Age differential CD13 and interferon expression in airway epithelia affect SARS-CoV-2 infection - effects of vitamin D
et al., Mucosal Immunology, doi:10.1016/j.mucimm.2023.08.002, Aug 2023
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
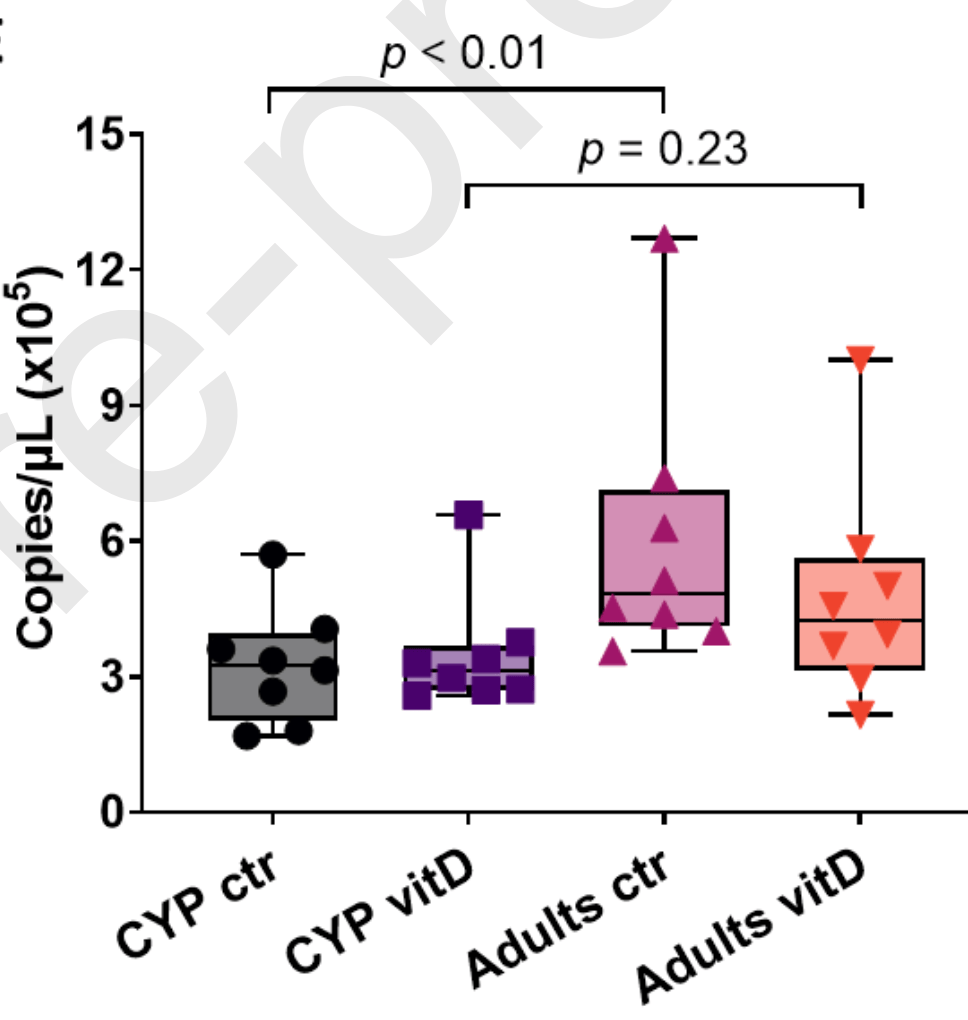
In vitro study showing that vitamin D reduced SARS-CoV-2 replication in adult nasal epithelial cells via increased type I interferon expression. Administration of vitamin D by systemic supplementation or direct nasal delivery may be beneficial for COVID-19. Results were age-dependent, with lower viral replication and treatment effect in pediatric epithelial cells.
29 preclinical studies support the efficacy of vitamin D for COVID-19:
Vitamin D has been identified by the European Food Safety Authority (EFSA) as having sufficient evidence for a causal relationship between intake and optimal immune system function27-30.
