A new investigation into the molecular mechanism of cholecalciferol towards reducing cytokines storm
, A., Octahedron Drug Research, doi:10.21608/odr.2024.308273.1043, Jan 2025
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
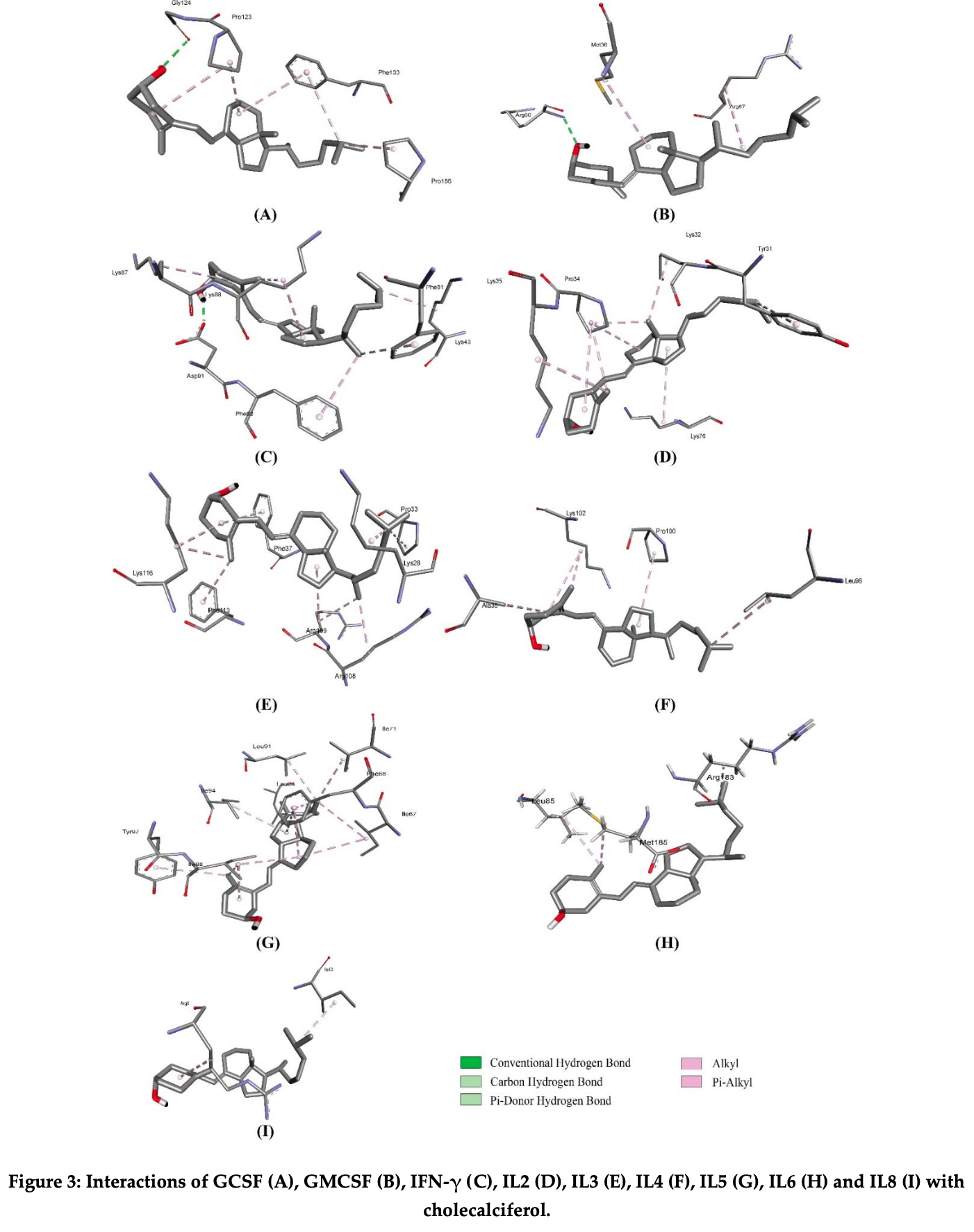
In silico study showing that cholecalciferol (vitamin D3) exhibits strong binding affinity to multiple cytokines involved in cytokine storm, with binding energies exceeding -6.5 kcal/mol. Molecular dynamics simulations revealed remarkable stability of cholecalciferol-cytokine complexes. Pharmacokinetic analysis suggests cholecalciferol has favorable absorption, distribution, metabolism, and excretion properties. Authors propose cholecalciferol may help reduce cytokine storm and alleviate severe COVID-19 symptoms by modulating the immune response and dampening excessive cytokine production.
