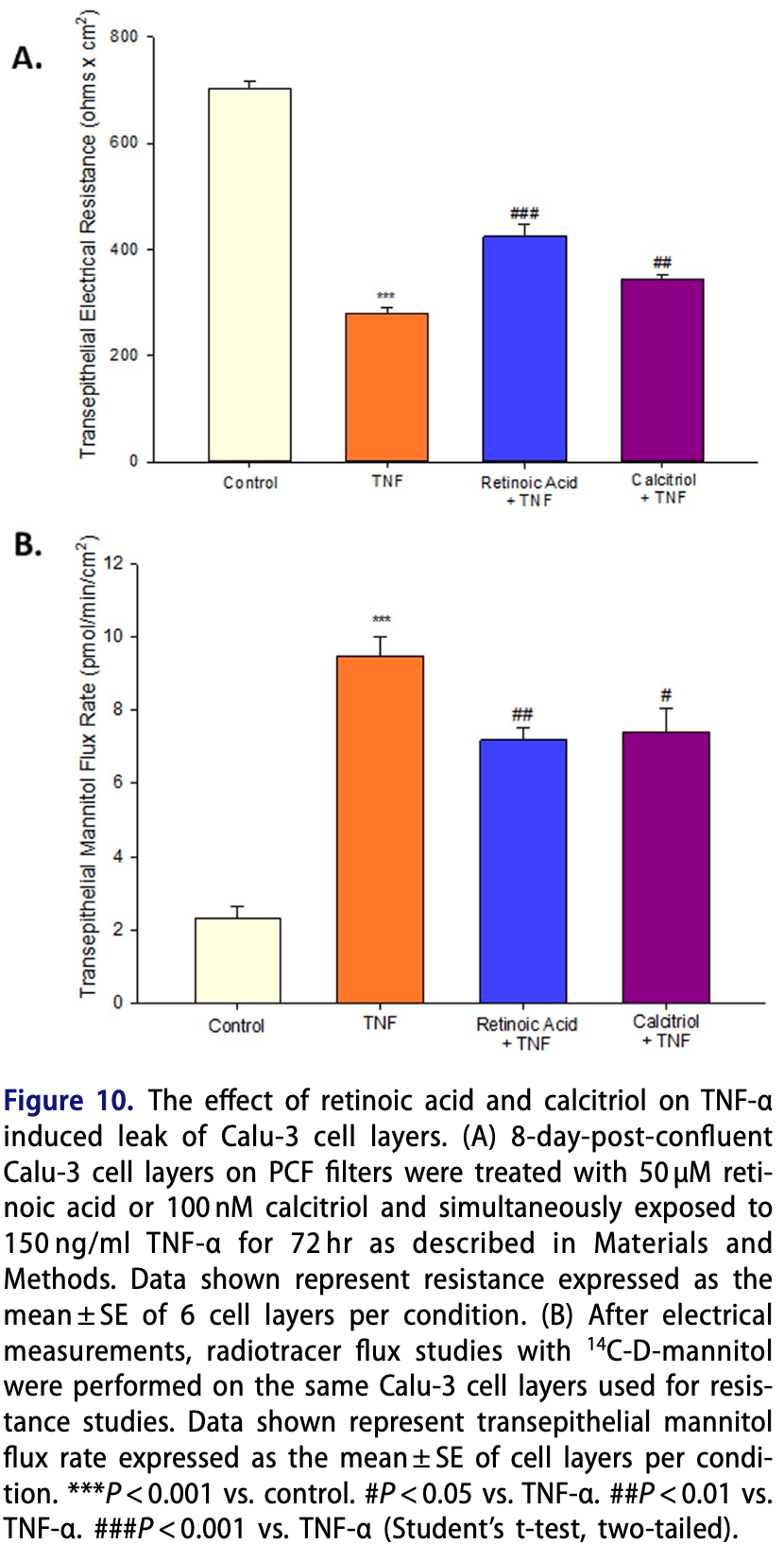
The multiphasic TNF-α-induced compromise of Calu-3 airway epithelial barrier function
et al., Experimental Lung Research, doi:10.1080/01902148.2023.2193637, Mar 2023
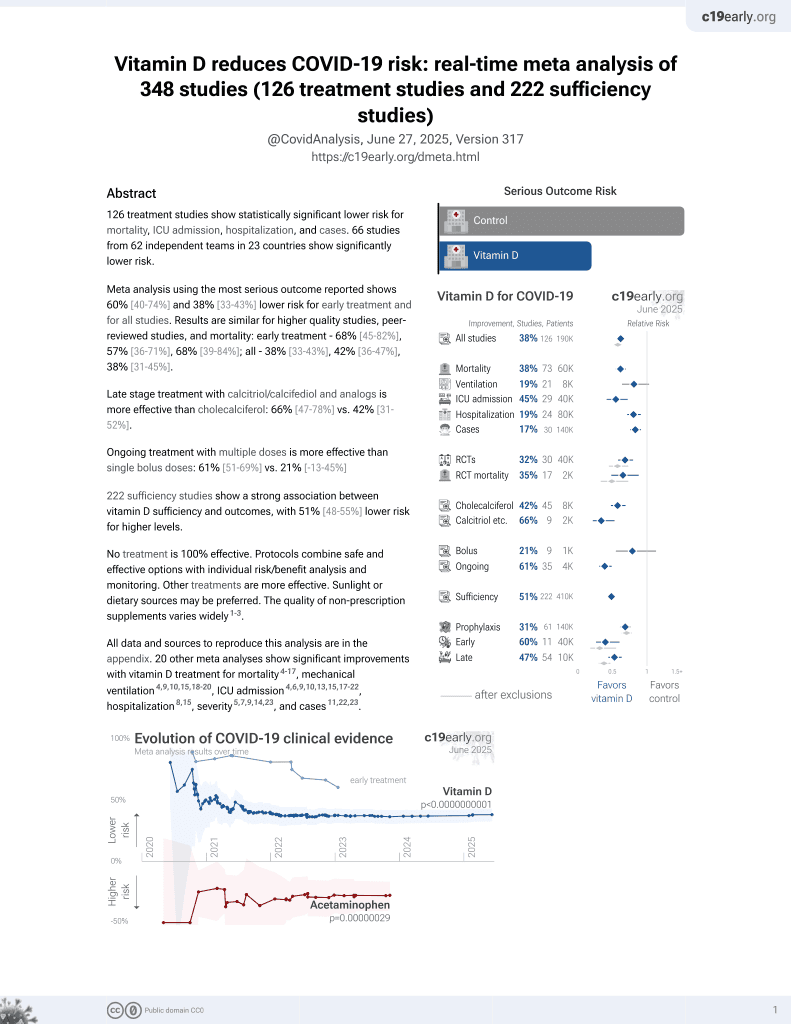
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study showing that TNF-α induced a multiphasic transepithelial leak in Calu-3 cell layers, and that vitamin A and vitamin D (calcitriol) were effective at reducing the barrier compromise caused by TNF-α.
