Discovery of re-purposed drugs that slow SARS-CoV-2 replication in human cells
et al., PLOS Pathogens, doi:10.1371/journal.ppat.1009840, Sep 2021
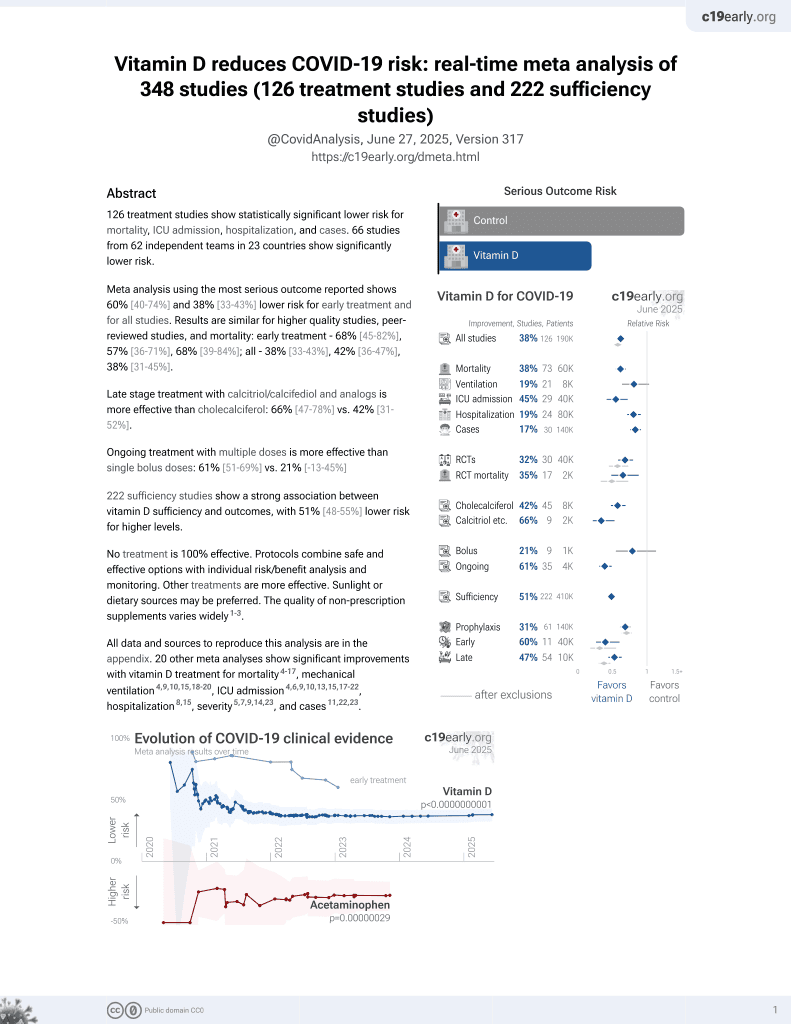
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
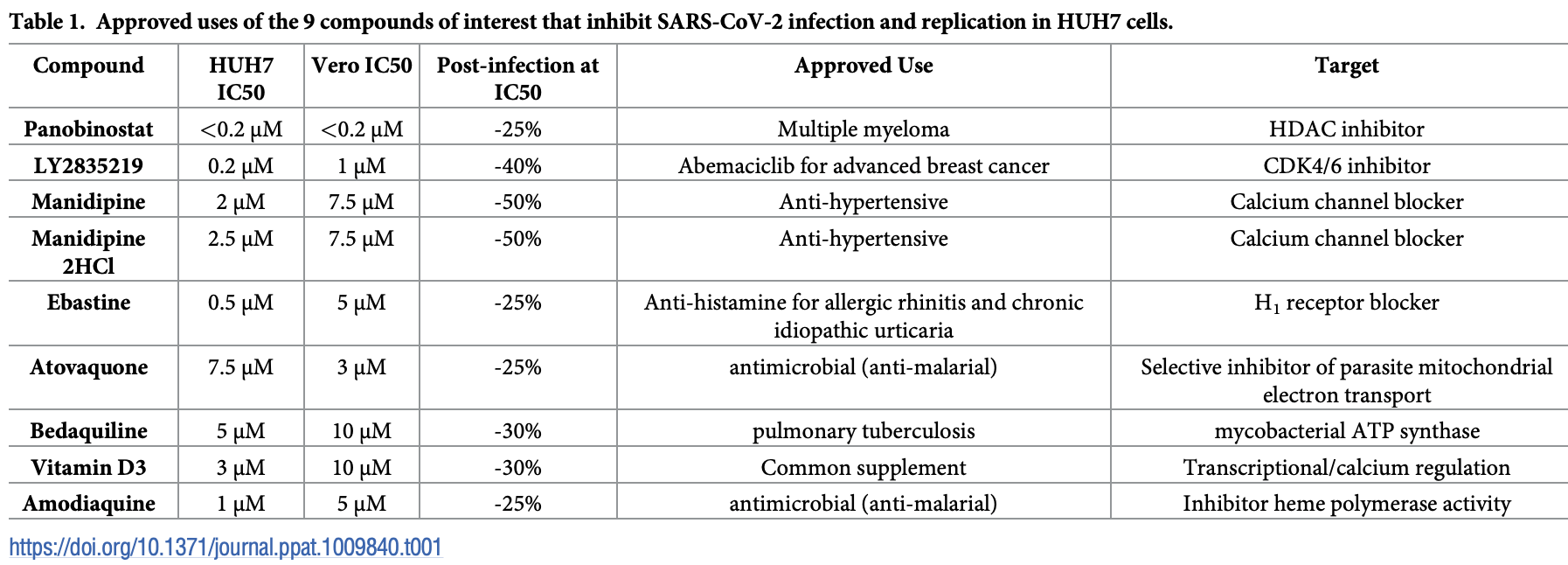
In vitro studying identifying 35 compounds that inhibit SARS-CoV-2 in Vero cells and hepatocytes when treated prior to infection, and several compounds that slow replication when treated after infection: vitamin D, amodiaquine, atovaquone, bedaquiline, ebastine, LY2835219, manidipine, and panobinosta. Authors use a nano-luciferase tagged version of the virus to quantify viral load.