29 preclinical studies support the efficacy of vitamin D for COVID-19:
Vitamin D has been identified by the European Food Safety Authority (EFSA) as having sufficient evidence for a causal relationship between intake and optimal immune system function27-30.
Vitamin D inhibits SARS-CoV-2 replication in vitro17,24, mitigates lung inflammation, damage, and lethality in mice with an MHV-3 model for β-CoV respiratory infections17,24, reduces SARS-CoV-2 replication in nasal epithelial cells via increased type I interferon expression20, downregulates proinflammatory cytokines IL-1β and TNF-α in SARS-CoV-2 spike protein-stimulated cells16, attenuates nucleocapsid protein-induced hyperinflammation by inactivating the NLRP3 inflammasome through the VDR-BRCC3 signaling pathway21, may be neuroprotective by protecting the blood-brain barrier, reducing neuroinflammation, and via immunomodulatory effects31, may mitigate hyperinflammation and cytokine storm by upregulating TLR10 expression which downregulates proinflammatory cytokines13, downregulates ACE2 and TMPRSS2 in human trophoblasts and minimizes spike protein-induced inflammation19, may minimize cytokine storm by dampening excessive cytokine production2, may suppress viral entry and replication via LL-37 induction11,12, and minimizes platelet aggregation mediated by SARS-CoV-2 spike protein via inhibiting integrin αIIbβ3 outside-in signaling15.
Cholecalciferol and calcifediol directly bind two allosteric pockets on the SARS-CoV-2 Spike RBD, bias the trimer toward a closed state, weaken ACE2 engagement, and reduce viral entry in cell models1.
Calcitriol may destabilize the Spike protein architecture and inhibit IL-17R dimerization, blocking viral entry and mitigating hyperinflammatory cytokine storm32.
Vitamin D improves regulatory immune cell levels and control of proinflammatory cytokines in severe COVID-1933.
Calcifediol inhibits SARS-CoV-2 papain-like protease (PLpro), a critical enzyme for viral replication14.
Symptomatic COVID-19 is associated with a lower frequency of natural killer (NK) cells and vitamin D has been shown to improve NK cell activity34,35.
1.
García-Marín et al., Exploring SARS-CoV-2 Spike RBD Pockets as Targets for Generic Drugs: A Combined Computational, Biophysical, and Biological Approach, ACS Omega, doi:10.1021/acsomega.5c05175.
2.
Alzahrani, A., A new investigation into the molecular mechanism of cholecalciferol towards reducing cytokines storm, Octahedron Drug Research, doi:10.21608/odr.2024.308273.1043.
3.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
4.
Morales-Bayuelo et al., New findings on ligand series used as SARS-CoV-2 virus inhibitors within the frameworks of molecular docking, molecular quantum similarity and chemical reactivity indices, F1000Research, doi:10.12688/f1000research.123550.3.
5.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
6.
Pandya et al., Unravelling Vitamin B12 as a potential inhibitor against SARS-CoV-2: A computational approach, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2022.100951.
7.
Mansouri et al., The impact of calcitriol and estradiol on the SARS-CoV-2 biological activity: a molecular modeling approach, Scientific Reports, doi:10.1038/s41598-022-04778-y.
8.
Song et al., Vitamin D3 and its hydroxyderivatives as promising drugs against COVID-19: a computational study, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1964601.
9.
Qayyum et al., Vitamin D and lumisterol novel metabolites can inhibit SARS-CoV-2 replication machinery enzymes, Endocrinology and Metabolism, doi:10.1152/ajpendo.00174.2021.