29 preclinical studies support the efficacy of vitamin D for COVID-19:
Vitamin D has been identified by the European Food Safety Authority (EFSA) as having sufficient evidence for a causal relationship between intake and optimal immune system function27-30.
Vitamin D inhibits SARS-CoV-2 replication in vitro17,24, mitigates lung inflammation, damage, and lethality in mice with an MHV-3 model for β-CoV respiratory infections17,24, reduces SARS-CoV-2 replication in nasal epithelial cells via increased type I interferon expression20, downregulates proinflammatory cytokines IL-1β and TNF-α in SARS-CoV-2 spike protein-stimulated cells16, attenuates nucleocapsid protein-induced hyperinflammation by inactivating the NLRP3 inflammasome through the VDR-BRCC3 signaling pathway21, may be neuroprotective by protecting the blood-brain barrier, reducing neuroinflammation, and via immunomodulatory effects31, may mitigate hyperinflammation and cytokine storm by upregulating TLR10 expression which downregulates proinflammatory cytokines13, downregulates ACE2 and TMPRSS2 in human trophoblasts and minimizes spike protein-induced inflammation19, may minimize cytokine storm by dampening excessive cytokine production2, may suppress viral entry and replication via LL-37 induction11,12, and minimizes platelet aggregation mediated by SARS-CoV-2 spike protein via inhibiting integrin αIIbβ3 outside-in signaling15.
Cholecalciferol and calcifediol directly bind two allosteric pockets on the SARS-CoV-2 Spike RBD, bias the trimer toward a closed state, weaken ACE2 engagement, and reduce viral entry in cell models1.
Calcitriol may destabilize the Spike protein architecture and inhibit IL-17R dimerization, blocking viral entry and mitigating hyperinflammatory cytokine storm32.
Vitamin D improves regulatory immune cell levels and control of proinflammatory cytokines in severe COVID-1933.
Calcifediol inhibits SARS-CoV-2 papain-like protease (PLpro), a critical enzyme for viral replication14.
Symptomatic COVID-19 is associated with a lower frequency of natural killer (NK) cells and vitamin D has been shown to improve NK cell activity34,35.
Study covers vitamin A and vitamin D.
1.
García-Marín et al., Exploring SARS-CoV-2 Spike RBD Pockets as Targets for Generic Drugs: A Combined Computational, Biophysical, and Biological Approach, ACS Omega, doi:10.1021/acsomega.5c05175.
2.
Alzahrani, A., A new investigation into the molecular mechanism of cholecalciferol towards reducing cytokines storm, Octahedron Drug Research, doi:10.21608/odr.2024.308273.1043.
3.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
4.
Morales-Bayuelo et al., New findings on ligand series used as SARS-CoV-2 virus inhibitors within the frameworks of molecular docking, molecular quantum similarity and chemical reactivity indices, F1000Research, doi:10.12688/f1000research.123550.3.
5.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
6.
Pandya et al., Unravelling Vitamin B12 as a potential inhibitor against SARS-CoV-2: A computational approach, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2022.100951.
7.
Mansouri et al., The impact of calcitriol and estradiol on the SARS-CoV-2 biological activity: a molecular modeling approach, Scientific Reports, doi:10.1038/s41598-022-04778-y.
8.
Song et al., Vitamin D3 and its hydroxyderivatives as promising drugs against COVID-19: a computational study, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1964601.
9.
Qayyum et al., Vitamin D and lumisterol novel metabolites can inhibit SARS-CoV-2 replication machinery enzymes, Endocrinology and Metabolism, doi:10.1152/ajpendo.00174.2021.
10.
Al-Mazaideh et al., Vitamin D is a New Promising Inhibitor to the Main Protease (Mpro) of COVID-19 by Molecular Docking, Journal of Pharmaceutical Research International, doi:10.9734/jpri/2021/v33i29B31603.
11.
Roth et al., Vitamin D-inducible antimicrobial peptide LL-37 binds SARS-CoV-2 Spike and accessory proteins ORF7a and ORF8, Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2025.1671738.
12.
Vercellino et al., Influence of Sex and 1,25α Dihydroxyvitamin D3 on SARS-CoV-2 Infection and Viral Entry, Pathogens, doi:10.3390/pathogens14080765.
13.
Knez et al., TLR10 overexpression modulates immune response in A549 lung epithelial cells challenged with SARS-CoV-2 S and N proteins, Frontiers in Immunology, doi:10.3389/fimmu.2024.1490478.
14.
Chen et al., In Vitro Characterization of Inhibition Function of Calcifediol to the Protease Activity of SARS-COV-2 PLpro, Journal of Medical Virology, doi:10.1002/jmv.70085.
15.
Wang et al., 1,25‐Dihydroxyvitamin D3 attenuates platelet aggregation potentiated by SARS‐CoV‐2 spike protein via inhibiting integrin αIIbβ3 outside‐in signaling, Cell Biochemistry and Function, doi:10.1002/cbf.4039.