29 preclinical studies support the efficacy of vitamin D for COVID-19:
Vitamin D has been identified by the European Food Safety Authority (EFSA) as having sufficient evidence for a causal relationship between intake and optimal immune system function27-30.
Vitamin D inhibits SARS-CoV-2 replication in vitro17,24, mitigates lung inflammation, damage, and lethality in mice with an MHV-3 model for β-CoV respiratory infections17,24, reduces SARS-CoV-2 replication in nasal epithelial cells via increased type I interferon expression20, downregulates proinflammatory cytokines IL-1β and TNF-α in SARS-CoV-2 spike protein-stimulated cells16, attenuates nucleocapsid protein-induced hyperinflammation by inactivating the NLRP3 inflammasome through the VDR-BRCC3 signaling pathway21, may be neuroprotective by protecting the blood-brain barrier, reducing neuroinflammation, and via immunomodulatory effects31, may mitigate hyperinflammation and cytokine storm by upregulating TLR10 expression which downregulates proinflammatory cytokines13, downregulates ACE2 and TMPRSS2 in human trophoblasts and minimizes spike protein-induced inflammation19, may minimize cytokine storm by dampening excessive cytokine production2, may suppress viral entry and replication via LL-37 induction11,12, and minimizes platelet aggregation mediated by SARS-CoV-2 spike protein via inhibiting integrin αIIbβ3 outside-in signaling15.
Cholecalciferol and calcifediol directly bind two allosteric pockets on the SARS-CoV-2 Spike RBD, bias the trimer toward a closed state, weaken ACE2 engagement, and reduce viral entry in cell models1.
Calcitriol may destabilize the Spike protein architecture and inhibit IL-17R dimerization, blocking viral entry and mitigating hyperinflammatory cytokine storm32.
Vitamin D improves regulatory immune cell levels and control of proinflammatory cytokines in severe COVID-1933.
Calcifediol inhibits SARS-CoV-2 papain-like protease (PLpro), a critical enzyme for viral replication14.
Symptomatic COVID-19 is associated with a lower frequency of natural killer (NK) cells and vitamin D has been shown to improve NK cell activity34,35.
Study covers vitamin D and atovaquone.
1.
García-Marín et al., Exploring SARS-CoV-2 Spike RBD Pockets as Targets for Generic Drugs: A Combined Computational, Biophysical, and Biological Approach, ACS Omega, doi:10.1021/acsomega.5c05175.
2.
Alzahrani, A., A new investigation into the molecular mechanism of cholecalciferol towards reducing cytokines storm, Octahedron Drug Research, doi:10.21608/odr.2024.308273.1043.
3.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
4.
Morales-Bayuelo et al., New findings on ligand series used as SARS-CoV-2 virus inhibitors within the frameworks of molecular docking, molecular quantum similarity and chemical reactivity indices, F1000Research, doi:10.12688/f1000research.123550.3.
5.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
6.
Pandya et al., Unravelling Vitamin B12 as a potential inhibitor against SARS-CoV-2: A computational approach, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2022.100951.
7.
Mansouri et al., The impact of calcitriol and estradiol on the SARS-CoV-2 biological activity: a molecular modeling approach, Scientific Reports, doi:10.1038/s41598-022-04778-y.
8.
Song et al., Vitamin D3 and its hydroxyderivatives as promising drugs against COVID-19: a computational study, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1964601.
9.
Qayyum et al., Vitamin D and lumisterol novel metabolites can inhibit SARS-CoV-2 replication machinery enzymes, Endocrinology and Metabolism, doi:10.1152/ajpendo.00174.2021.
10.
Al-Mazaideh et al., Vitamin D is a New Promising Inhibitor to the Main Protease (Mpro) of COVID-19 by Molecular Docking, Journal of Pharmaceutical Research International, doi:10.9734/jpri/2021/v33i29B31603.
11.
Roth et al., Vitamin D-inducible antimicrobial peptide LL-37 binds SARS-CoV-2 Spike and accessory proteins ORF7a and ORF8, Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2025.1671738.
12.
Vercellino et al., Influence of Sex and 1,25α Dihydroxyvitamin D3 on SARS-CoV-2 Infection and Viral Entry, Pathogens, doi:10.3390/pathogens14080765.
13.
Knez et al., TLR10 overexpression modulates immune response in A549 lung epithelial cells challenged with SARS-CoV-2 S and N proteins, Frontiers in Immunology, doi:10.3389/fimmu.2024.1490478.
14.
Chen et al., In Vitro Characterization of Inhibition Function of Calcifediol to the Protease Activity of SARS-COV-2 PLpro, Journal of Medical Virology, doi:10.1002/jmv.70085.
15.
Wang et al., 1,25‐Dihydroxyvitamin D3 attenuates platelet aggregation potentiated by SARS‐CoV‐2 spike protein via inhibiting integrin αIIbβ3 outside‐in signaling, Cell Biochemistry and Function, doi:10.1002/cbf.4039.
16.