Vitamin D inhibits SARS-CoV-2 replication in vitro17,24, mitigates lung inflammation, damage, and lethality in mice with an MHV-3 model for β-CoV respiratory infections17,24, reduces SARS-CoV-2 replication in nasal epithelial cells via increased type I interferon expression20, downregulates proinflammatory cytokines IL-1β and TNF-α in SARS-CoV-2 spike protein-stimulated cells16, attenuates nucleocapsid protein-induced hyperinflammation by inactivating the NLRP3 inflammasome through the VDR-BRCC3 signaling pathway21, may be neuroprotective by protecting the blood-brain barrier, reducing neuroinflammation, and via immunomodulatory effects31, may mitigate hyperinflammation and cytokine storm by upregulating TLR10 expression which downregulates proinflammatory cytokines13, downregulates ACE2 and TMPRSS2 in human trophoblasts and minimizes spike protein-induced inflammation19, may minimize cytokine storm by dampening excessive cytokine production2, may suppress viral entry and replication via LL-37 induction11,12, and minimizes platelet aggregation mediated by SARS-CoV-2 spike protein via inhibiting integrin αIIbβ3 outside-in signaling15.
Cholecalciferol and calcifediol directly bind two allosteric pockets on the SARS-CoV-2 Spike RBD, bias the trimer toward a closed state, weaken ACE2 engagement, and reduce viral entry in cell models1.
Calcitriol may destabilize the Spike protein architecture and inhibit IL-17R dimerization, blocking viral entry and mitigating hyperinflammatory cytokine storm32.
Vitamin D improves regulatory immune cell levels and control of proinflammatory cytokines in severe COVID-1933.
Calcifediol inhibits SARS-CoV-2 papain-like protease (PLpro), a critical enzyme for viral replication14.
Symptomatic COVID-19 is associated with a lower frequency of natural killer (NK) cells and vitamin D has been shown to improve NK cell activity34,35.
1.
García-Marín et al., Exploring SARS-CoV-2 Spike RBD Pockets as Targets for Generic Drugs: A Combined Computational, Biophysical, and Biological Approach, ACS Omega, doi:10.1021/acsomega.5c05175.
2.
Alzahrani, A., A new investigation into the molecular mechanism of cholecalciferol towards reducing cytokines storm, Octahedron Drug Research, doi:10.21608/odr.2024.308273.1043.
3.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
4.
Morales-Bayuelo et al., New findings on ligand series used as SARS-CoV-2 virus inhibitors within the frameworks of molecular docking, molecular quantum similarity and chemical reactivity indices, F1000Research, doi:10.12688/f1000research.123550.3.
5.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
6.
Pandya et al., Unravelling Vitamin B12 as a potential inhibitor against SARS-CoV-2: A computational approach, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2022.100951.
7.
Mansouri et al., The impact of calcitriol and estradiol on the SARS-CoV-2 biological activity: a molecular modeling approach, Scientific Reports, doi:10.1038/s41598-022-04778-y.
8.
Song et al., Vitamin D3 and its hydroxyderivatives as promising drugs against COVID-19: a computational study, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1964601.
9.
Qayyum et al., Vitamin D and lumisterol novel metabolites can inhibit SARS-CoV-2 replication machinery enzymes, Endocrinology and Metabolism, doi:10.1152/ajpendo.00174.2021.
10.
Al-Mazaideh et al., Vitamin D is a New Promising Inhibitor to the Main Protease (Mpro) of COVID-19 by Molecular Docking, Journal of Pharmaceutical Research International, doi:10.9734/jpri/2021/v33i29B31603.
11.
Roth et al., Vitamin D-inducible antimicrobial peptide LL-37 binds SARS-CoV-2 Spike and accessory proteins ORF7a and ORF8, Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2025.1671738.
12.
Vercellino et al., Influence of Sex and 1,25α Dihydroxyvitamin D3 on SARS-CoV-2 Infection and Viral Entry, Pathogens, doi:10.3390/pathogens14080765.
13.
Knez et al., TLR10 overexpression modulates immune response in A549 lung epithelial cells challenged with SARS-CoV-2 S and N proteins, Frontiers in Immunology, doi:10.3389/fimmu.2024.1490478.
14.
Chen et al., In Vitro Characterization of Inhibition Function of Calcifediol to the Protease Activity of SARS-COV-2 PLpro, Journal of Medical Virology, doi:10.1002/jmv.70085.
15.
Wang et al., 1,25‐Dihydroxyvitamin D3 attenuates platelet aggregation potentiated by SARS‐CoV‐2 spike protein via inhibiting integrin αIIbβ3 outside‐in signaling, Cell Biochemistry and Function, doi:10.1002/cbf.4039.
16.
Alcalá-Santiago et al., Disentangling the Immunomodulatory Effects of Vitamin D on the SARS-CoV-2 Virus by In Vitro Approaches, The 14th European Nutrition Conference FENS 2023, doi:10.3390/proceedings2023091415.
17.
Campolina-Silva et al., Dietary Vitamin D Mitigates Coronavirus-Induced Lung Inflammation and Damage in Mice, Viruses, doi:10.3390/v15122434.
18.