16.
Alcalá-Santiago et al., Disentangling the Immunomodulatory Effects of Vitamin D on the SARS-CoV-2 Virus by In Vitro Approaches, The 14th European Nutrition Conference FENS 2023, doi:10.3390/proceedings2023091415.
17.
Campolina-Silva et al., Dietary Vitamin D Mitigates Coronavirus-Induced Lung Inflammation and Damage in Mice, Viruses, doi:10.3390/v15122434.
18.
Moatasim et al., Potent Antiviral Activity of Vitamin B12 against Severe Acute Respiratory Syndrome Coronavirus 2, Middle East Respiratory Syndrome Coronavirus, and Human Coronavirus 229E, Microorganisms, doi:10.3390/microorganisms11112777.
19.
Vargas-Castro et al., Calcitriol prevents SARS-CoV spike-induced inflammation in human trophoblasts through downregulating ACE2 and TMPRSS2 expression, The Journal of Steroid Biochemistry and Molecular Biology, doi:10.1016/j.jsbmb.2024.106625.
20.
Sposito et al., Age differential CD13 and interferon expression in airway epithelia affect SARS-CoV-2 infection - effects of vitamin D, Mucosal Immunology, doi:10.1016/j.mucimm.2023.08.002.
21.
Chen (B) et al., Vitamin D3 attenuates SARS‐CoV‐2 nucleocapsid protein‐caused hyperinflammation by inactivating the NLRP3 inflammasome through the VDR‐BRCC3 signaling pathway in vitro and in vivo, MedComm, doi:10.1002/mco2.318.
22.
Rybakovsky et al., Calcitriol modifies tight junctions, improves barrier function, and reduces TNF‐α‐induced barrier leak in the human lung‐derived epithelial cell culture model, 16HBE 14o‐, Physiological Reports, doi:10.14814/phy2.15592.
23.
DiGuilio et al., The multiphasic TNF-α-induced compromise of Calu-3 airway epithelial barrier function, Experimental Lung Research, doi:10.1080/01902148.2023.2193637.
24.
Pickard et al., Discovery of re-purposed drugs that slow SARS-CoV-2 replication in human cells, PLOS Pathogens, doi:10.1371/journal.ppat.1009840.
25.
Mok et al., Calcitriol, the active form of vitamin D, is a promising candidate for COVID-19 prophylaxis, bioRxiv, doi:10.1101/2020.06.21.162396.
26.
Fernandes de Souza et al., Lung Inflammation Induced by Inactivated SARS-CoV-2 in C57BL/6 Female Mice Is Controlled by Intranasal Instillation of Vitamin D, Cells, doi:10.3390/cells12071092.
27.
Galmés et al., Suboptimal Consumption of Relevant Immune System Micronutrients Is Associated with a Worse Impact of COVID-19 in Spanish Populations, Nutrients, doi:10.3390/nu14112254.
28.
Galmés (B) et al., Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework, Nutrients, doi:10.3390/nu12092738.
29.
EFSA, Scientific Opinion on the substantiation of a health claim related to vitamin D and contribution to the normal function of the immune system pursuant to Article 14 of Regulation (EC) No 1924/2006, EFSA Journal, doi:10.2903/j.efsa.2015.4096.
30.
EFSA (B), Scientific Opinion on the substantiation of health claims related to vitamin D and normal function of the immune system and inflammatory response (ID 154, 159), maintenance of normal muscle function (ID 155) and maintenance of normal cardiovascular function (ID 159) pursuant to Article 13(1) of Regulation (E, EFSA Journal, doi:10.2903/j.efsa.2010.1468.
31.
Gotelli et al., Understanding the immune-endocrine effects of vitamin D in SARS-CoV-2 infection: a role in protecting against neurodamage?, Neuroimmunomodulation, doi:10.1159/000533286.
32.
Fadel et al., Targeting asparagine and cysteine in SARS-CoV-2 variants and human pro-inflammatory mediators to alleviate COVID-19 severity; a cross-section and in-silico study, Scientific Reports, doi:10.1038/s41598-025-19359-y.
33.
Saheb Sharif-Askari et al., Increased blood immune regulatory cells in severe COVID-19 with autoantibodies to type I interferons, Scientific Reports, doi:10.1038/s41598-023-43675-w.
DiGuilio et al., 31 Mar 2023, peer-reviewed, 5 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
The multiphasic TNF-α-induced compromise of Calu-3 airway epithelial barrier function
Experimental Lung Research, doi:10.1080/01902148.2023.2193637
Purpose: Airway epithelial barrier leak and the involvement of proinflammatory cytokines play a key role in a variety of diseases. This study evaluates barrier compromise by the inflammatory mediator Tumor Necrosis Factor-α (TNF-α) in the human airway epithelial Calu-3 model. Methods: We examined the effects of TNF-α on barrier function in Calu-3 cell layers using Transepithelial Electrical Resistance (TER) and transepithelial diffusion of radiolabeled probe molecules. Western immunoblot analyses of tight junctional (TJ) proteins in detergent soluble fractions were performed. Results: TNF-α dramatically reduced TER and increased paracellular permeability of both 14C-D-mannitol and the larger 5 kDa probe, 14C-inulin. A time course of the effects shows two separate actions on barrier function. An initial compromise of barrier function occurs 2-4 hours after TNF-α exposure, followed by complete recovery of barrier function by 24 hrs. Beginning 48 hrs. post-exposure, a second more sustained barrier compromise ensues, in which leakiness persists through 144 hrs. There were no changes in TJ proteins observed at 3 hrs. post exposure, but significant increases in claudins-2, -3, -4, and -5, as well as a decrease in occludin were seen at 72 hrs. post TNF-α exposure. Both the 2-4 hr. and the 72 hr. TNF-α induced leaks are shown to be mediated by the ERK signaling pathway. Conclusion: TNF-α induced a multiphasic transepithelial leak in Calu-3 cell layers that was shown to be ERK mediated, as well as involve changes in the TJ complex. The micronutrients, retinoic acid and calcitriol, were effective at reducing this barrier compromise caused by TNF-α. The significance of these results for airway disease and for COVID-19 specifically are discussed.