Alcalá-Santiago et al., Disentangling the Immunomodulatory Effects of Vitamin D on the SARS-CoV-2 Virus by In Vitro Approaches, The 14th European Nutrition Conference FENS 2023, doi:10.3390/proceedings2023091415.
17.
Campolina-Silva et al., Dietary Vitamin D Mitigates Coronavirus-Induced Lung Inflammation and Damage in Mice, Viruses, doi:10.3390/v15122434.
18.
Moatasim et al., Potent Antiviral Activity of Vitamin B12 against Severe Acute Respiratory Syndrome Coronavirus 2, Middle East Respiratory Syndrome Coronavirus, and Human Coronavirus 229E, Microorganisms, doi:10.3390/microorganisms11112777.
19.
Vargas-Castro et al., Calcitriol prevents SARS-CoV spike-induced inflammation in human trophoblasts through downregulating ACE2 and TMPRSS2 expression, The Journal of Steroid Biochemistry and Molecular Biology, doi:10.1016/j.jsbmb.2024.106625.
20.
Sposito et al., Age differential CD13 and interferon expression in airway epithelia affect SARS-CoV-2 infection - effects of vitamin D, Mucosal Immunology, doi:10.1016/j.mucimm.2023.08.002.
21.
Chen (B) et al., Vitamin D3 attenuates SARS‐CoV‐2 nucleocapsid protein‐caused hyperinflammation by inactivating the NLRP3 inflammasome through the VDR‐BRCC3 signaling pathway in vitro and in vivo, MedComm, doi:10.1002/mco2.318.
22.
Rybakovsky et al., Calcitriol modifies tight junctions, improves barrier function, and reduces TNF‐α‐induced barrier leak in the human lung‐derived epithelial cell culture model, 16HBE 14o‐, Physiological Reports, doi:10.14814/phy2.15592.
23.
DiGuilio et al., The multiphasic TNF-α-induced compromise of Calu-3 airway epithelial barrier function, Experimental Lung Research, doi:10.1080/01902148.2023.2193637.
24.
Pickard et al., Discovery of re-purposed drugs that slow SARS-CoV-2 replication in human cells, PLOS Pathogens, doi:10.1371/journal.ppat.1009840.
25.
Mok et al., Calcitriol, the active form of vitamin D, is a promising candidate for COVID-19 prophylaxis, bioRxiv, doi:10.1101/2020.06.21.162396.
26.
Fernandes de Souza et al., Lung Inflammation Induced by Inactivated SARS-CoV-2 in C57BL/6 Female Mice Is Controlled by Intranasal Instillation of Vitamin D, Cells, doi:10.3390/cells12071092.
27.
Galmés et al., Suboptimal Consumption of Relevant Immune System Micronutrients Is Associated with a Worse Impact of COVID-19 in Spanish Populations, Nutrients, doi:10.3390/nu14112254.
28.
Galmés (B) et al., Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework, Nutrients, doi:10.3390/nu12092738.
29.
EFSA, Scientific Opinion on the substantiation of a health claim related to vitamin D and contribution to the normal function of the immune system pursuant to Article 14 of Regulation (EC) No 1924/2006, EFSA Journal, doi:10.2903/j.efsa.2015.4096.
30.
EFSA (B), Scientific Opinion on the substantiation of health claims related to vitamin D and normal function of the immune system and inflammatory response (ID 154, 159), maintenance of normal muscle function (ID 155) and maintenance of normal cardiovascular function (ID 159) pursuant to Article 13(1) of Regulation (E, EFSA Journal, doi:10.2903/j.efsa.2010.1468.
31.
Gotelli et al., Understanding the immune-endocrine effects of vitamin D in SARS-CoV-2 infection: a role in protecting against neurodamage?, Neuroimmunomodulation, doi:10.1159/000533286.
32.
Fadel et al., Targeting asparagine and cysteine in SARS-CoV-2 variants and human pro-inflammatory mediators to alleviate COVID-19 severity; a cross-section and in-silico study, Scientific Reports, doi:10.1038/s41598-025-19359-y.
33.
Saheb Sharif-Askari et al., Increased blood immune regulatory cells in severe COVID-19 with autoantibodies to type I interferons, Scientific Reports, doi:10.1038/s41598-023-43675-w.
Pickard et al., 9 Sep 2021, peer-reviewed, 7 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Discovery of re-purposed drugs that slow SARS-CoV-2 replication in human cells
PLOS Pathogens, doi:10.1371/journal.ppat.1009840
COVID-19 vaccines based on the Spike protein of SARS-CoV-2 have been developed that appear to be largely successful in stopping infection. However, therapeutics that can help manage the disease are still required until immunity has been achieved globally. The identification of repurposed drugs that stop SARS-CoV-2 replication could have enormous utility in stemming the disease. Here, using a nano-luciferase tagged version of the virus (SARS-CoV-2-ΔOrf7a-NLuc) to quantitate viral load, we evaluated a range of human cell types for their ability to be infected and support replication of the virus, and performed a screen of 1971 FDA-approved drugs. Hepatocytes, kidney glomerulus, and proximal tubule cells were particularly effective in supporting SARS-CoV-2 replication, which is in-line with reported proteinuria and liver damage in patients with COVID-19. Using the nano-luciferase as a measure of virus replication we identified 35 drugs that reduced replication in Vero cells and human hepatocytes when treated prior to SARS-CoV-2 infection and found amodiaquine, atovaquone, bedaquiline, ebastine, LY2835219, manidipine, panobinostat, and vitamin D3 to be effective in slowing SARS-CoV-2 replication in human cells when used to treat infected cells. In conclusion, our study has identified strong candidates for drug repurposing, which could prove powerful additions to the treatment of COVID.