Moatasim et al., Potent Antiviral Activity of Vitamin B12 against Severe Acute Respiratory Syndrome Coronavirus 2, Middle East Respiratory Syndrome Coronavirus, and Human Coronavirus 229E, Microorganisms, doi:10.3390/microorganisms11112777.
19.
Vargas-Castro et al., Calcitriol prevents SARS-CoV spike-induced inflammation in human trophoblasts through downregulating ACE2 and TMPRSS2 expression, The Journal of Steroid Biochemistry and Molecular Biology, doi:10.1016/j.jsbmb.2024.106625.
20.
Sposito et al., Age differential CD13 and interferon expression in airway epithelia affect SARS-CoV-2 infection - effects of vitamin D, Mucosal Immunology, doi:10.1016/j.mucimm.2023.08.002.
21.
Chen (B) et al., Vitamin D3 attenuates SARS‐CoV‐2 nucleocapsid protein‐caused hyperinflammation by inactivating the NLRP3 inflammasome through the VDR‐BRCC3 signaling pathway in vitro and in vivo, MedComm, doi:10.1002/mco2.318.
22.
Rybakovsky et al., Calcitriol modifies tight junctions, improves barrier function, and reduces TNF‐α‐induced barrier leak in the human lung‐derived epithelial cell culture model, 16HBE 14o‐, Physiological Reports, doi:10.14814/phy2.15592.
23.
DiGuilio et al., The multiphasic TNF-α-induced compromise of Calu-3 airway epithelial barrier function, Experimental Lung Research, doi:10.1080/01902148.2023.2193637.
24.
Pickard et al., Discovery of re-purposed drugs that slow SARS-CoV-2 replication in human cells, PLOS Pathogens, doi:10.1371/journal.ppat.1009840.
25.
Mok et al., Calcitriol, the active form of vitamin D, is a promising candidate for COVID-19 prophylaxis, bioRxiv, doi:10.1101/2020.06.21.162396.
26.
Fernandes de Souza et al., Lung Inflammation Induced by Inactivated SARS-CoV-2 in C57BL/6 Female Mice Is Controlled by Intranasal Instillation of Vitamin D, Cells, doi:10.3390/cells12071092.
27.
Galmés et al., Suboptimal Consumption of Relevant Immune System Micronutrients Is Associated with a Worse Impact of COVID-19 in Spanish Populations, Nutrients, doi:10.3390/nu14112254.
28.
Galmés (B) et al., Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework, Nutrients, doi:10.3390/nu12092738.
29.
EFSA, Scientific Opinion on the substantiation of a health claim related to vitamin D and contribution to the normal function of the immune system pursuant to Article 14 of Regulation (EC) No 1924/2006, EFSA Journal, doi:10.2903/j.efsa.2015.4096.
30.
EFSA (B), Scientific Opinion on the substantiation of health claims related to vitamin D and normal function of the immune system and inflammatory response (ID 154, 159), maintenance of normal muscle function (ID 155) and maintenance of normal cardiovascular function (ID 159) pursuant to Article 13(1) of Regulation (E, EFSA Journal, doi:10.2903/j.efsa.2010.1468.
31.
Gotelli et al., Understanding the immune-endocrine effects of vitamin D in SARS-CoV-2 infection: a role in protecting against neurodamage?, Neuroimmunomodulation, doi:10.1159/000533286.
32.
Fadel et al., Targeting asparagine and cysteine in SARS-CoV-2 variants and human pro-inflammatory mediators to alleviate COVID-19 severity; a cross-section and in-silico study, Scientific Reports, doi:10.1038/s41598-025-19359-y.
33.
Saheb Sharif-Askari et al., Increased blood immune regulatory cells in severe COVID-19 with autoantibodies to type I interferons, Scientific Reports, doi:10.1038/s41598-023-43675-w.
Sposito et al., 11 Aug 2023, peer-reviewed, 10 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Journal Pre-proofs Article Age differential CD13 and interferon expression in airway epithelia affect SARS-CoV-2 infection -effects of vitamin D
doi:10.1016/j.mucimm.2023.08.002
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONTRIBUTORS CMH, PSM and FS designed the study. FS performed most experiments, including cell culture, transfections, luciferase experiments, protein Co-IPs, Western blots, ChIP, PCRs, and analysed data. SN performed the NanoString experiments. SHP, CAWD, JD, GB, and NJL performed the SARS-CoV-2 infections. AC contributed to the gene expression and DNA methylation data analysis. FS, CMH, and PSM wrote the manuscript. All authors read, edited, and approved the final version of the manuscript.
