Vitamin D enhances type I IFN signaling in COVID-19 patients
et al., Scientific Reports, doi:10.1038/s41598-022-22307-9, Oct 2022
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
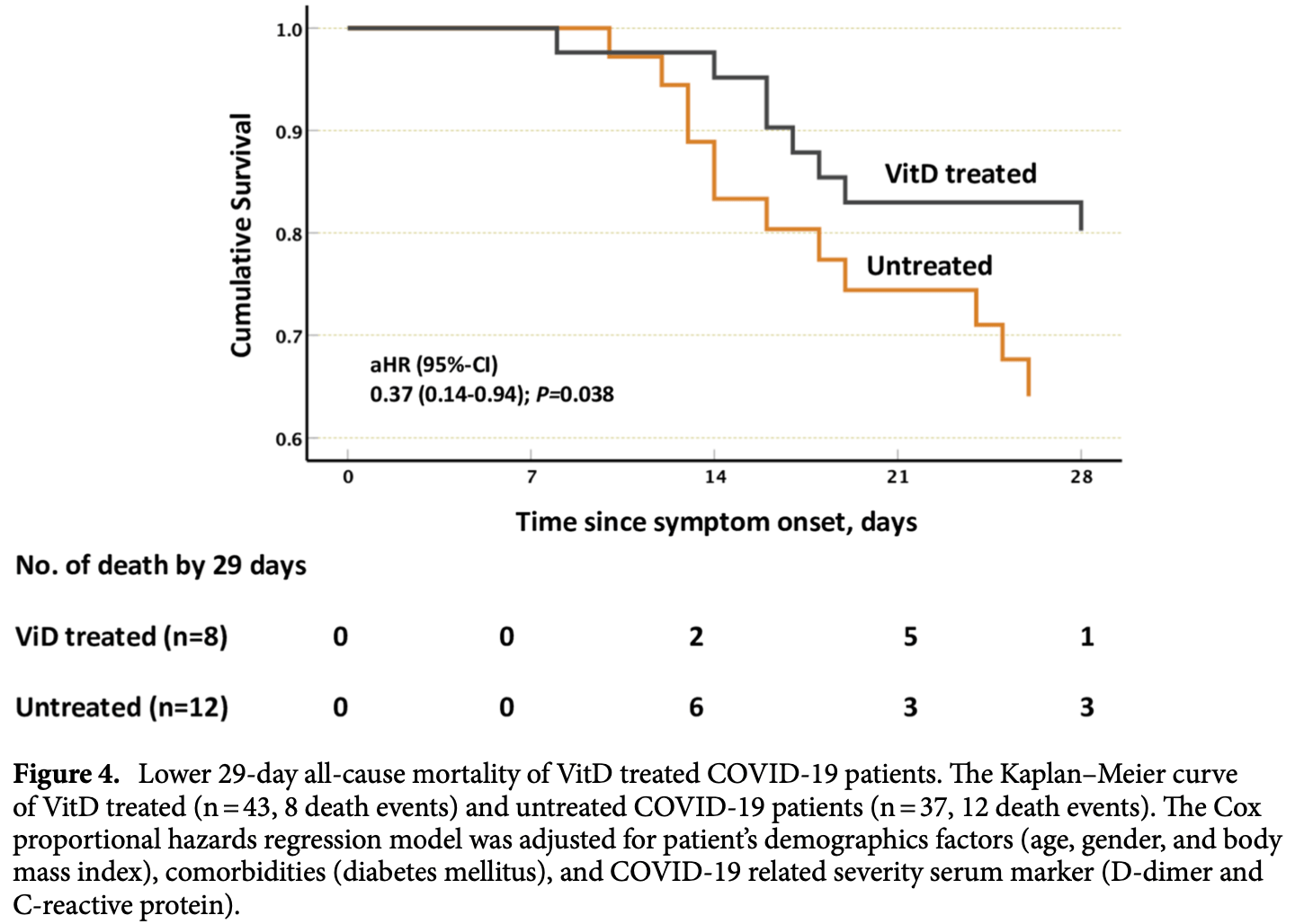
Retrospective 80 ICU patients, and in vitro study with human airway epithelial cells, showing that vitamin D enhances host IFN-a/β signaling. Significantly lower mortality was seen with vitamin D treatment.
Cholecalciferol was used in this study.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 45% [34‑54%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
This is the 102nd of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
40 studies are RCTs, which show efficacy with p=0.0000001.
This study is excluded in the after exclusion results of meta-analysis:
very late stage study using cholecalciferol instead of calcifediol or calcitriol.
|
risk of death, 63.0% lower, HR 0.37, p = 0.04, treatment 8 of 43 (18.6%), control 12 of 37 (32.4%), NNT 7.2, Cox proportional hazards, day 29.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hafezi et al., 22 Oct 2022, retrospective, United Arab Emirates, peer-reviewed, 8 authors, study period September 2020 - January 2021, dosage 50,000IU days 1, 8, 15.