Generation of functional SARS-CoV-2 virus DNA encoding the genome of SARS-CoV-2 and SARS-CoV-2-ΔOrf7a-NLuc were purchased from Vectorbuilder Inc. (Chicago, US). Transfection of DNA encoding the viruses failed to generate replicative virus when electroporated into 293T cells. We therefore produced RNA molecules which encoded the virus in vitro. Briefly, virus encoding DNA (1 μg) was transcribed using T7 mMessenger mMachine (Thermo) with a GTP:Cap ratio of 2:1 used in a 20 μL reaction. In addition, RNA encoding the SARS-CoV-2 nucleocapsid was also generated by PCR using primers P1 and P2 (S5 Table ) . It has been reported that this aids the recovery of replicative virus [18] . Viral RNA genomes (10 μl) and 2.5 μL nucleocapsid RNA were electroporated into 293T cells within the BSL3 laboratory. Viral RNAs were electroporated (5,000,000 cells, 1100 V, 20 ms and 2 pulses) and grown in T75 cm 2 flasks and 24 well plates. Cells grown in 24 well plates for 24-120 hrs were used to monitor changes in NLuc activity.
Virus production, maintenance and assessment of titer Culture medium was collected from 293T cells 6 days after electroporation. This virus (P0) was used to infect cells of interest. As virus replication was slow in 293T cells, virus stocks were maintained by passage in Vero cells grown in DMEM supplemented with 2% FBS. Medium (1 mL) was used to infect Vero cells in order to generate P1 virus. Replication was assessed by measuring NLuc activity over 10 days. For..
References
Banerjee, Blanco, Bruce, Honson, Chen et al., SARS-CoV-2 Disrupts Splicing, Translation, and Protein Trafficking to Suppress Host Defenses, Cell, doi:10.1016/j.cell.2020.10.004
Bozic, Guzman, Benet, Sanchez-Campos, Garcia-Monzon et al., Hepatocyte vitamin D receptor regulates lipid metabolism and mediates experimental diet-induced steatosis, J Hepatol, doi:10.1016/j.jhep.2016.05.031
Braun, Lutgehetmann, Pfefferle, Wong, Carsten et al., SARS-CoV-2 renal tropism associates with acute kidney injury, Lancet, doi:10.1016/S0140-6736%2820%2931759-1
Calverley, Kadler, Pickard, Dynamic High-Sensitivity Quantitation of Procollagen-I by Endogenous CRISPR-Cas9 NanoLuciferase Tagging, Cells, doi:10.3390/cells9092070
Campbell, Michel, Bremard-Oury, Crampette, Bousquet, Overview of allergic mechanisms. Ebastine has more than an antihistamine effect, Drugs, doi:10.2165/00003495-199600521-00005
Cantuti-Castelvetri, Ojha, Pedro, Djannatian, Franz et al., Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity, Science, doi:10.1126/science.abd2985
Chatenoud, Ferran, Bach, The anti-CD3-induced syndrome: a consequence of massive in vivo cell activation, Curr Top Microbiol Immunol, doi:10.1007/978-3-642-50998-8%5F9
Chen, Tao, Shen, Miao, Li et al., A drug screening toolkit based on the -1 ribosomal frameshifting of SARS-CoV-2, Heliyon, doi:10.1016/j.heliyon.2020.e04793
Chu, Chan, Yuen, Shuai, Yuan et al., Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study, Lancet Microbe, doi:10.1016/S2666-5247%2820%2930004-5
Dittmar, Lee, Whig, Segrist, Li et al., Drug repurposing screens reveal cell-typespecific entry pathways and FDA-approved drugs active against SARS-Cov-2, Cell Rep, doi:10.1016/j.celrep.2021.108959
Fajgenbaum, June, Cytokine Storm, N Engl J Med, doi:10.1056/NEJMra2026131
Farag, Wang, Boys, Eitson, Ohlson et al., Identification of Atovaquone, Ouabain and Mebendazole as FDA Approved Drugs Tar-geting SARS-CoV-2, doi:10.26434/chemrxiv.12003930.v4
Ferraz, Gomes, Sn, Trossini, Ligand and structure-based virtual screening applied to the SARS-CoV-2 main protease: an in silico repurposing study, Future Med Chem, doi:10.4155/fmc-2020-0165
Fry, Pudney, Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4'-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80), Biochem Pharmacol, doi:10.1016/0006-2952%2892%2990213-3
Ghahremanpour, Tirado-Rives, Deshmukh, Ippolito, Zhang et al., Identification of 14 Known Drugs as Inhibitors of the Main Protease of SARS-CoV-2, ACS Med Chem Lett, doi:10.1021/acsmedchemlett.0c00521
Group, A randomised trial of treatments to prevent death in patients hospitalised with COVID-19 (coronavirus), doi:10.1186/ISRCTN50189673
Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Group, RECOVERY): a randomised, controlled, open-label, platform trial, doi:10.1016/S0140-6736%2820%2932013-4
He, Garmire, Prediction of repurposed drugs for treating lung injury in COVID-19, doi:10.12688/f1000research.23996.2
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736%2820%2930183-5
Jeon, Ko, Lee, Choi, Byun et al., Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs, Antimicrob Agents Chemother, doi:10.1128/AAC.00819-20
Lai, Millet, Daniel, Freed, Whittaker, The SARS-CoV Fusion Peptide Forms an Extended Bipartite Fusion Platform that Perturbs Membrane Order in a Calcium-Dependent Manner, J Mol Biol, doi:10.1016/j.jmb.2017.10.017
Lancet Diabetes, Vitamin D and COVID-19: why the controversy?, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587%2821%2900003-6
Li, Moore, Vasilieva, Sui, Wong et al., Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus, Nature, doi:10.1038/nature02145