References
Amoozadeh, Xiao, Waheed, Szászi, Tumor necrosis factor-α induces a biphasic change in claudin-2 expression in tubular epithelial cells: role in barrier functions, Am J Physiol Cell Physiol, doi:10.1152/ajpcell.00388.2014
Anwer, Branchard, Szászi, Tumor necrosis factor-α induces claudin-3 upregulation in kidney tubular epithelial cells through NF-κB and CREB1, Am J Physiol Cell Physiol, doi:10.1152/ajpcell.00185.2020
Aveleira, Lin, Abcouwer, Ambrósio, Antonetti, TNF-α signals through PKCζ/ NF-κB to alter the tight junction complex and increase retinal endothelial cell permeability, Diabetes, doi:10.2337/db09-1606
Baltes, Nau, Lampen, All-trans retinoic acid enhances differentiation and influences permeability of intestinal Caco-2 cells under serum-free conditions, Dev Growth Differ, doi:10.1111/j.1440-169x.2004.00765.x
Callaghan, Rybakovsky, Ferrick, Thomas, Mullin, Retinoic acid improves baseline barrier function and attenuates TNF-α induced barrier leak in human bronchial epithelial cell culture model, 16HBE 14o, PLoS One, doi:10.1371/journal.pone.0242536
Castillo, Costa, Barrios, Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2020.105751
Chen, Zhu, Chen, 25-Dihydroxyvitamin D3 preserves intestinal epithelial barrier function from TNF-α induced injury via suppression of NF-kB p65 mediated MLCK-P-MLC signaling pathway, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2015.03.125
Chirayath, Gajdzik, Hulla, Graf, Cross et al., Vitamin D increases tight-junction conductance and paracellular Ca2+ transport in Caco-2 cell cultures, Am J Physiol, doi:10.1152/ajpgi.1998.274.2.G389
Coyne, Gambling, Boucher, Carson, Johnson, Role of claudin interactions in airway tight junctional permeability, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00182.2003
De Sá, Backert, Nattramilarasu, Vitamin D reverses disruption of gut epithelial barrier function caused by Campylobacter jejuni, IJMS, doi:10.3390/ijms22168872
Dey, Borkotoky, Banerjee, In silico identification of Tretinoin as a SARS-CoV-2 envelope (E) protein ion channel inhibitor, Comput Biol Med, doi:10.1016/j.compbiomed.2020.104063
Diguilio, Rybakovsky, Abdavies, Micronutrient improvement of epithelial barrier function in various disease states: a case for adjuvant therapy, IJMS, doi:10.3390/ijms23062995
Diyya, Thomas, Multiple micronutrient supplementation: as a supportive therapy in the treatment of COVID-19, Biomed Res Int, doi:10.1155/2022/3323825
Eisenhut, Shin, Pathways in the pathophysiology of coronavirus 19 lung disease accessible to prevention and treatment, Front Physiol, doi:10.3389/fphys.2020.00872
Elias, Friend, Vitamin-A induced mucous metaplasia. An in vitro system for modulating tight and gap junction differentiation, J Cell Biol, doi:10.1083/jcb.68.2.173
Ewaschuk, Diaz, Meddings, Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function, Am J Physiol Gastrointest Liver Physiol, doi:10.1152/ajpgi.90227.2008
Fiorino, Gallo, Zippi, Cytokine storm in aged people with CoV-2: possible role of vitamins as therapy or preventive strategy, Aging Clin Exp Res, doi:10.1007/s40520-020-01669-y
Gaspar, De Matos, Cortes, Pollen proteases play multiple roles in allergic disorders, IJMS, doi:10.3390/ijms21103578
Gorodeski, Eckert, Pal, Utian, Rorke, Retinoids regulate tight junctional resistance of cultured human cervical cells, Am J Physiol, doi:10.1152/ajpcell.1997.273.5.C1707
Groeger, Jarzina, Windhorst, Meyle, Influence of retinoic acid on human gingival epithelial barriers, J Periodontal Res, doi:10.1111/jre.12351
Han, Fink, Uchiyama, Yang, Delude, Increased iNOS activity is essential for pulmonary epithelial tight junction dysfunction in endotoxemic mice, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00187.2003
Hao, Ning, Kuz, Vorhies, Yan et al., Long-term modeling of SARS-CoV-2 infection of in vitro cultured polarized human airway epithelium, mBio, doi:10.1128/mBio.02852-20
Hardyman, Wilkinson, Martin, TNF-αmediated bronchial barrier disruption and regulation by src-family kinase activation, J Allergy Clin Immunol, doi:10.1016/j.jaci.2013.03.005
Haws, Finkbeiner, Widdicombe, Wine, CFTR in Calu-3 human airway cells: channel properties and role in cAMP-activated Cl-conductance
He, Wang, Sheng, Zha, Graham et al., Contributions of myosin light chain kinase to regulation of epithelial paracellular permeability and mucosal homeostasis, IJMS, doi:10.3390/ijms21030993
Infante, Buoso, Pieri, Low Vitamin D Status at Admission as a Risk Factor for Poor Survival in Hospitalized Patients With COVID-19: An Italian Retrospective Study, J Am Nutr Assoc, doi:10.1080/07315724.2021.1877580
Kaminsky, Al-Sadi, Ma, IL-1β and the intestinal epithelial tight junction barrier, Front Immunol, doi:10.3389/fimmu.2021.767456
Katz, Yue, Xue, Increased risk for COVID-19 in patients with vitamin D deficiency, Nutrition, doi:10.1016/j.nut.2020.111106
Kondo, Sato, Kusumi, Claudin-1 expression is induced by tumor necrosis factor-α in human pancreatic cancer cells, Int J Mol Med