10.
Al-Mazaideh et al., Vitamin D is a New Promising Inhibitor to the Main Protease (Mpro) of COVID-19 by Molecular Docking, Journal of Pharmaceutical Research International, doi:10.9734/jpri/2021/v33i29B31603.
11.
Roth et al., Vitamin D-inducible antimicrobial peptide LL-37 binds SARS-CoV-2 Spike and accessory proteins ORF7a and ORF8, Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2025.1671738.
12.
Vercellino et al., Influence of Sex and 1,25α Dihydroxyvitamin D3 on SARS-CoV-2 Infection and Viral Entry, Pathogens, doi:10.3390/pathogens14080765.
13.
Knez et al., TLR10 overexpression modulates immune response in A549 lung epithelial cells challenged with SARS-CoV-2 S and N proteins, Frontiers in Immunology, doi:10.3389/fimmu.2024.1490478.
14.
Chen et al., In Vitro Characterization of Inhibition Function of Calcifediol to the Protease Activity of SARS-COV-2 PLpro, Journal of Medical Virology, doi:10.1002/jmv.70085.
15.
Wang et al., 1,25‐Dihydroxyvitamin D3 attenuates platelet aggregation potentiated by SARS‐CoV‐2 spike protein via inhibiting integrin αIIbβ3 outside‐in signaling, Cell Biochemistry and Function, doi:10.1002/cbf.4039.
16.
Alcalá-Santiago et al., Disentangling the Immunomodulatory Effects of Vitamin D on the SARS-CoV-2 Virus by In Vitro Approaches, The 14th European Nutrition Conference FENS 2023, doi:10.3390/proceedings2023091415.
17.
Campolina-Silva et al., Dietary Vitamin D Mitigates Coronavirus-Induced Lung Inflammation and Damage in Mice, Viruses, doi:10.3390/v15122434.
18.
Moatasim et al., Potent Antiviral Activity of Vitamin B12 against Severe Acute Respiratory Syndrome Coronavirus 2, Middle East Respiratory Syndrome Coronavirus, and Human Coronavirus 229E, Microorganisms, doi:10.3390/microorganisms11112777.
19.
Vargas-Castro et al., Calcitriol prevents SARS-CoV spike-induced inflammation in human trophoblasts through downregulating ACE2 and TMPRSS2 expression, The Journal of Steroid Biochemistry and Molecular Biology, doi:10.1016/j.jsbmb.2024.106625.
20.
Sposito et al., Age differential CD13 and interferon expression in airway epithelia affect SARS-CoV-2 infection - effects of vitamin D, Mucosal Immunology, doi:10.1016/j.mucimm.2023.08.002.
21.
Chen (B) et al., Vitamin D3 attenuates SARS‐CoV‐2 nucleocapsid protein‐caused hyperinflammation by inactivating the NLRP3 inflammasome through the VDR‐BRCC3 signaling pathway in vitro and in vivo, MedComm, doi:10.1002/mco2.318.
22.
Rybakovsky et al., Calcitriol modifies tight junctions, improves barrier function, and reduces TNF‐α‐induced barrier leak in the human lung‐derived epithelial cell culture model, 16HBE 14o‐, Physiological Reports, doi:10.14814/phy2.15592.
23.
DiGuilio et al., The multiphasic TNF-α-induced compromise of Calu-3 airway epithelial barrier function, Experimental Lung Research, doi:10.1080/01902148.2023.2193637.
24.
Pickard et al., Discovery of re-purposed drugs that slow SARS-CoV-2 replication in human cells, PLOS Pathogens, doi:10.1371/journal.ppat.1009840.
25.
Mok et al., Calcitriol, the active form of vitamin D, is a promising candidate for COVID-19 prophylaxis, bioRxiv, doi:10.1101/2020.06.21.162396.
26.
Fernandes de Souza et al., Lung Inflammation Induced by Inactivated SARS-CoV-2 in C57BL/6 Female Mice Is Controlled by Intranasal Instillation of Vitamin D, Cells, doi:10.3390/cells12071092.
27.