Contact: rhalwani@sharjah.ac.ae.
Vitamin D enhances type I IFN signaling in COVID-19 patients
Scientific Reports, doi:10.1038/s41598-022-22307-9
The ability of Vitamin D (VitD) to modulate antiviral responses through induction of antimicrobial peptide is well established. However, the effect of VitD on host responses to SARS-CoV-2 is not well investigated. We here report the ability of VitD to enhance host IFN-alpha/beta (a/β) signaling both in vitro and among severe COVID-19 patients treated with VitD. Blood and saliva specimens were obtained from severe COVID-19 patients treated (43 patients), or not (37 patients), with vitD, during their stay in intensive care unit. Patients were followed up to 29 days following admission, and patient survival outcomes were collected. Higher activity levels of RIG-1/MDA-5 and JAK-STAT signaling pathways were observed with significantly higher gene and protein levels of antiviral interferon stimulating genes (ISGs) such as MX-1 and ISG-15; both in vitro, following treatment of PBMCs with vitD, and in whole blood and saliva specimens of VitD treated patients. Moreover, VitD treated patients had lower risk of all-cause mortality by day 29 compared to untreated patients (adjusted hazard ratio, 0.37, 95% confidence interval of 0.14-0.94; P = 0.038). The herein uncovered regulatory role of VitD on type I IFNs suggests the importance of insuring a normal level of VitD for the prevention and probably treatment of SARS-CoV-2 infection. Additional mechanistic studies, however, are needed to fully elucidate the antiviral effects of VitD particularly in the setting of COVID-19 infection. Innate immunity is critical for controlling SARS-CoV-2 infection; and patients with dysregulated innate immunity are prone to development of severe disease 1 . Type I interferons (IFN-α, and β) represent key elements of antiviral innate immunity. Several reports have shown that efficient induction of IFN-α/β signaling and the resultant interferon stimulating genes (ISGs) are essential for the control and resolution of SARS-CoV-2 infection 2 . SARS-CoV-2 is recognized in the cytosol of human epithelial cells by single-stranded (ss)RNA cytosolic sensing proteins (RIG-1 and MDA5) 3,4 . This will then lead to the downstream activation of interferon regulatory factors (IRF)3, or IRF7, and rapid production of IFN α/β cytokines, which exhibit key antiviral activity, thereby limiting viral proliferation and spreading 5 . IFN α/β cytokines bind to a dimeric receptor composed of IFNAR1 and IFNAR2 subunits; consequently, triggering the formation of transcription complex, IFN-stimulated gene factor 3 (ISGF3). ISGF3, which consists of phosphorylated signal transducer and activator of transcription STAT(1), STAT2 and IRF9, migrates to the nucleus, binds to interferon-stimulated response elements (ISREs), and activates transcription of anti-viral ISGs 6-8 . Collectively, the efficient induction of IFN-α/β signaling and ISGs in virus-infected cells is fundamental for the antiviral response of a host. As such, studies have reported that COVID-19 patients with a genetic defect in the production of..
Author contributions
Funding The authors extend their appreciation to the Deanship of Scientific Research, king Saud University for funding through Vice Deanship of Scientific Research Chairs; Research Chair of Prince Abdullah Ben Khalid Celiac Disease research chair; Riyadh, Kingdom of Saudi Arabia.
Competing interests The authors declare no competing interests.