Kong, Zhang, Musch, Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier, Am J Physiol Gastrointest Liver Physiol, doi:10.1152/ajpgi.00398.2007
Krug, Amasheh, Richter, Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability, Mol Biol Cell, doi:10.1091/mbc.e09-01-0080
Kryvenko, Vadász, Molecular mechanisms of Na, K-ATPase dysregulation driving alveolar epithelial barrier failure in severe COVID-19, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00056.2021
Lagha, Grenier, Tea polyphenols protect gingival keratinocytes against TNF-α induced tight junction barrier dysfunction and attenuate the inflammatory response of monocytes/macrophages, Cytokine, doi:10.1016/j.cyto.2018.12.009
Lee, Kim, Preventive effects of thinned apple extracts on TNF-α induced intestinal tight junction dysfunction in Caco-2 cells through myosin light chain kinase suppression, Foods, doi:10.3390/foods11121714
Liu, Sun, Wang, Zhang, Zhao et al., Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis, Int J Infect Dis, doi:10.1016/j.ijid.2020.12.077
Ma, Boivin, Ye, Pedram, Said, Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression, Am J Physiol Gastrointest Liver Physiol, doi:10.1152/ajpgi.00412.2004
Ma, Iwamoto, Hoa, TNF-alpha induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation, Am J Physiol Gastrointest Liver Physiol, doi:10.1152/ajpgi.00173.2003
Ma, Zhou, Heianza, Qi, Habitual use of vitamin D supplements and risk of coronavirus disease 2019 (COVID-19) infection: a prospective study in UK Biobank, Am J Clin Nutr, doi:10.1093/ajcn/nqaa381
Marano, Lewis, Garulacan, Soler, Mullin, Tumor necrosis factor-alpha increases sodium and chloride conductance across the tight junction of CACO-2 BBE, a human intestinal epithelial cell line, J Membr Biol, doi:10.1007/s002329900333
Martineau, Jolliffe, Hooper, Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, BMJ, doi:10.1136/bmj.i6583
Mawson, Croft, Gonzalez-Fernandez, Liver damage and exposure to toxic concentrations of endogenous retinoids in the pathogenesis of COVID-19 disease: hypothesis, Viral Immunol, doi:10.1089/vim.2020.0330
Mehta, Mcauley, Brown, COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet, doi:10.1016/S0140-6736(20)30628-0
Merzon, Tworowski, Gorohovski, Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study, Febs J, doi:10.1111/febs.15495
Midha, Kumar, Kumar, Madan, Mega doses of retinol: a possible immunomodulation in Covid-19 illness in resource-limited settings, Rev Med Virol, doi:10.1002/rmv.2204
Morita, Miyakawa, Jeremiah, All-trans retinoic acid exhibits antiviral effect against SARS-CoV-2 by Inhibiting 3CLpro activity, Viruses, doi:10.3390/v13081669
Mullin, Marano, Laughlin, Nuciglio, Stevenson et al., Different size limitations for increased transepithelial paracellular solute flux across phorbol ester and tumor necrosis factor-treated epithelial cell sheets, J Cell Physiol, doi:10.1002/(SICI)1097-4652(199705)171:2â•›<â•›226:
Mullin, Snock, Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability, Cancer Res
Munshi, Hussein, Toraih, Vitamin D insufficiency as a potential culprit in critical COVID-19 patients, J Med Virol, doi:10.1002/jmv.26360
Nimer, Khabour, Swedan, Kofahi, The impact of vitamin and mineral supplements usage prior to COVID-19 infection on disease severity and hospitalization, Bosn J Basic Med Sci, doi:10.17305/bjbms.2021.7009
Pasman, Baptista, Van Riet, Development of an in vitro airway epithelial-endothelial cell culture model on a flexible porous poly (trimethylene carbonate) membrane based on calu-3 airway epithelial cells and lung microvascular endothelial cells, Membranes, doi:10.3390/membranes11030197
Petecchia, Sabatini, Usai, Caci, Varesio et al., Cytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ ERK1/2-pathway, Lab Invest, doi:10.1038/labinvest.2012.67
Prasad, Alomar, Almuqri, Rudayni, Kumar, Genomics-guided identification of potential modulators of SARS-CoV-2 entry proteases, TMPRSS2 and Cathepsins B/L, PLoS One, doi:10.1371/journal.pone.0256141
Rabito, Tchao, Valentich, Leighton, Distribution and characteristics of the occluding junctions in a monolayer of a cell line (MDCK) derived from canine kidney, J Membr Biol, doi:10.1007/BF01871696
Rabito, Tchao, Valentich, Leighton, Effect of cell-substratum interaction on hemicyst formation by MDCK cells, Vitro, doi:10.1007/BF02626458
Ramos, Lin, Liu, Antonetti, The EPAC-Rap1 pathway prevents and reverses cytokine induced retinal vascular permeability, J Biol Chem, doi:10.1074/jbc.M117.815381
Rastogi, Bhansali, Khare, Short term high-dose vitamin D supplementation for COVID-19 disease: a randomized, placebo-controlled, study (SHADE study), Postgrad Med J, doi:10.1136/postgradmedj-2020-139065
Renata, Arely, Gabriela, Esther, Immunomodulatory role of microelements in COVID-19 outcome: a relationship with nutritional status, Biol Trace Elem Res, doi:10.1007/s12011-022-03290-8