DECLARATION OF COMPETING INTERESTS The authors have no competing interests to declare.
AKNOWLEDGMENTS
References
Berni Canani, Comegna, Paparo, Cernera, Bruno et al., Age-Related Differences in the Expression of Most Relevant Mediators of SARS-CoV-2 Infection in Human Respiratory and Gastrointestinal Tract, Front. Pediatr, doi:10.3389/fped.2021.697390
Berry, Hesketh, Power, Hyppönen, Vitamin D status has a linear association with seasonal infections and lung function in British adults, Br. J. Nutr, doi:10.1017/S0007114511001991
Brockman-Schneider, Pickles, Gern, Effects of Vitamin D on Airway Epithelial Cell Morphology and Rhinovirus Replication, doi:10.1371/journal.pone.0086755
Brunvoll, Nygaard, Ellingjord-Dale, Holland, Istre et al., Prevention of covid-19 and other acute respiratory infections with cod liver oil supplementation, a low dose vitamin D supplement: quadruple blinded, randomised placebo controlled trial, BMJ, doi:10.1136/bmj-2022-071245
Cantuti-Castelvetri, Ojha, Pedro, Djannatian, Franz et al., Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity, Science, doi:10.1126/science.abd2985
Crouse, Kalinke, Oxenius, Regulation of antiviral T cell responses by type I interferons, Nat. Rev. Immunol, doi:10.1038/nri3806
Dror, Morozov, Daoud, Namir, Orly et al., Pre-infection 25-hydroxyvitamin D3 levels and association with severity of COVID-19 illness, doi:10.1101/2021.06.04.21258358
Felsenstein, Hedrich, SARS-CoV-2 infections in children and young people, Clin. Immunol, doi:10.1016/j.clim.2020.108588
Felsenstein, Herbert, Mcnamara, Hedrich, COVID-19: Immunology and treatment options, Clin. Immunol, doi:10.1016/j.clim.2020.108448
Gallagher, Vitamin, None, Endocrinol. Metab. Clin. North Am, doi:10.1016/j.ecl.2013.02.004
Gao, Ding, Dong, Zhang, Kursat Azkur et al., Risk factors for severe and critically ill COVID-19 patients: A review, Allergy, doi:10.1111/all.14657
Ghelani, Alesi, Mousa, Vitamin D and COVID-19: An Overview of Recent Evidence, Int. J. Mol. Sci, doi:10.3390/ijms221910559
Gibney, Nolan, Epigenetics and gene expression, Heredity, doi:10.1038/hdy.2010.54
Greiller, Suri, Jolliffe, Kebadze, Hirsman et al., Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2018.11.013
Hafezi, Saheb Sharif-Askari, Saheb Sharif-Askari, Ali Hussain Alsayed, Alsafar et al., Vitamin D enhances type I IFN signaling in COVID-19 patients, Sci. Rep, doi:10.1038/s41598-022-22307-9
Hedrich, Bream, Cell type-specific regulation of IL-10 expression in inflammation and disease, Immunol. Res, doi:10.1007/s12026-009-8150-5
Hedrich, Mäbert, Rauen, Tsokos, DNA methylation in systemic lupus erythematosus, Epigenomics, doi:10.2217/epi-2016-0096
Hedrich, Ramakrishnan, Dabitao, Wang, Ranatunga et al., Dynamic DNA methylation patterns across the mouse and human IL10 genes during CD4+ T cell activation; influence of IL-27, Mol. Immunol, doi:10.1016/j.molimm.2010.09.009
Ho, Most, Perl, Diaz, Casazza et al., Incidence and Risk Factors for Severe Outcomes in Pediatric Patients With COVID-19
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Jolliffe, Holt, Greenig, Talaei, Perdek et al., Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and covid-19: phase 3 randomised controlled trial (CORONAVIT), BMJ, doi:10.1136/bmj-2022-071230
Ju, Zhu, Lei, Yan, Zhu et al., TTLL12 Inhibits the Activation of Cellular Antiviral Signaling through Interaction with VISA/MAVS, J. Immunol, doi:10.4049/jimmunol.1601194
Lachance, Arbour, Cashman, Talbot, Involvement of Aminopeptidase N (CD13) in Infection of Human Neural Cells by Human Coronavirus 229E, J. Virol, doi:10.1128/JVI.72.8.6511-6519.1998
Lim, Ng, Tam, Liu, Human Coronaviruses: A Review of Virus-Host Interactions, doi:10.3390/diseases4030026