References
Alavi Darazam, Role of interferon therapy in severe COVID-19: The COVIFERON randomized controlled trial, Sci. Rep
Alsafar, COVID-19 Disease severity and death in relation to vitamin D status among SARS-CoV-2-positive UAE residents, Nutrients
Bastard, Autoantibodies against type I IFNs in patients with life-threatening COVID-19, Science
Beard, Bearden, Striker, Vitamin D and the anti-viral state, J. Clin. Virol
Carlberg, In vivo response of the human epigenome to vitamin D: A proof-of-principle study, J. Steroid Biochem. Mol. Biol
Casanova, A global effort to define the human genetics of protective immunity to SARS-CoV-2 infection, Cell
Castillo, Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J. Steroid Biochem. Mol. Biol
Cereda, Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy Italy, Nutrition
Chan, treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset, J. Infect. Dis
Crafa, Influence of 25-hydroxy-cholecalciferol levels on SARS-CoV-2 infection and COVID-19 severity: A systematic review and meta-analysis, EClinicalMedicine
Dai, Activation of TLR3/interferon signaling pathway by bluetongue virus results in HIV inhibition in macrophages, FASEB J
Dudoit, Yang, Callow, Speed, Statistical methods for identifying differentially expressed genes in replicated cDNA microarray experiments, Stat. Sin
Dzimianski, Scholte, Bergeron, Pegan, ISG15: It's complicated, J. Mol. Biol
Feng, Interferon-stimulated gene (ISG)-expression screening reveals the specific antibunyaviral activity of ISG20, J. Virol
García-Morato, Impaired control of multiple viral infections in a family with complete IRF9 deficiency, J. Allergy Clin. Immunol
Goel, SARS-CoV-2 Switches 'on'MAPK and NFκB signaling via the reduction of nuclear DUSP1 and DUSP5 expression, Front. Pharmacol
Grant, Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths, Nutrients
Hanel, Common and personal target genes of the micronutrient vitamin D in primary immune cells from human peripheral blood, Sci. Rep
Hughey, Butte, Robust meta-analysis of gene expression using the elastic net, Nucleic Acids Res
Hung, Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial, The Lancet
Jadhav, Gokhale, Seervi, Patil, Alagarasu, Immunomodulatory effect of 1, 25 dihydroxy vitamin D3 on the expression of RNA sensing pattern recognition receptor genes and cytokine response in dengue virus infected U937-DC-SIGN cells and THP-1 macrophages, Int. Immunopharmacol
Kariuki, Mapping variation in cellular and transcriptional response to 1,25-dihydroxyvitamin D3 in peripheral blood mononuclear cells, PLoS ONE
Kuchipudi, 18S rRNA is a reliable normalisation gene for real time PCR based on influenza virus infected cells, Virol. J
Lee, Shin, The type I interferon response in COVID-19: Implications for treatment, Nat. Rev. Immunol
Li, Leung, Qureshi, Darnell, Stark, Formation of STAT1-STAT2 heterodimers and their role in the activation of IRF-1 Gene Transcription by Interferon-α(*), J. Biol. Chem
Malhani, Combination of (interferon beta-1b, lopinavir/ritonavir and ribavirin) versus favipiravir in hospitalized patients with non-critical COVID-19: A cohort study, PLOS ONE
Masood, Upregulated type I interferon responses in asymptomatic COVID-19 infection are associated with improved clinical outcome, Sci. Rep
Matsuyama, Kubli, Yoshinaga, Pfeffer, Mak, An aberrant STAT pathway is central to COVID-19, Cell Death Differ
Mdkhana, Nucleic acid-sensing pathways during SARS-CoV-2 infection: Expectations versus reality, J. Inflamm. Res
Muhammad, Saheb Sharif-Askari, Cui, Hamad, Halwani, SARS-CoV-2 infection-induced promoter hypomethylation as an epigenetic modulator of heat shock protein A1L (HSPA1L) gene, Front. Genet
Paun, Functional characterization of murine interferon regulatory factor 5 (IRF-5) and its role in the innate antiviral response, J. Biol. Chem
Rehwinkel, Gack, RIG-I-like receptors: Their regulation and roles in RNA sensing, Nat. Rev. Immunol
Ritchie, limma powers differential expression analyses for RNA-sequencing and microarray studies, Nucleic Acids Res
Rubin, Sorting out whether vitamin D deficiency raises COVID-19 risk, JAMA
Sapkota, COVID-19 salivary signature: Diagnostic and research opportunities, J. Clin. Pathol
Seuter, Neme, Carlberg, Epigenome-wide effects of vitamin D and their impact on the transcriptome of human monocytes involve CTCF, Nucleic Acids Res
Sharif-Askari, Low vitamin D serum level is associated with HDL-C Dyslipidemia and increased serum thrombomodulin levels of insulin-resistant individuals, Diabetes Metab. Syndrome Obes. Targets Ther
Sharif-Askari, Upregulation of interleukin-19 in severe asthma: A potential saliva biomarker for asthma severity, ERJ Open Res
Sharif-Askari, Vitamin D modulates systemic inflammation in patients with severe COVID-19, Life Sci
Smyth Gordon, Linear models and empirical bayes methods for assessing differential expression in microarray experiments, Stat. Appl. Genet. Mol. Biol
Suzannah, TRIM69 inhibits vesicular stomatitis Indiana virus, J. Virol
Teymoori-Rad, Shokri, Salimi, Marashi, The interplay between vitamin D and viral infections, Rev. Med. Virol
Uzé, Schreiber, Piehler, Pellegrini, The receptor of the type I interferon family
Verhelst, Parthoens, Schepens, Fiers, Saelens, Interferon-inducible protein Mx1 inhibits influenza virus by interfering with functional viral ribonucleoprotein complex assembly, J. Virol
Warwick, A hierarchical regulatory network analysis of the vitamin D induced transcriptome reveals novel regulators and complete VDR dependency in monocytes, Sci. Rep
White, Emerging roles of vitamin d-induced antimicrobial peptides in antiviral innate immunity, Nutrients
Yamada, RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells, Nat. Immunol
Yan, Phosphorylated interferon-alpha receptor 1 subunit (IFNaR1) acts as a docking site for the latent form of the 113 kDa STAT2 protein, EMBO J
Zhang, Inborn errors of type I IFN immunity in patients with life-threatening COVID-19, Science
Zhang, Saliva in the diagnosis of diseases, Int. J. Oral Sci
DOI record:
{