Rodriguez, Heyman, Candalh, Blaton, Bouchaud, Tumour necrosis factor-alpha induces morphological and functional alterations of intestinal HT29 cl.19A cell monolayers, Cytokine, doi:10.1006/cyto.1995.0060
Rong, Liu, Effect of all-trans retinoic acid on the barrier function in human retinal pigment epithelial cells, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2011.03.080
Rybakovsky, Buleza, Hoxha, Spontaneous and cytokine induced hole formation in epithelial cell layers: implications for barrier function studies with the gingival cell culture, Gie-3B11, and other epithelial models, Trends Cell Mol Biol
Rybakovsky, Valenzano, Deis, Diguilio, Thomas et al., Improvement of human-oral-epithelial-barrier function and of tight junctions by micronutrients, J Agric Food Chem, doi:10.1021/acs.jafc.7b04203
Sarohan, Akelma, Araç, Aslan, Cen, Retinol depletion in COVID-19, Clin Nutr Open Sci, doi:10.1016/j.nutos.2022.05.007
Schmitz, Fromm, Bentzel, Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6, J Cell Sci, doi:10.1242/jcs.112.1.137
Sengupta, Roldan, Kiener, A new immortalized human alveolar epithelial cell model to study lung injury and toxicity on a breathing lung-onchip system, Front Toxicol, doi:10.3389/ftox.2022.840606
Shen, Finkbeiner, Wine, Mrsny, Widdicombe, Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl-secretion
Shen, Tight junctions on the move: molecular mechanisms for epithelial barrier regulation, Ann N Y Acad Sci, doi:10.1111/j.1749-6632.2012.06613.x
Shepley-Mctaggart, Sagum, Oliva, SARS-CoV-2 envelope (E) protein interacts with PDZ-domain-2 of host tight junction protein ZO1, PLoS One, doi:10.1371/journal.pone.0251955
Shi, Lai, Teboul, COVID-19 ARDS is characterized by higher extravascular lung water than non-COVID-19 ARDS: the PiCCOVID study, Crit Care, doi:10.1186/s13054-021-03594-6
Shirvaliloo, The blood-gas barrier in COVID-19: an overview of the effects of SARS-CoV-2 infection on the alveolar epithelial and endothelial cells of the lung, Tissue Barriers, doi:10.1080/21688370.2021.1937013
Tatsuta, Kan, Ishii, Effects of cigarette smoke on barrier function and tight junction proteins in the bronchial epithelium: protective role of cathelicidin LL-37, Respir Res, doi:10.1186/s12931-019-1226-4
Tenenbaum, Matalon, Adam, Dexamethasone prevents alteration of tight junction-associated proteins and barrier function in porcine choroid plexus epithelial cells after infection with Streptococcus suis in vitro, Brain Res, doi:10.1016/j.brainres.2008.06.118
Tepasse, Vollenberg, Fobker, Vitamin A plasma levels in COVID-19 patients: a prospective multicenter study and hypothesis, Nutrients, doi:10.3390/nu13072173
Togami, Yamaguchi, Chono, Tada, Evaluation of permeability alteration and epithelial-mesenchymal transition induced by transforming growth factor-β 1 in A549, NCI-H441, and Calu-3 cells: development of an in vitro model of respiratory epithelial cells in idiopathic pulmonary fibrosis, J Pharmacol Toxicol Methods, doi:10.1016/j.vascn.2017.02.023
Trasino, A role for retinoids in the treatment of COVID-19?, Clin Exp Pharmacol Physiol, doi:10.1111/1440-1681.13354
Ueda, Ueda, Fukuda, Lipid hydroperoxide induced tumor necrosis factor (TNF)-α, vascular endothelial growth factor and neovascularization in the rabbit cornea: effect of TNF inhibition, Angiogenesis, doi:10.1023/A:1018377621102
Valentich, Tchao, Leighton, Hemicyst formation stimulated by cyclic AMP in dog kidney cell line MDCK, J Cell Physiol, doi:10.1002/jcp.1041000210
Voelkle, Gregoriano, Neyer, Prevalence of micronutrient deficiencies in patients hospitalized with COVID-19: an observational cohort study, Nutrients, doi:10.3390/nu14091862
Wine, Finkbeiner, Haws, CFTR and other Cl-channels in human airway cells, Jpn J Physiol
Wynne, Zou, Linck, Hoover, Ma et al., Regulation of lung epithelial sodium channels by cytokines and chemokines, Front Immunol, doi:10.3389/fimmu.2017.00766
Zech, Pouvreau, Cotinet, Goureau, Varlet et al., Effect of cytokines and nitric oxide on tight junctions in cultured rat retinal pigment epithelium, Invest Ophthalmol Vis Sci
Zeni, Doepker, Schulze-Topphoff, Huewel, Tenenbaum et al., MMPs contribute to TNF-alpha induced alteration of the blood-cerebrospinal fluid barrier in vitro, Am J Physiol Cell Physiol, doi:10.1152/ajpcell.00470.2006
Zhao, Zhang, Wu, Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS induced acute colitis in mice, BMC Gastroenterol, doi:10.1186/1471-230X-12-57
DOI record:
{
"DOI": "10.1080/01902148.2023.2193637",
"ISSN": [
"0190-2148",
"1521-0499"
],
"URL": "http://dx.doi.org/10.1080/01902148.2023.2193637",
"alternative-id": [
"10.1080/01902148.2023.2193637"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=ielu20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=ielu20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-09-09"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Revised",
"name": "revised",
"order": 1,