Mahmoodpoor, Sanaie, Roudbari, Sabzevari, Sohrabifar et al., Understanding the role of telomere attrition and epigenetic signatures in COVID-19 severity, Gene, doi:10.1016/j.gene.2021.146069
Martineau, Jolliffe, Hooper, Greenberg, Aloia et al., Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, BMJ, doi:10.1136/bmj.i6583
Mcnab, Mayer-Barber, Sher, Wack, O'garra, Type I interferons in infectious disease, Nat. Rev. Immunol, doi:10.1038/nri3787
Merzon, Tworowski, Gorohovski, Vinker, Golan Cohen et al., Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study, FEBS J, doi:10.1111/febs.15495
Moore, Le, Fan, None, DNA Methylation and Its Basic Function, doi:10.1038/npp.2012.112
Müller, Brighton, Carson, Fischer, Jaspers, Culturing of Human Nasal Epithelial Cells at the Air Liquid Interface, J. Vis. Exp, doi:10.3791/50646
Nomura, Kiyota, Suzaki, Kataoka, Ohe et al., Human Coronavirus 229E Binds to CD13 in Rafts and Enters the Cell through Caveolae, J. Virol, doi:10.1128/JVI.78.16.8701-8708.2004
Ong, Booth, Parnell, Vitamin D and its Effects on DNA Methylation in Development, Aging, and Disease, doi:10.1002/mnfr.202000437
Pike, Genome-wide principles of gene regulation by the vitamin D receptor and its activating ligand, Mol. Cell. Endocrinol, doi:10.1016/j.mce.2011.05.012
Ramagopalan, Heger, Berlanga, Maugeri, Lincoln et al., A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution, Genome Res, doi:10.1101/gr.107920.110
Ricci, Pagliuca, D'ascanio, Innammorato, Vitis et al., Circulating Vitamin D levels status and clinical prognostic indices in COVID-19 patients, Respir. Res, doi:10.1186/s12931-021-01666-3
Ruiz-Aravena, Mckee, Gamble, Lunn, Morris et al., Ecology, evolution and spillover of coronaviruses from bats, Nat. Rev. Microbiol, doi:10.1038/s41579-021-00652-2
Saheb Sharif-Askari, Saheb Sharif-Askari, Alabed, Temsah, Al et al., Airways Expression of SARS-CoV-2 Receptor, ACE2, and TMPRSS2 Is Lower in Children Than Adults and Increases with Smoking and COPD, Mol. Ther. -Methods Clin. Dev, doi:10.1016/j.omtm.2020.05.013
Smith, Sandrini, Datta, Freestone, Shafeeq et al., Respiratory Syncytial Virus Increases the Virulence of Streptococcus pneumoniae by Binding to Penicillin Binding Protein 1a. A New Paradigm in Respiratory Infection, Am. J. Respir. Crit. Care Med, doi:10.1164/rccm.201311-2110OC
Statsenko, Al Zahmi, Habuza, Almansoori, Smetanina et al., Impact of Age and Sex on COVID-19 Severity Assessed From Radiologic and Clinical Findings, doi:10.3389/fcimb.2021.777070
Stölting, Baillon, Frise, Bonner, Hewitt et al., Distinct airway epithelial immune responses after infection with SARS-CoV-2 compared to H1N1, Mucosal Immunol, doi:10.1038/s41385-022-00545-4
Tesser, De Carvalho, Sandrin-Garcia, Pin, Pastore et al., Higher interferon score and normal complement levels may identify a distinct clinical subset in children with systemic lupus erythematosus, Arthritis Res. Ther, doi:10.1186/s13075-020-02161-8
Varikasuvu, Thangappazham, Vykunta, Duggina, Manne et al., COVID-19 and vitamin D (Co-VIVID study): a systematic review and metaanalysis of randomized controlled trials, Expert Rev. Anti Infect. Ther, doi:10.1080/14787210.2022.2035217
Wan, Oliver, Wang, Zhu, Zack et al., Characterization of tissue-specific differential DNA methylation suggests distinct modes of positive and negative gene expression regulation, BMC Genomics, doi:10.1186/s12864-015-1271-4
Wang, Horbinski, Wu, Liu, Sheng et al., NanoStringDiff: a novel statistical method for differential expression analysis based on NanoString nCounter data, Nucleic Acids Res, doi:10.1093/nar/gkw677
Wang, Liu, Hu, Wang, Liu et al., Epigenetic regulation of aging: implications for interventions of aging and diseases, Signal Transduct. Target. Ther, doi:10.1038/s41392-022-01211-8
Zhu, Zhang, Wang, Li, Yang et al., A Novel Coronavirus from Patients with Pneumonia in China, doi:10.1056/NEJMoa2001017