"DOI": "10.1038/s41598-022-22307-9",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-022-22307-9",
"abstract": "<jats:title>Abstract</jats:title><jats:p>The ability of Vitamin D (VitD) to modulate antiviral responses through induction of antimicrobial peptide is well established. However, the effect of VitD on host responses to SARS-CoV-2 is not well investigated. We here report the ability of VitD to enhance host IFN-alpha/beta (a/β) signaling both in vitro and among severe COVID-19 patients treated with VitD. Blood and saliva specimens were obtained from severe COVID-19 patients treated (43 patients), or not (37 patients), with vitD, during their stay in intensive care unit. Patients were followed up to 29 days following admission, and patient survival outcomes were collected. Higher activity levels of RIG-1/MDA-5 and JAK-STAT signaling pathways were observed with significantly higher gene and protein levels of antiviral interferon stimulating genes (ISGs) such as MX-1 and ISG-15; both in vitro, following treatment of PBMCs with vitD, and in whole blood and saliva specimens of VitD treated patients. Moreover, VitD treated patients had lower risk of all-cause mortality by day 29 compared to untreated patients (adjusted hazard ratio, 0.37, 95% confidence interval of 0.14–0.94; P = 0.038). The herein uncovered regulatory role of VitD on type I IFNs suggests the importance of insuring a normal level of VitD for the prevention and probably treatment of SARS-CoV-2 infection. Additional mechanistic studies, however, are needed to fully elucidate the antiviral effects of VitD particularly in the setting of COVID-19 infection.</jats:p>",
"alternative-id": [
"22307"
],
"article-number": "17778",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "10 March 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "12 October 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "22 October 2022"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Hafezi",
"given": "Shirin",
"sequence": "first"
},
{
"affiliation": [],
"family": "Saheb Sharif-Askari",
"given": "Fatemeh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saheb Sharif-Askari",
"given": "Narjes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ali Hussain Alsayed",
"given": "Hawra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alsafar",
"given": "Habiba",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al Anouti",
"given": "Fatme",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hamid",
"given": "Qutayba",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Halwani",
"given": "Rabih",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
10,
22
]
],
"date-time": "2022-10-22T13:05:16Z",
"timestamp": 1666443916000
},
"deposited": {
"date-parts": [
[
2022,
10,
22
]
],
"date-time": "2022-10-22T13:09:56Z",
"timestamp": 1666444196000
},
"indexed": {
"date-parts": [
[
2022,
10,
23
]
],
"date-time": "2022-10-23T04:55:42Z",
"timestamp": 1666500942669
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
10,
22
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
22
]
],
"date-time": "2022-10-22T00:00:00Z",
"timestamp": 1666396800000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
22
]
],
"date-time": "2022-10-22T00:00:00Z",
"timestamp": 1666396800000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-022-22307-9.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-22307-9",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-22307-9.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2022,
10,
22
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
22
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.cell.2020.05.016",
"author": "J-L Casanova",
"doi-asserted-by": "publisher",
"first-page": "1194",
"journal-title": "Cell",
"key": "22307_CR1",
"unstructured": "Casanova, J.-L. et al. A global effort to define the human genetics of protective immunity to SARS-CoV-2 infection. Cell 181, 1194–1199 (2020).",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/s41577-020-00429-3",
"author": "JS Lee",
"doi-asserted-by": "publisher",
"first-page": "585",
"journal-title": "Nat. Rev. Immunol.",
"key": "22307_CR2",
"unstructured": "Lee, J. S. & Shin, E.-C. The type I interferon response in COVID-19: Implications for treatment. Nat. Rev. Immunol. 20, 585–586 (2020).",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.2147/JIR.S277716",
"author": "B Mdkhana",
"doi-asserted-by": "publisher",
"first-page": "199",
"journal-title": "J. Inflamm. Res.",
"key": "22307_CR3",
"unstructured": "Mdkhana, B. et al. Nucleic acid-sensing pathways during SARS-CoV-2 infection: Expectations versus reality. J. Inflamm. Res. 14, 199–216 (2021).",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1038/s41590-021-00942-0",
"author": "T Yamada",
"doi-asserted-by": "publisher",
"first-page": "820",
"journal-title": "Nat. Immunol.",
"key": "22307_CR4",
"unstructured": "Yamada, T. et al. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat. Immunol. 22, 820–828 (2021).",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1038/s41577-020-0288-3",
"author": "J Rehwinkel",
"doi-asserted-by": "publisher",
"first-page": "537",
"journal-title": "Nat. Rev. Immunol.",
"key": "22307_CR5",
"unstructured": "Rehwinkel, J. & Gack, M. U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 20, 537–551 (2020).",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1074/jbc.271.10.5790",
"author": "X Li",
"doi-asserted-by": "publisher",
"first-page": "5790",
"journal-title": "J. Biol. Chem.",
"key": "22307_CR6",
"unstructured": "Li, X., Leung, S., Qureshi, S., Darnell, J. E. & Stark, G. R. Formation of STAT1-STAT2 heterodimers and their role in the activation of IRF-1 Gene Transcription by Interferon-α(∗). J. Biol. Chem. 271, 5790–5794 (1996).",
"volume": "271",
"year": "1996"
},
{
"DOI": "10.1007/978-3-540-71329-6_5",
"doi-asserted-by": "crossref",
"key": "22307_CR7",
"unstructured": "Uzé, G., Schreiber, G., Piehler, J. & Pellegrini, S. The receptor of the type I interferon family. in Interferon: The 50th Anniversary (ed. Pitha, P.M.) 71–95 (Springer Berlin Heidelberg, Berlin, Heidelberg, 2007)."