"value": "2023-03-03"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2023-03-09"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2023-03-31"
}
],
"author": [
{
"affiliation": [
{
"name": "Lankenau Institute for Medical Research, Wynnewood, PA, USA"
}
],
"family": "DiGuilio",
"given": "Katherine M.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Lankenau Institute for Medical Research, Wynnewood, PA, USA"
}
],
"family": "Rybakovsky",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Chemistry, Drexel University, Philadelphia, PA, USA"
}
],
"family": "Baek",
"given": "Yoongyeong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Lankenau Institute for Medical Research, Wynnewood, PA, USA"
}
],
"family": "Valenzano",
"given": "Mary Carmen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Lankenau Institute for Medical Research, Wynnewood, PA, USA"
}
],
"family": "Mullin",
"given": "James M.",
"sequence": "additional"
}
],
"container-title": "Experimental Lung Research",
"container-title-short": "Experimental Lung Research",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2023,
3,
31
]
],
"date-time": "2023-03-31T14:27:10Z",
"timestamp": 1680272830000
},
"deposited": {
"date-parts": [
[
2023,
3,
31
]
],
"date-time": "2023-03-31T14:27:17Z",
"timestamp": 1680272837000
},
"indexed": {
"date-parts": [
[
2023,
4,
1
]
],
"date-time": "2023-04-01T04:49:39Z",
"timestamp": 1680324579762
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
3,
31
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
31
]
],
"date-time": "2023-03-31T00:00:00Z",
"timestamp": 1680220800000
}
}
],
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/01902148.2023.2193637",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "1-14",
"prefix": "10.1080",
"published": {
"date-parts": [
[
2023,
3,
31
]
]
},
"published-online": {
"date-parts": [
[
2023,
3,
31
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1006/cyto.1995.0060",
"doi-asserted-by": "publisher",
"key": "CIT0001"
},
{
"DOI": "10.1007/s002329900333",
"doi-asserted-by": "publisher",
"key": "CIT0002"
},
{
"DOI": "10.1152/ajpgi.00412.2004",
"doi-asserted-by": "publisher",
"key": "CIT0003"
},
{
"author": "Mullin JM",
"first-page": "2172",
"issue": "7",
"journal-title": "Cancer Res",
"key": "CIT0004",
"volume": "50",
"year": "1990"
},
{
"DOI": "10.1016/j.jaci.2013.03.005",
"doi-asserted-by": "publisher",
"key": "CIT0005"
},
{
"DOI": "10.1371/journal.pone.0242536",
"doi-asserted-by": "publisher",
"key": "CIT0006"
},
{
"DOI": "10.1152/ajpcell.00470.2006",
"doi-asserted-by": "publisher",
"key": "CIT0007"
},
{
"DOI": "10.1016/j.cyto.2018.12.009",
"doi-asserted-by": "publisher",
"key": "CIT0008"
},
{
"author": "Rybakovsky E",
"first-page": "99",
"journal-title": "Trends Cell Mol Biol",
"key": "CIT0009",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1023/A:1018377621102",
"doi-asserted-by": "publisher",
"key": "CIT0010"
},
{
"DOI": "10.2337/db09-1606",
"doi-asserted-by": "publisher",
"key": "CIT0011"
},
{
"DOI": "10.3390/ijms23062995",
"doi-asserted-by": "publisher",
"key": "CIT0012"
},
{
"DOI": "10.1111/j.1749-6632.2012.06613.x",
"doi-asserted-by": "publisher",
"key": "CIT0013"
},
{
"DOI": "10.3389/fimmu.2021.767456",
"doi-asserted-by": "publisher",
"key": "CIT0014"
},
{
"DOI": "10.3390/ijms21030993",
"doi-asserted-by": "publisher",
"key": "CIT0015"
},
{
"DOI": "10.3389/ftox.2022.840606",
"doi-asserted-by": "publisher",
"key": "CIT0016"
},
{
"DOI": "10.1371/journal.pone.0251955",
"doi-asserted-by": "publisher",
"key": "CIT0017"
},
{
"DOI": "10.3390/membranes11030197",
"doi-asserted-by": "publisher",
"key": "CIT0018"
},
{
"DOI": "10.1128/mBio.02852-20",
"doi-asserted-by": "publisher",
"key": "CIT0019"
},
{
"DOI": "10.1080/21688370.2021.1937013",
"doi-asserted-by": "publisher",
"key": "CIT0020"
},
{
"author": "Wine JJ",
"first-page": "S199",
"issue": "2",
"journal-title": "Jpn J Physiol",
"key": "CIT0021",
"volume": "44",
"year": "1994"
},
{
"DOI": "10.1152/ajplung.1994.266.5.L493",
"doi-asserted-by": "publisher",
"key": "CIT0022"
},
{
"DOI": "10.1152/ajplung.1994.266.5.L502",
"doi-asserted-by": "publisher",
"key": "CIT0023"
},
{
"DOI": "10.1016/j.vascn.2017.02.023",
"doi-asserted-by": "publisher",
"key": "CIT0024"
},
{
"DOI": "10.1186/s12931-019-1226-4",
"doi-asserted-by": "publisher",
"key": "CIT0025"
},
{
"DOI": "10.3390/ijms21103578",
"doi-asserted-by": "publisher",
"key": "CIT0026"
},
{
"DOI": "10.1152/ajplung.00056.2021",
"doi-asserted-by": "publisher",
"key": "CIT0027"
},
{
"DOI": "10.3389/fimmu.2017.00766",
"doi-asserted-by": "publisher",
"key": "CIT0028"
},
{
"DOI": "10.1186/s13054-021-03594-6",
"doi-asserted-by": "publisher",
"key": "CIT0029"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"doi-asserted-by": "publisher",
"key": "CIT0030"
},
{
"DOI": "10.3389/fphys.2020.00872",
"doi-asserted-by": "publisher",
"key": "CIT0031"
},
{
"DOI": "10.1038/labinvest.2012.67",
"doi-asserted-by": "publisher",
"key": "CIT0032"
},
{
"DOI": "10.1152/ajplung.00187.2003",