Liu, Li, Liu, Yao, Wang et al., SARS-CoV-2 cell tropism and multiorgan infection, Cell Discov, doi:10.1038/s41421-021-00249-2
Ma, Sacco, Hurst, Townsend, Hu et al., Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease, Cell Res, doi:10.1038/s41422-020-0356-z
Maier, Neuman, Bickerton, Keep, Alrashedi et al., Extensive coronavirusinduced membrane rearrangements are not a determinant of pathogenicity, Sci Rep, doi:10.1038/srep27126
Mirabelli, Wotring, Zhang, Mccarty, Fursmidt et al., Morphological Cell Profiling of SARS-CoV-2 Infection Identifies Drug Repurposing Candidates for COVID-19, bioRxiv, doi:10.1101/2020.05.27.117184
Nhu, Labroussaa, Ebert, Kovski, Stalder et al., Rapid reconstruction of SARS-CoV-2 using a synthetic genomics platform, Nature, doi:10.1038/s41586-020-2294-9
Pickard, Chang, Alachkar, Calverley, Garva et al., Preservation of circadian rhythms by the protein folding chaperone, BiP, FASEB J, doi:10.1096/fj.201802366RR
Pushpakom, Iorio, Eyers, Escott, Hopper et al., Drug repurposing: progress, challenges and recommendations, Nat Rev Drug Discov, doi:10.1038/nrd.2018.168
Ramirez-Salinas, Martinez-Archundia, Correa-Basurto, Garcia-Machorro, Repositioning of Ligands That Target the Spike Glycoprotein as Potential Drugs for SARS-CoV-2 in an In Silico Study, Molecules, doi:10.3390/molecules25235615
Riva, Yuan, Yin, Martin-Sancho, Matsunaga et al., Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing, Nature, doi:10.1038/s41586-020-2577-1
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Med, doi:10.1007/s00134-020-05991-x
Snijder, Van Der Meer, Zevenhoven-Dobbe, Onderwater, Van Der Meulen et al., Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex, J Virol, doi:10.1128/JVI.02501-05
Touret, Gilles, Barral, Nougairede, Van Helden et al., In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication, Sci Rep, doi:10.1038/s41598-020-70143-6
Triantos, Aggeletopoulou, Thomopoulos, Mouzaki, Vitamin D-liver disease association: Biological basis and mechanisms of action, Hepatology, doi:10.1002/hep.31699
Wang, Davis, Xu, chronic liverdisease in the United States, doi:10.1016/j.eclinm.2020.100688
Wysocki, Lores, Ye, Soler, Batlle, Kidney and Lung ACE2 Expression after an ACE Inhibitor or an Ang II Receptor Blocker: Implications for COVID-19, J Am Soc Nephrol, doi:10.1681/ASN.2020050667
Xie, Muruato, Lokugamage, Narayanan, Zhang et al., An Infectious cDNA Clone of SARS-CoV-2, Cell Host Microbe, doi:10.1016/j.chom.2020.04.004
Xie, Muruato, Zhang, Lokugamage, Fontes-Garfias et al., A nanoluciferase SARS-CoV-2 for rapid neutralization testing and screening of anti-infective drugs for COVID-19, Nat Commun, doi:10.1038/s41467-020-19055-7
Yamamoto, Ichinohe, Watanabe, Kobayashi, Zhang et al., The Antimalarial Compound Atovaquone Inhibits Zika and Dengue Virus Infection by Blocking E Protein-Mediated Membrane Fusion, Viruses, doi:10.3390/v12121475
Yeo, Kaushal, Yeo, Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible?, Lancet Gastroenterol Hepatol, doi:10.1016/S2468-1253%2820%2930048-0
Zhang, Chung, Oldenburg, A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays, J Biomol Screen, doi:10.1177/108705719900400206
Zhang, Shi, Wang, Liver injury in COVID-19: management and challenges, Lancet Gastroenterol Hepatol, doi:10.1016/S2468-1253%2820%2930057-1
Zhou, Yang, Wang, Hu, Zhang et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature, doi:10.1038/s41586-020-2012-7
DOI record:
{
"DOI": "10.1371/journal.ppat.1009840",
"ISSN": [
"1553-7374"
],
"URL": "http://dx.doi.org/10.1371/journal.ppat.1009840",
"abstract": "<jats:p>COVID-19 vaccines based on the Spike protein of SARS-CoV-2 have been developed that appear to be largely successful in stopping infection. However, therapeutics that can help manage the disease are still required until immunity has been achieved globally. The identification of repurposed drugs that stop SARS-CoV-2 replication could have enormous utility in stemming the disease. Here, using a nano-luciferase tagged version of the virus (SARS-CoV-2-ΔOrf7a-NLuc) to quantitate viral load, we evaluated a range of human cell types for their ability to be infected and support replication of the virus, and performed a screen of 1971 FDA-approved drugs. Hepatocytes, kidney glomerulus, and proximal tubule cells were particularly effective in supporting SARS-CoV-2 replication, which is in-line with reported proteinuria and liver damage in patients with COVID-19. Using the nano-luciferase as a measure of virus replication we identified 35 drugs that reduced replication in Vero cells and human hepatocytes when treated prior to SARS-CoV-2 infection and found amodiaquine, atovaquone, bedaquiline, ebastine, LY2835219, manidipine, panobinostat, and vitamin D3 to be effective in slowing SARS-CoV-2 replication in human cells when used to treat infected cells. In conclusion, our study has identified strong candidates for drug repurposing, which could prove powerful additions to the treatment of COVID.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-9757-143X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Pickard",
"given": "Adam",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-9403-0363",
"affiliation": [],