},
{
"DOI": "10.1002/j.1460-2075.1996.tb00444.x",
"author": "H Yan",
"doi-asserted-by": "publisher",
"first-page": "1064",
"journal-title": "EMBO J.",
"key": "22307_CR8",
"unstructured": "Yan, H. et al. Phosphorylated interferon-alpha receptor 1 subunit (IFNaR1) acts as a docking site for the latent form of the 113 kDa STAT2 protein. EMBO J. 15, 1064–1074 (1996).",
"volume": "15",
"year": "1996"
},
{
"key": "22307_CR9",
"unstructured": "Zhang, Q., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 370, eabd4570 (2020)."
},
{
"key": "22307_CR10",
"unstructured": "Bastard, P., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370, eabd4585 (2020)."
},
{
"DOI": "10.1371/journal.pone.0252984",
"doi-asserted-by": "crossref",
"key": "22307_CR11",
"unstructured": "Malhani, A., et al. Combination of (interferon beta-1b, lopinavir/ritonavir and ribavirin) versus favipiravir in hospitalized patients with non-critical COVID-19: A cohort study. PLOS ONE 16, e0252984 (2021)."
},
{
"DOI": "10.1038/s41598-021-86859-y",
"author": "I Alavi Darazam",
"doi-asserted-by": "publisher",
"first-page": "8059",
"journal-title": "Sci. Rep.",
"key": "22307_CR12",
"unstructured": "Alavi Darazam, I. et al. Role of interferon therapy in severe COVID-19: The COVIFERON randomized controlled trial. Sci. Rep. 11, 8059 (2021).",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)31042-4",
"author": "IF-N Hung",
"doi-asserted-by": "publisher",
"first-page": "1695",
"journal-title": "The Lancet",
"key": "22307_CR13",
"unstructured": "Hung, I.F.-N. et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. The Lancet 395, 1695–1704 (2020).",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiv392",
"author": "JF-W Chan",
"doi-asserted-by": "publisher",
"first-page": "1904",
"journal-title": "J. Infect. Dis.",
"key": "22307_CR14",
"unstructured": "Chan, J.F.-W. et al. treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 212, 1904–1913 (2015).",
"volume": "212",
"year": "2015"
},
{
"DOI": "10.1016/j.jcv.2010.12.006",
"author": "JA Beard",
"doi-asserted-by": "publisher",
"first-page": "194",
"journal-title": "J. Clin. Virol.",
"key": "22307_CR15",
"unstructured": "Beard, J. A., Bearden, A. & Striker, R. Vitamin D and the anti-viral state. J. Clin. Virol. 50, 194–200 (2011).",
"volume": "50",
"year": "2011"
},
{
"DOI": "10.2147/DMSO.S245742",
"author": "FS Sharif-Askari",
"doi-asserted-by": "publisher",
"first-page": "1599",
"journal-title": "Diabetes Metab. Syndrome Obes. Targets Ther.",
"key": "22307_CR16",
"unstructured": "Sharif-Askari, F. S. et al. Low vitamin D serum level is associated with HDL-C Dyslipidemia and increased serum thrombomodulin levels of insulin-resistant individuals. Diabetes Metab. Syndrome Obes. Targets Ther. 13, 1599 (2020).",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2021.100967",
"doi-asserted-by": "crossref",
"key": "22307_CR17",
"unstructured": "Crafa, A., et al. Influence of 25-hydroxy-cholecalciferol levels on SARS-CoV-2 infection and COVID-19 severity: A systematic review and meta-analysis. EClinicalMedicine 37(2021)."