"doi-asserted-by": "publisher",
"key": "CIT0033"
},
{
"DOI": "10.1242/jcs.112.1.137",
"doi-asserted-by": "publisher",
"key": "CIT0034"
},
{
"author": "Zech JC",
"first-page": "1600",
"issue": "9",
"journal-title": "Invest Ophthalmol Vis Sci",
"key": "CIT0035",
"volume": "39",
"year": "1998"
},
{
"DOI": "10.1152/ajpcell.00388.2014",
"doi-asserted-by": "publisher",
"key": "CIT0036"
},
{
"DOI": "10.1152/ajpgi.00173.2003",
"doi-asserted-by": "publisher",
"key": "CIT0037"
},
{
"DOI": "10.1002/(SICI)1097-4652(199705)171:2 < 226:",
"doi-asserted-by": "publisher",
"key": "CIT0038"
},
{
"DOI": "10.1016/j.bbrc.2015.03.125",
"doi-asserted-by": "publisher",
"key": "CIT0039"
},
{
"DOI": "10.1091/mbc.e09-01-0080",
"doi-asserted-by": "publisher",
"key": "CIT0040"
},
{
"DOI": "10.1002/jcp.1041000210",
"doi-asserted-by": "publisher",
"key": "CIT0041"
},
{
"DOI": "10.1007/BF01871696",
"doi-asserted-by": "publisher",
"key": "CIT0042"
},
{
"DOI": "10.1007/BF02626458",
"doi-asserted-by": "publisher",
"key": "CIT0043"
},
{
"DOI": "10.1152/ajpgi.90227.2008",
"doi-asserted-by": "publisher",
"key": "CIT0044"
},
{
"DOI": "10.1074/jbc.M117.815381",
"doi-asserted-by": "publisher",
"key": "CIT0045"
},
{
"DOI": "10.1152/ajpcell.00185.2020",
"doi-asserted-by": "publisher",
"key": "CIT0046"
},
{
"author": "Kondo J",
"first-page": "645",
"issue": "5",
"journal-title": "Int J Mol Med",
"key": "CIT0047",
"volume": "22",
"year": "2008"
},
{
"DOI": "10.1152/ajplung.00182.2003",
"doi-asserted-by": "publisher",
"key": "CIT0048"
},
{
"DOI": "10.1016/j.brainres.2008.06.118",
"doi-asserted-by": "publisher",
"key": "CIT0049"
},
{
"DOI": "10.3390/foods11121714",
"doi-asserted-by": "publisher",
"key": "CIT0050"
},
{
"DOI": "10.1021/acs.jafc.7b04203",
"doi-asserted-by": "publisher",
"key": "CIT0051"
},
{
"DOI": "10.1111/jre.12351",
"doi-asserted-by": "publisher",
"key": "CIT0052"
},
{
"DOI": "10.1016/j.bbrc.2011.03.080",
"doi-asserted-by": "publisher",
"key": "CIT0053"
},
{
"DOI": "10.1083/jcb.68.2.173",
"doi-asserted-by": "publisher",
"key": "CIT0054"
},
{
"DOI": "10.1111/j.1440-169x.2004.00765.x",
"doi-asserted-by": "publisher",
"key": "CIT0055"
},
{
"DOI": "10.1152/ajpcell.1997.273.5.C1707",
"doi-asserted-by": "publisher",
"key": "CIT0056"
},
{
"DOI": "10.1152/ajpgi.00398.2007",
"doi-asserted-by": "publisher",
"key": "CIT0057"
},
{
"DOI": "10.1186/1471-230X-12-57",
"doi-asserted-by": "publisher",
"key": "CIT0058"
},
{
"DOI": "10.3390/ijms22168872",
"doi-asserted-by": "publisher",
"key": "CIT0059"
},
{
"DOI": "10.1152/ajpgi.1998.274.2.G389",
"doi-asserted-by": "publisher",
"key": "CIT0060"
},
{
"DOI": "10.1016/j.nut.2020.111106",
"doi-asserted-by": "publisher",
"key": "CIT0061"
},
{
"DOI": "10.1080/07315724.2021.1877580",
"doi-asserted-by": "publisher",
"key": "CIT0062"
},
{
"DOI": "10.1016/j.ijid.2020.12.077",
"doi-asserted-by": "publisher",
"key": "CIT0063"
},
{
"DOI": "10.1002/jmv.26360",
"doi-asserted-by": "publisher",
"key": "CIT0064"
},
{
"DOI": "10.1111/febs.15495",
"doi-asserted-by": "publisher",
"key": "CIT0065"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"doi-asserted-by": "publisher",
"key": "CIT0066"
},
{
"DOI": "10.1136/bmj.i6583",
"doi-asserted-by": "publisher",
"key": "CIT0067"
},
{
"DOI": "10.1093/ajcn/nqaa381",
"doi-asserted-by": "publisher",
"key": "CIT0068"
},
{
"DOI": "10.1136/postgradmedj-2020-139065",
"doi-asserted-by": "publisher",
"key": "CIT0069"
},
{
"DOI": "10.17305/bjbms.2021.7009",
"doi-asserted-by": "publisher",
"key": "CIT0070"
},
{
"DOI": "10.1007/s12011-022-03290-8",
"doi-asserted-by": "publisher",
"key": "CIT0071"
},
{
"DOI": "10.3390/nu14091862",
"doi-asserted-by": "publisher",
"key": "CIT0072"
},
{
"DOI": "10.3390/nu13072173",
"doi-asserted-by": "publisher",
"key": "CIT0073"
},
{
"DOI": "10.1016/j.nutos.2022.05.007",
"doi-asserted-by": "publisher",
"key": "CIT0074"
},
{
"DOI": "10.1155/2022/3323825",
"doi-asserted-by": "publisher",
"key": "CIT0075"
},
{
"DOI": "10.1002/rmv.2204",
"doi-asserted-by": "publisher",
"key": "CIT0076"
},
{
"DOI": "10.1111/1440-1681.13354",
"doi-asserted-by": "publisher",
"key": "CIT0077"
},
{
"DOI": "10.1007/s40520-020-01669-y",
"doi-asserted-by": "publisher",
"key": "CIT0078"
},
{
"DOI": "10.3390/v13081669",
"doi-asserted-by": "publisher",
"key": "CIT0079"
},
{
"DOI": "10.1371/journal.pone.0256141",
"doi-asserted-by": "publisher",
"key": "CIT0080"
},
{
"DOI": "10.1016/j.compbiomed.2020.104063",
"doi-asserted-by": "publisher",
"key": "CIT0081"
},
{
"DOI": "10.1089/vim.2020.0330",
"doi-asserted-by": "publisher",
"key": "CIT0082"
}
],
"reference-count": 82,
"references-count": 82,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/01902148.2023.2193637"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Clinical Biochemistry",
"Pulmonary and Respiratory Medicine",
"Molecular Biology"
],
"subtitle": [],
"title": "The multiphasic TNF-α-induced compromise of Calu-3 airway epithelial barrier function",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01"
}
diguilio2