"authenticated-orcid": true,
"family": "Calverley",
"given": "Ben C.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7283-9759",
"affiliation": [],
"authenticated-orcid": true,
"family": "Chang",
"given": "Joan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7752-8936",
"affiliation": [],
"authenticated-orcid": true,
"family": "Garva",
"given": "Richa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gago",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Yinhui",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4977-4683",
"affiliation": [],
"authenticated-orcid": true,
"family": "Kadler",
"given": "Karl E.",
"sequence": "additional"
}
],
"container-title": "PLOS Pathogens",
"container-title-short": "PLoS Pathog",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plospathogens.org"
]
},
"created": {
"date-parts": [
[
2021,
9,
9
]
],
"date-time": "2021-09-09T17:27:10Z",
"timestamp": 1631208430000
},
"deposited": {
"date-parts": [
[
2021,
9,
9
]
],
"date-time": "2021-09-09T17:28:12Z",
"timestamp": 1631208492000
},
"editor": [
{
"affiliation": [],
"family": "Pekosz",
"given": "Andrew",
"sequence": "first"
}
],
"funder": [
{
"award": [
"110126/Z/15/Z"
],
"name": "wellcome"
},
{
"DOI": "10.13039/100010269",
"award": [
"203128/Z/16/Z"
],
"doi-asserted-by": "crossref",
"name": "Wellcome"
},
{
"name": "NIHR Manchester Research Centre"
},
{
"name": "Fungal Infection Trust"
}
],
"indexed": {
"date-parts": [
[
2023,
8,
25
]
],
"date-time": "2023-08-25T09:24:09Z",
"timestamp": 1692955449472
},
"is-referenced-by-count": 11,
"issue": "9",
"issued": {
"date-parts": [
[
2021,
9,
9
]
]
},
"journal-issue": {
"issue": "9",
"published-online": {
"date-parts": [
[
2021,
9,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
9
]
],
"date-time": "2021-09-09T00:00:00Z",
"timestamp": 1631145600000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.ppat.1009840",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e1009840",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2021,
9,
9
]
]
},
"published-online": {
"date-parts": [
[
2021,
9,
9
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"DOI": "10.1038/s41421-021-00249-2",
"article-title": "SARS-CoV-2 cell tropism and multiorgan infection",
"author": "J Liu",
"doi-asserted-by": "crossref",
"first-page": "17",
"issue": "1",
"journal-title": "Cell Discov",
"key": "ppat.1009840.ref001",
"volume": "7",
"year": "2021"
},
{
"article-title": "The anti-CD3-induced syndrome: a consequence of massive in vivo cell activation",
"author": "L Chatenoud",
"first-page": "121",
"journal-title": "Curr Top Microbiol Immunol",
"key": "ppat.1009840.ref002",
"volume": "174",
"year": "1991"
},
{
"DOI": "10.1056/NEJMra2026131",
"article-title": "Cytokine Storm",
"author": "DC Fajgenbaum",
"doi-asserted-by": "crossref",
"first-page": "2255",
"issue": "23",
"journal-title": "N Engl J Med",
"key": "ppat.1009840.ref003",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-05991-x",
"article-title": "Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China",
"author": "Q Ruan",
"doi-asserted-by": "crossref",
"first-page": "846",
"issue": "5",
"journal-title": "Intensive Care Med",
"key": "ppat.1009840.ref004",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1038/nature02145",
"article-title": "Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus",
"author": "W Li",
"doi-asserted-by": "crossref",
"first-page": "450",
"issue": "6965",
"journal-title": "Nature",
"key": "ppat.1009840.ref005",
"volume": "426",
"year": "2003"
},
{
"DOI": "10.1126/science.abd2985",
"article-title": "Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity",
"author": "L Cantuti-Castelvetri",
"doi-asserted-by": "crossref",
"first-page": "856",
"issue": "6518",
"journal-title": "Science",
"key": "ppat.1009840.ref006",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1038/srep27126",
"article-title": "Extensive coronavirus-induced membrane rearrangements are not a determinant of pathogenicity",
"author": "HJ Maier",
"doi-asserted-by": "crossref",
"first-page": "27126",
"journal-title": "Sci Rep",
"key": "ppat.1009840.ref007",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1016/j.cell.2020.10.004",
"article-title": "SARS-CoV-2 Disrupts Splicing, Translation, and Protein Trafficking to Suppress Host Defenses",
"author": "AK Banerjee",
"doi-asserted-by": "crossref",
"first-page": "1325",
"issue": "5",
"journal-title": "Cell",
"key": "ppat.1009840.ref008",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.3390/cells9092070",
"article-title": "Dynamic High-Sensitivity Quantitation of Procollagen-I by Endogenous CRISPR-Cas9 NanoLuciferase Tagging",
"author": "BC Calverley",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "Cells",
"key": "ppat.1009840.ref009",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"article-title": "A pneumonia outbreak associated with a new coronavirus of probable bat origin",
"author": "P Zhou",
"doi-asserted-by": "crossref",
"first-page": "270",
"issue": "7798",
"journal-title": "Nature",
"key": "ppat.1009840.ref010",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2577-1",
"article-title": "Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing",