},
{
"DOI": "10.3390/nu13051714",
"doi-asserted-by": "crossref",
"key": "22307_CR18",
"unstructured": "AlSafar, H., et al. COVID-19 Disease severity and death in relation to vitamin D status among SARS-CoV-2-positive UAE residents. Nutrients 13 (2021)."
},
{
"DOI": "10.20944/preprints202003.0235.v2",
"doi-asserted-by": "crossref",
"key": "22307_CR19",
"unstructured": "Grant, W.B., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 12(2020)."
},
{
"DOI": "10.1016/j.lfs.2022.120909",
"author": "FS Sharif-Askari",
"doi-asserted-by": "publisher",
"journal-title": "Life Sci.",
"key": "22307_CR20",
"unstructured": "Sharif-Askari, F. S. et al. Vitamin D modulates systemic inflammation in patients with severe COVID-19. Life Sci. 307, 120909 (2022).",
"volume": "307",
"year": "2022"
},
{
"DOI": "10.1002/rmv.2032",
"author": "M Teymoori-Rad",
"doi-asserted-by": "publisher",
"journal-title": "Rev. Med. Virol.",
"key": "22307_CR21",
"unstructured": "Teymoori-Rad, M., Shokri, F., Salimi, V. & Marashi, S. M. The interplay between vitamin D and viral infections. Rev. Med. Virol. 29, e2032 (2019).",
"volume": "29",
"year": "2019"
},
{
"DOI": "10.3390/nu14020284",
"doi-asserted-by": "crossref",
"key": "22307_CR22",
"unstructured": "White, J.H. Emerging roles of vitamin d-induced antimicrobial peptides in antiviral innate immunity. Nutrients 14 (2022)."
},
{
"DOI": "10.1128/JVI.02140-17",
"doi-asserted-by": "crossref",
"key": "22307_CR23",
"unstructured": "Feng, J., et al. Interferon-stimulated gene (ISG)-expression screening reveals the specific antibunyaviral activity of ISG20. J. Virol. 92, e02140–02117."
},
{
"DOI": "10.1128/JVI.00951-19",
"doi-asserted-by": "crossref",
"key": "22307_CR24",
"unstructured": "Rihn Suzannah, J., et al. TRIM69 inhibits vesicular stomatitis Indiana virus. J. Virol. 93, e00951–00919."
},
{
"key": "22307_CR25",
"unstructured": "Dubai Health Authority, https://services.dha.gov.ae/sheryan/wps/portal/home/circular-details?circularRefNo=CIR-2020-00000259&isPublicCircular=true&fromHome=true, Access date: 8 August, 2020."
},
{
"key": "22307_CR26",
"unstructured": "Interim Guidelines for Collecting and Handling of Clinical Specimens for COVID-19 Testing, https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html, Accessed 9 Sep 2020."
},
{
"DOI": "10.3389/fgene.2021.622271",
"doi-asserted-by": "crossref",
"key": "22307_CR27",
"unstructured": "Muhammad, J.S., Saheb Sharif-Askari, N., Cui, Z.-G., Hamad, M. & Halwani, R. SARS-CoV-2 infection-induced promoter hypomethylation as an epigenetic modulator of heat shock protein A1L (HSPA1L) gene. Front. Genet. 12(2021)."
},
{
"DOI": "10.3389/fphar.2021.631879",
"author": "S Goel",
"doi-asserted-by": "publisher",
"first-page": "404",
"journal-title": "Front. Pharmacol.",
"key": "22307_CR28",
"unstructured": "Goel, S. et al. SARS-CoV-2 Switches ‘on’MAPK and NFκB signaling via the reduction of nuclear DUSP1 and DUSP5 expression. Front. Pharmacol. 12, 404 (2021).",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1186/1743-422X-9-230",
"author": "SV Kuchipudi",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Virol. J.",
"key": "22307_CR29",
"unstructured": "Kuchipudi, S. V. et al. 18S rRNA is a reliable normalisation gene for real time PCR based on influenza virus infected cells. Virol. J. 9, 1–7 (2012).",
"volume": "9",
"year": "2012"
},
{
"DOI": "10.1093/nar/gkv229",
"author": "JJ Hughey",
"doi-asserted-by": "publisher",
"first-page": "e79",
"journal-title": "Nucleic Acids Res.",
"key": "22307_CR30",
"unstructured": "Hughey, J. J. & Butte, A. J. Robust meta-analysis of gene expression using the elastic net. Nucleic Acids Res. 43, e79–e79 (2015).",
"volume": "43",
"year": "2015"
},
{
"DOI": "10.1093/nar/gkv007",
"author": "ME Ritchie",
"doi-asserted-by": "publisher",
"first-page": "e47",
"journal-title": "Nucleic Acids Res.",
"key": "22307_CR31",
"unstructured": "Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47–e47 (2015).",
"volume": "43",
"year": "2015"
},
{
"key": "22307_CR32",
"unstructured": "Dudoit, S., Yang, Y.H., Callow, M.J. & Speed, T.P. Statistical methods for identifying differentially expressed genes in replicated cDNA microarray experiments. Stat. Sin. 111–139 (2002)."