Galmés et al., Suboptimal Consumption of Relevant Immune System Micronutrients Is Associated with a Worse Impact of COVID-19 in Spanish Populations, Nutrients, doi:10.3390/nu14112254.
28.
Galmés (B) et al., Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework, Nutrients, doi:10.3390/nu12092738.
29.
EFSA, Scientific Opinion on the substantiation of a health claim related to vitamin D and contribution to the normal function of the immune system pursuant to Article 14 of Regulation (EC) No 1924/2006, EFSA Journal, doi:10.2903/j.efsa.2015.4096.
30.
EFSA (B), Scientific Opinion on the substantiation of health claims related to vitamin D and normal function of the immune system and inflammatory response (ID 154, 159), maintenance of normal muscle function (ID 155) and maintenance of normal cardiovascular function (ID 159) pursuant to Article 13(1) of Regulation (E, EFSA Journal, doi:10.2903/j.efsa.2010.1468.
31.
Gotelli et al., Understanding the immune-endocrine effects of vitamin D in SARS-CoV-2 infection: a role in protecting against neurodamage?, Neuroimmunomodulation, doi:10.1159/000533286.
32.
Fadel et al., Targeting asparagine and cysteine in SARS-CoV-2 variants and human pro-inflammatory mediators to alleviate COVID-19 severity; a cross-section and in-silico study, Scientific Reports, doi:10.1038/s41598-025-19359-y.
33.
Saheb Sharif-Askari et al., Increased blood immune regulatory cells in severe COVID-19 with autoantibodies to type I interferons, Scientific Reports, doi:10.1038/s41598-023-43675-w.
Alzahrani et al., 1 Jan 2025, multiple countries, peer-reviewed, 1 author.
Contact: alzharaniaar@bu.edu.sa (corresponding author).
In silico studies are an important part of preclinical research, however results may be very different in vivo.
A new investigation into the molecular mechanism of cholecalciferol towards reducing cytokines storm
Cytokine storm, also referred to as cytokine release syndrome (CRS), is a condition characterized by an excessive production of inflammatory signals by the immune system, potentially leading to organ failure and death. This phenomenon has garnered significant attention due to its association with the COVID-19 pandemic, wherein it appears to contribute to severe symptoms in certain individuals infected with the SARS-CoV-2 virus. In efforts to combat cytokine storm, researchers have explored natural substances as potential therapeutics. In this study, we investigated the efficacy of cholecalciferol in targeting key cytokines involved in cytokine storm. Through molecular docking analyses, molecular dynamics simulations, and assessment of pharmacokinetic properties, we evaluated the stability and potential of cholecalciferol in mitigating cytokine storm. Our findings indicate that cholecalciferol exhibits strong binding affinity with several cytokines, with binding energies exceeding -6.5 kcal/mol. Furthermore, post-molecular dynamics analysis revealed remarkable stability of cholecalciferol with these cytokines. Pharmacokinetic measurements further supported its potential as a therapeutic agent, demonstrating favorable characteristics in terms of absorption, distribution, metabolism, and excretion. This research suggests that cholecalciferol may hold promise in reducing cytokine storm and alleviating severe symptoms associated with conditions such as COVID-19.
Ethical consideration: All the participants in this study gave their informed permission.
Conflicts of Interest No conflicts of interest are disclosed.