"author": "L Riva",
"doi-asserted-by": "crossref",
"first-page": "113",
"issue": "7827",
"journal-title": "Nature",
"key": "ppat.1009840.ref011",
"volume": "586",
"year": "2020"
},
{
"DOI": "10.1016/j.celrep.2021.108959",
"article-title": "Drug repurposing screens reveal cell-type-specific entry pathways and FDA-approved drugs active against SARS-Cov-2",
"author": "M Dittmar",
"doi-asserted-by": "crossref",
"first-page": "108959",
"issue": "1",
"journal-title": "Cell Rep",
"key": "ppat.1009840.ref012",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1128/AAC.00819-20",
"article-title": "Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs",
"author": "S Jeon",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "Antimicrob Agents Chemother",
"key": "ppat.1009840.ref013",
"volume": "64",
"year": "2020"
},
{
"article-title": "Morphological Cell Profiling of SARS-CoV-2 Infection Identifies Drug Repurposing Candidates for COVID-19",
"author": "C Mirabelli",
"journal-title": "bioRxiv",
"key": "ppat.1009840.ref014",
"year": "2020"
},
{
"DOI": "10.1016/j.heliyon.2020.e04793",
"article-title": "A drug screening toolkit based on the -1 ribosomal frameshifting of SARS-CoV-2",
"author": "Y Chen",
"doi-asserted-by": "crossref",
"first-page": "e04793",
"issue": "8",
"journal-title": "Heliyon",
"key": "ppat.1009840.ref015",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2294-9",
"article-title": "Rapid reconstruction of SARS-CoV-2 using a synthetic genomics platform",
"author": "T Thi Nhu Thao",
"doi-asserted-by": "crossref",
"first-page": "561",
"issue": "7813",
"journal-title": "Nature",
"key": "ppat.1009840.ref016",
"volume": "582",
"year": "2020"
},
{
"DOI": "10.1016/j.chom.2020.04.004",
"article-title": "An Infectious cDNA Clone of SARS-CoV-2",
"author": "X Xie",
"doi-asserted-by": "crossref",
"first-page": "841",
"issue": "5",
"journal-title": "Cell Host Microbe",
"key": "ppat.1009840.ref017",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-19055-7",
"article-title": "A nanoluciferase SARS-CoV-2 for rapid neutralization testing and screening of anti-infective drugs for COVID-19",
"author": "X Xie",
"doi-asserted-by": "crossref",
"first-page": "5214",
"issue": "1",
"journal-title": "Nat Commun",
"key": "ppat.1009840.ref018",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1177/108705719900400206",
"article-title": "A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays",
"author": "JH Zhang",
"doi-asserted-by": "crossref",
"first-page": "67",
"issue": "2",
"journal-title": "J Biomol Screen",
"key": "ppat.1009840.ref019",
"volume": "4",
"year": "1999"
},
{
"DOI": "10.1038/s41598-020-70143-6",
"article-title": "In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication",
"author": "F Touret",
"doi-asserted-by": "crossref",
"first-page": "13093",
"issue": "1",
"journal-title": "Sci Rep",
"key": "ppat.1009840.ref020",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/S2666-5247(20)30004-5",
"article-title": "Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study",
"author": "H Chu",
"doi-asserted-by": "crossref",
"first-page": "e14",
"issue": "1",
"journal-title": "Lancet Microbe",
"key": "ppat.1009840.ref021",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1681/ASN.2020050667",
"article-title": "Kidney and Lung ACE2 Expression after an ACE Inhibitor or an Ang II Receptor Blocker: Implications for COVID-19",
"author": "J Wysocki",
"doi-asserted-by": "crossref",
"first-page": "1941",
"issue": "9",
"journal-title": "J Am Soc Nephrol",
"key": "ppat.1009840.ref022",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "C Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"issue": "10223",
"journal-title": "Lancet",
"key": "ppat.1009840.ref023",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S2468-1253(20)30057-1",
"article-title": "Liver injury in COVID-19: management and challenges",
"author": "C Zhang",
"doi-asserted-by": "crossref",
"first-page": "428",
"issue": "5",
"journal-title": "Lancet Gastroenterol Hepatol",
"key": "ppat.1009840.ref024",
"volume": "5",
"year": "2020"
},
{
"article-title": "COVID-19 risk, disparities and outcomes in patients with chronic liverdisease in the United States",
"author": "Q Wang",
"journal-title": "EClinicalMedicine",
"key": "ppat.1009840.ref025",
"year": "2020"
},
{
"DOI": "10.1016/S2468-1253(20)30048-0",
"article-title": "Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible?",
"author": "C Yeo",
"doi-asserted-by": "crossref",
"first-page": "335",
"issue": "4",
"journal-title": "Lancet Gastroenterol Hepatol",
"key": "ppat.1009840.ref026",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31759-1",
"article-title": "SARS-CoV-2 renal tropism associates with acute kidney injury",
"author": "F Braun",
"doi-asserted-by": "crossref",
"first-page": "597",
"issue": "10251",
"journal-title": "Lancet",
"key": "ppat.1009840.ref027",
"volume": "396",
"year": "2020"
},
{
"DOI": "10.1186/ISRCTN50189673",
"doi-asserted-by": "crossref",
"key": "ppat.1009840.ref028",
"unstructured": "Group RC. A randomised trial of treatments to prevent death in patients hospitalised with COVID-19 (coronavirus). ISRCTN registry. https://doi.org/10.1186/ISRCTN50189673."