},
{
"DOI": "10.2202/1544-6115.1027",
"author": "K Smyth Gordon",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Stat. Appl. Genet. Mol. Biol.",
"key": "22307_CR33",
"unstructured": "Smyth Gordon, K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, 1–25 (2004).",
"volume": "3",
"year": "2004"
},
{
"DOI": "10.1016/j.jsbmb.2018.01.002",
"author": "C Carlberg",
"doi-asserted-by": "publisher",
"first-page": "142",
"journal-title": "J. Steroid Biochem. Mol. Biol.",
"key": "22307_CR34",
"unstructured": "Carlberg, C. et al. In vivo response of the human epigenome to vitamin D: A proof-of-principle study. J. Steroid Biochem. Mol. Biol. 180, 142–148 (2018).",
"volume": "180",
"year": "2018"
},
{
"DOI": "10.1038/s41598-020-78288-0",
"author": "A Hanel",
"doi-asserted-by": "publisher",
"first-page": "21051",
"journal-title": "Sci. Rep.",
"key": "22307_CR35",
"unstructured": "Hanel, A. et al. Common and personal target genes of the micronutrient vitamin D in primary immune cells from human peripheral blood. Sci. Rep. 10, 21051 (2020).",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0159779",
"author": "SN Kariuki",
"doi-asserted-by": "publisher",
"journal-title": "PLoS ONE",
"key": "22307_CR36",
"unstructured": "Kariuki, S. N. et al. Mapping variation in cellular and transcriptional response to 1,25-dihydroxyvitamin D3 in peripheral blood mononuclear cells. PLoS ONE 11, e0159779 (2016).",
"volume": "11",
"year": "2016"
},
{
"DOI": "10.1093/nar/gkv1519",
"author": "S Seuter",
"doi-asserted-by": "publisher",
"first-page": "4090",
"journal-title": "Nucleic Acids Res.",
"key": "22307_CR37",
"unstructured": "Seuter, S., Neme, A. & Carlberg, C. Epigenome-wide effects of vitamin D and their impact on the transcriptome of human monocytes involve CTCF. Nucleic Acids Res. 44, 4090–4104 (2016).",
"volume": "44",
"year": "2016"
},
{
"DOI": "10.1136/jclinpath-2020-206834",
"author": "D Sapkota",
"doi-asserted-by": "publisher",
"first-page": "344",
"journal-title": "J. Clin. Pathol.",
"key": "22307_CR38",
"unstructured": "Sapkota, D. et al. COVID-19 salivary signature: Diagnostic and research opportunities. J. Clin. Pathol. 74, 344 (2021).",
"volume": "74",
"year": "2021"
},
{
"DOI": "10.1038/s41418-020-00633-7",
"author": "T Matsuyama",
"doi-asserted-by": "publisher",
"first-page": "3209",
"journal-title": "Cell Death Differ.",
"key": "22307_CR39",
"unstructured": "Matsuyama, T., Kubli, S. P., Yoshinaga, S. K., Pfeffer, K. & Mak, T. W. An aberrant STAT pathway is central to COVID-19. Cell Death Differ. 27, 3209–3225 (2020).",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1016/j.intimp.2018.07.019",
"author": "NJ Jadhav",
"doi-asserted-by": "publisher",
"first-page": "237",
"journal-title": "Int. Immunopharmacol.",
"key": "22307_CR40",
"unstructured": "Jadhav, N. J., Gokhale, S., Seervi, M., Patil, P. S. & Alagarasu, K. Immunomodulatory effect of 1, 25 dihydroxy vitamin D3 on the expression of RNA sensing pattern recognition receptor genes and cytokine response in dengue virus infected U937-DC-SIGN cells and THP-1 macrophages. Int. Immunopharmacol. 62, 237–243 (2018).",
"volume": "62",
"year": "2018"
},
{
"DOI": "10.1016/j.jaci.2019.02.019",
"author": "M Bravo García-Morato",
"doi-asserted-by": "publisher",
"first-page": "309",
"journal-title": "J. Allergy Clin. Immunol.",
"key": "22307_CR41",
"unstructured": "Bravo García-Morato, M. et al. Impaired control of multiple viral infections in a family with complete IRF9 deficiency. J. Allergy Clin. Immunol. 144, 309-312.e310 (2019).",
"volume": "144",
"year": "2019"
},
{
"DOI": "10.1128/JVI.01682-12",
"author": "J Verhelst",
"doi-asserted-by": "publisher",
"first-page": "13445",
"journal-title": "J. Virol.",
"key": "22307_CR42",
"unstructured": "Verhelst, J., Parthoens, E., Schepens, B., Fiers, W. & Saelens, X. Interferon-inducible protein Mx1 inhibits influenza virus by interfering with functional viral ribonucleoprotein complex assembly. J. Virol. 86, 13445–13455 (2012).",