References
Aranow, Vitamin D and the immune system, doi:10.2310/JIM.0b013e31821b8755?casa_token=4uAPj6fIyF8
Borel, Caillaud, Cano, Vitamin D bioavailability: state of the art, Crit Rev Food Sci Nutr
Computer, MOLECULAR-DYNAMICS SIMULATIONS -the University of Groningen research portal
Daina, Michielin, Zoete, SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules, Sci Reports
Dallakyan, Olson, Small-molecule library screening by docking with PyRx, Methods Mol Biol
Daneshkhah, Agrawal, Eshein, Subramanian, Roy et al., Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients, Aging Clin Exp Res, doi:10.1007/s40520-020-01677-y
Goncalves-Mendes, Talvas, Dualé, Guttmann, Corbin et al., Impact of Vitamin D Supplementation on Influenza Vaccine Response and Immune Functions in Deficient Elderly Persons: A Randomized Placebo-Controlled Trial, Front Immunol
Grant, Giovannucci, The possible roles of solar ultraviolet-B radiation and vitamin D in reducing case-fatality rates from the 1918-1919 influenza pandemic in the United States, Dermatoendocrinol
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Hughes, Nibbs, A guide to chemokines and their receptors, Febs J [Internet
Janoušek, Pilařová, Macáková, Nomura, Veiga-Matos et al., Vitamin D: sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites, Crit Rev Clin Lab Sci
Jarczak, Nierhaus, Cytokine Storm-Definition, Causes, and Implications, Int J Mol Sci
Khosroshahi, Rokni, Mokhtari, Noorbakhsh, Immunology, immunopathogenesis and immunotherapeutics of COVID-19; an overview, Int Immunopharmacol
Kim, Chen, Cheng, Gindulyte, He et al., PubChem in 2021: new data content and improved web interfaces, Nucleic Acids Res, doi:10.1093/nar/gkaa971
Mark, Nilsson, Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K, J Phys Chem A, doi:10.1021/jp003020w
Martineau, Jolliffe, Hooper, Greenberg, Aloia et al., Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, BMJ
Morán, Parra-Medina, Cardona, Quintero-Ronderos, Rodríguez, Cytokines, chemokines and growth factors
Nnoaham, Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis, Int J Epidemiol
Rigsby, Parker, Using the PyMOL application to reinforce visual understanding of protein structure, Biochem Mol Biol Educ
Saito, Nagao, Nishikawa, Kinugawa, Molecular collective dynamics in solid para-hydrogen and orthodeuterium: The Parrinello-Rahman-type path integral centroid molecular dynamics approach, J Chem Phys
Trott, Olson, AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading, J Comput Chem
Yu, Mackerell, Computer-Aided Drug Design Methods, Methods Mol Biol
Zambrano, Otth, Mujica, Concha, Maccioni, Interleukin-3 prevents neuronal death induced by amyloid peptide, BMC Neurosci
Zhang, Cytokines, Inflammation and Pain, Int Anesthesiol Clin
DOI record:
{
"DOI": "10.21608/odr.2024.308273.1043",
"ISSN": [
"2812-636X"
],
"URL": "http://dx.doi.org/10.21608/odr.2024.308273.1043",
"author": [
{
"affiliation": [],
"family": "Alzahrani",
"given": "Abdulaziz Ahmed",
"sequence": "first"
}
],
"container-title": "Octahedron Drug Research",
"container-title-short": "Octahedron Drug Research",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"odr.journals.ekb.eg"
]
},
"created": {
"date-parts": [
[
2025,
1,
22
]
],
"date-time": "2025-01-22T12:12:07Z",
"timestamp": 1737547927000
},
"deposited": {
"date-parts": [
[
2025,
1,
22
]
],
"date-time": "2025-01-22T12:43:51Z",
"timestamp": 1737549831000
},
"indexed": {
"date-parts": [
[
2025,
1,
23
]
],
"date-time": "2025-01-23T05:24:19Z",
"timestamp": 1737609859218,
"version": "3.33.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
1,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
1,
1
]
]
},
"published-print": {
"date-parts": [
[
2025,
1,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://odr.journals.ekb.eg/article_405505_1a124fcb3df7bb23104aa27d412be60c.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "9060",
"original-title": [],
"page": "16-26",
"prefix": "10.21608",
"published": {
"date-parts": [
[
2025,
1,
1
]
]
},
"published-online": {
"date-parts": [
[
2025,
1,
1
]
]
},
"published-print": {
"date-parts": [
[
2025,
1,
1
]
]
},
"publisher": "Egyptian Knowledge Bank",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://odr.journals.ekb.eg/article_405505.html"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A new investigation into the molecular mechanism of cholecalciferol towards reducing cytokines storm.",
"type": "journal-article",
"update-policy": "https://doi.org/10.21608/crossmark_policy",
"volume": "6"
}