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in Hospitalized Patients with Covid-19",
"author": "RC Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "ppat.1009840.ref029",
"volume": "384",
"year": "2021"
},
{
"article-title": "Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Group RC",
"journal-title": "The Lancet",
"key": "ppat.1009840.ref030"
},
{
"DOI": "10.1038/nrd.2018.168",
"article-title": "Drug repurposing: progress, challenges and recommendations",
"author": "S Pushpakom",
"doi-asserted-by": "crossref",
"first-page": "41",
"issue": "1",
"journal-title": "Nat Rev Drug Discov",
"key": "ppat.1009840.ref031",
"volume": "18",
"year": "2019"
},
{
"DOI": "10.12688/f1000research.23996.2",
"article-title": "Prediction of repurposed drugs for treating lung injury in COVID-19",
"author": "B He",
"doi-asserted-by": "crossref",
"first-page": "609",
"journal-title": "F1000Res",
"key": "ppat.1009840.ref032",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/0006-2952(92)90213-3",
"article-title": "Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4’-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80)",
"author": "M Fry",
"doi-asserted-by": "crossref",
"first-page": "1545",
"issue": "7",
"journal-title": "Biochem Pharmacol",
"key": "ppat.1009840.ref033",
"volume": "43",
"year": "1992"
},
{
"DOI": "10.3390/v12121475",
"article-title": "The Antimalarial Compound Atovaquone Inhibits Zika and Dengue Virus Infection by Blocking E Protein-Mediated Membrane Fusion",
"author": "M Yamamoto",
"doi-asserted-by": "crossref",
"issue": "12",
"journal-title": "Viruses",
"key": "ppat.1009840.ref034",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.3390/molecules25235615",
"article-title": "Repositioning of Ligands That Target the Spike Glycoprotein as Potential Drugs for SARS-CoV-2 in an In Silico Study",
"author": "GL Ramirez-Salinas",
"doi-asserted-by": "crossref",
"issue": "23",
"journal-title": "Molecules",
"key": "ppat.1009840.ref035",
"volume": "25",
"year": "2020"
},
{
"article-title": "Identification of Atovaquone, Ouabain and Mebendazole as FDA Approved Drugs Tar-geting SARS-CoV-2 (Version 4)",
"author": "A Farag",
"journal-title": "ChemRxiv",
"key": "ppat.1009840.ref036",
"year": "2020"
},
{
"DOI": "10.4155/fmc-2020-0165",
"article-title": "Ligand and structure-based virtual screening applied to the SARS-CoV-2 main protease: an in silico repurposing study",
"author": "WR Ferraz",
"doi-asserted-by": "crossref",
"first-page": "1815",
"issue": "20",
"journal-title": "Future Med Chem",
"key": "ppat.1009840.ref037",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1128/JVI.02501-05",
"article-title": "Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex",
"author": "EJ Snijder",
"doi-asserted-by": "crossref",
"first-page": "5927",
"issue": "12",
"journal-title": "J Virol",
"key": "ppat.1009840.ref038",
"volume": "80",
"year": "2006"
},
{
"DOI": "10.1038/s41422-020-0356-z",
"article-title": "Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease",
"author": "C Ma",
"doi-asserted-by": "crossref",
"first-page": "678",
"issue": "8",
"journal-title": "Cell Res",
"key": "ppat.1009840.ref039",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1021/acsmedchemlett.0c00521",
"article-title": "Identification of 14 Known Drugs as Inhibitors of the Main Protease of SARS-CoV-2",
"author": "MM Ghahremanpour",
"doi-asserted-by": "crossref",
"first-page": "2526",
"issue": "12",
"journal-title": "ACS Med Chem Lett",
"key": "ppat.1009840.ref040",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.jmb.2017.10.017",
"article-title": "The SARS-CoV Fusion Peptide Forms an Extended Bipartite Fusion Platform that Perturbs Membrane Order in a Calcium-Dependent Manner",
"author": "AL Lai",
"doi-asserted-by": "crossref",
"first-page": "3875",
"issue": "24",
"journal-title": "J Mol Biol",
"key": "ppat.1009840.ref041",
"volume": "429",
"year": "2017"
},
{
"article-title": "Overview of allergic mechanisms. Ebastine has more than an antihistamine effect",
"author": "A Campbell",
"first-page": "15",
"issue": "Suppl 1",
"journal-title": "Drugs",
"key": "ppat.1009840.ref042",
"volume": "52",
"year": "1996"
},
{
"DOI": "10.1016/j.jhep.2016.05.031",
"article-title": "Hepatocyte vitamin D receptor regulates lipid metabolism and mediates experimental diet-induced steatosis",
"author": "M Bozic",
"doi-asserted-by": "crossref",
"first-page": "748",
"issue": "4",
"journal-title": "J Hepatol",
"key": "ppat.1009840.ref043",
"volume": "65",
"year": "2016"
},
{
"article-title": "Vitamin D—liver disease association: Biological basis and mechanisms of action",
"author": "C Triantos",
"journal-title": "Hepatology",
"key": "ppat.1009840.ref044",
"year": "2021"
},
{
"DOI": "10.1016/S2213-8587(21)00003-6",
"article-title": "Vitamin D and COVID-19: why the controversy?",
"author": "E The Lancet Diabetes",
"doi-asserted-by": "crossref",
"first-page": "53",
"issue": "2",
"journal-title": "Lancet Diabetes Endocrinol",
"key": "ppat.1009840.ref045",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1096/fj.201802366RR",
"article-title": "Preservation of circadian rhythms by the protein folding chaperone, BiP",
"author": "A Pickard",
"doi-asserted-by": "crossref",
"first-page": "7479",
"issue": "6",
"journal-title": "FASEB J",
"key": "ppat.1009840.ref046",
"volume": "33",
"year": "2019"
}
],
"reference-count": 46,
"references-count": 46,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.ppat.1009840"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Genetics",
"Molecular Biology",
"Immunology",
"Microbiology",
"Parasitology"
],
"subtitle": [],
"title": "Discovery of re-purposed drugs that slow SARS-CoV-2 replication in human cells",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.ppat.corrections_policy",
"volume": "17"
}