"volume": "86",
"year": "2012"
},
{
"DOI": "10.1016/j.jmb.2019.03.013",
"author": "JV Dzimianski",
"doi-asserted-by": "publisher",
"first-page": "4203",
"journal-title": "J. Mol. Biol.",
"key": "22307_CR43",
"unstructured": "Dzimianski, J. V., Scholte, F. E. M., Bergeron, É. & Pegan, S. D. ISG15: It’s complicated. J. Mol. Biol. 431, 4203–4216 (2019).",
"volume": "431",
"year": "2019"
},
{
"DOI": "10.1038/s41598-021-86032-5",
"author": "T Warwick",
"doi-asserted-by": "publisher",
"first-page": "6518",
"journal-title": "Sci. Rep.",
"key": "22307_CR44",
"unstructured": "Warwick, T. et al. A hierarchical regulatory network analysis of the vitamin D induced transcriptome reveals novel regulators and complete VDR dependency in monocytes. Sci. Rep. 11, 6518 (2021).",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1074/jbc.M800501200",
"author": "A Paun",
"doi-asserted-by": "publisher",
"first-page": "14295",
"journal-title": "J. Biol. Chem.",
"key": "22307_CR45",
"unstructured": "Paun, A. et al. Functional characterization of murine interferon regulatory factor 5 (IRF-5) and its role in the innate antiviral response. J. Biol. Chem. 283, 14295–14308 (2008).",
"volume": "283",
"year": "2008"
},
{
"DOI": "10.1096/fj.15-273128",
"author": "M Dai",
"doi-asserted-by": "publisher",
"first-page": "4978",
"journal-title": "FASEB J.",
"key": "22307_CR46",
"unstructured": "Dai, M. et al. Activation of TLR3/interferon signaling pathway by bluetongue virus results in HIV inhibition in macrophages. FASEB J. 29, 4978–4988 (2015).",
"volume": "29",
"year": "2015"
},
{
"DOI": "10.1038/ijos.2016.38",
"author": "C-Z Zhang",
"doi-asserted-by": "publisher",
"first-page": "133",
"journal-title": "Int. J. Oral Sci.",
"key": "22307_CR47",
"unstructured": "Zhang, C.-Z. et al. Saliva in the diagnosis of diseases. Int. J. Oral Sci. 8, 133–137 (2016).",
"volume": "8",
"year": "2016"
},
{
"DOI": "10.1183/23120541.00984-2020",
"author": "F Saheb Sharif-Askari",
"doi-asserted-by": "publisher",
"first-page": "00984",
"journal-title": "ERJ Open Res.",
"key": "22307_CR48",
"unstructured": "Saheb Sharif-Askari, F. et al. Upregulation of interleukin-19 in severe asthma: A potential saliva biomarker for asthma severity. ERJ Open Res. 7, 00984–02020 (2021).",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-02489-4",
"author": "KI Masood",
"doi-asserted-by": "publisher",
"first-page": "22958",
"journal-title": "Sci. Rep.",
"key": "22307_CR49",
"unstructured": "Masood, K. I. et al. Upregulated type I interferon responses in asymptomatic COVID-19 infection are associated with improved clinical outcome. Sci. Rep. 11, 22958 (2021).",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.24127",
"author": "R Rubin",
"doi-asserted-by": "publisher",
"first-page": "329",
"journal-title": "JAMA",
"key": "22307_CR50",
"unstructured": "Rubin, R. Sorting out whether vitamin D deficiency raises COVID-19 risk. JAMA 325, 329–330 (2021).",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"author": "M Entrenas Castillo",
"doi-asserted-by": "publisher",
"journal-title": "J. Steroid Biochem. Mol. Biol.",
"key": "22307_CR51",
"unstructured": "Entrenas Castillo, M. et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 203, 105751 (2020).",
"volume": "203",
"year": "2020"
},
{
"DOI": "10.1016/j.nut.2020.111055",
"author": "E Cereda",
"doi-asserted-by": "publisher",
"journal-title": "Nutrition",
"key": "22307_CR52",
"unstructured": "Cereda, E. et al. Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy Italy. Nutrition 82, 111055 (2021).",
"volume": "82",
"year": "2021"
}
],
"reference-count": 52,
"references-count": 52,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-022-22307-9"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Vitamin D enhances type I IFN signaling in COVID-19 patients",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "12"
}
