Lung Inflammation Induced by Inactivated SARS-CoV-2 in C57BL/6 Female Mice Is Controlled by Intranasal Instillation of Vitamin D
et al., Cells, doi:10.3390/cells12071092, Apr 2023
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
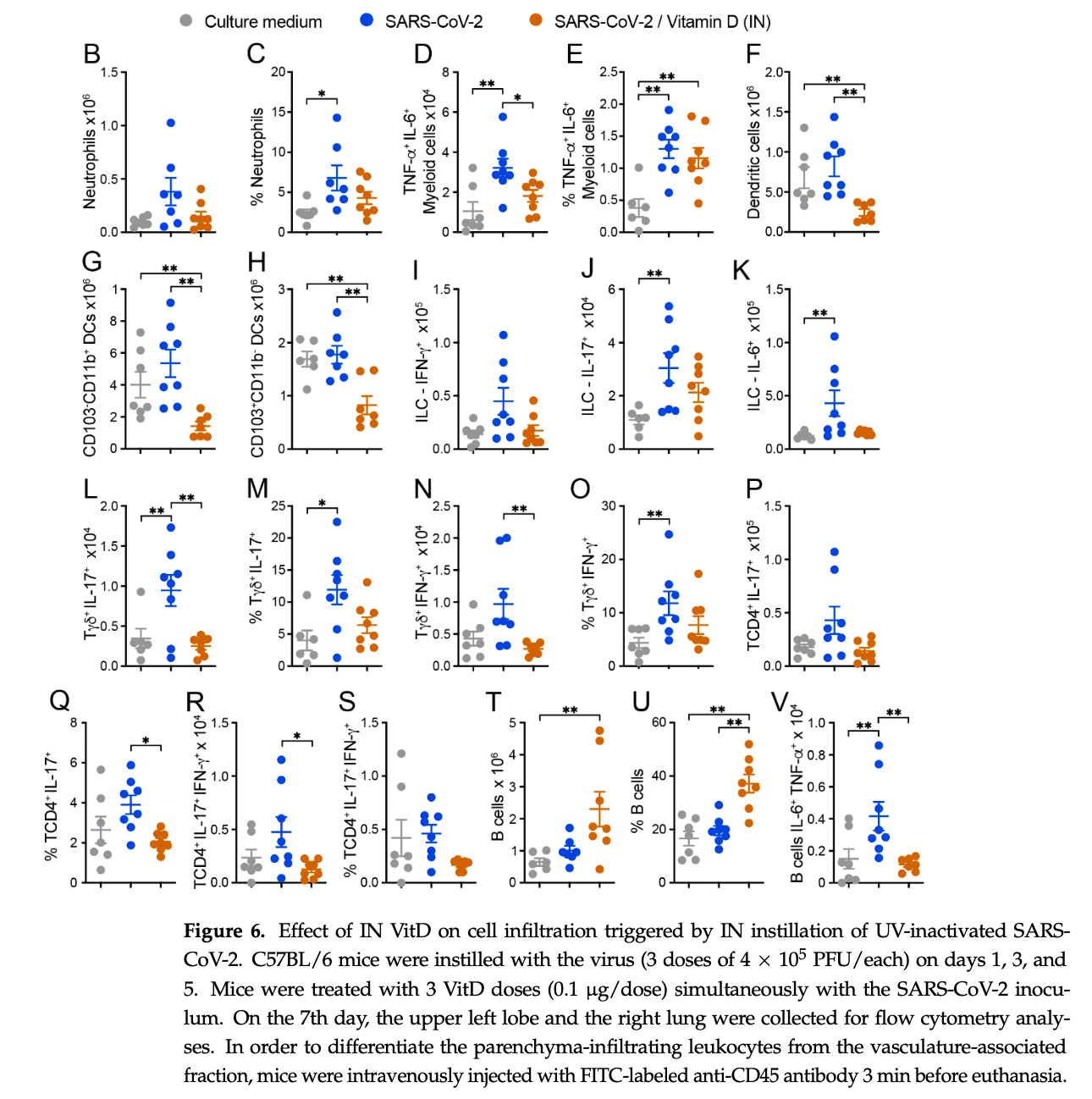
C57BL/6 mouse study showing intranasal administration of vitamin D decreased inflammation following intranasal inactivated SARS-CoV-2. Authors suggest a promising potential of intranasal vitamin D to control pulmonary inflammation associated with SARS-CoV-2.
29 preclinical studies support the efficacy of vitamin D for COVID-19:
Vitamin D has been identified by the European Food Safety Authority (EFSA) as having sufficient evidence for a causal relationship between intake and optimal immune system function27-30.
Vitamin D inhibits SARS-CoV-2 replication in vitro17,24, mitigates lung inflammation, damage, and lethality in mice with an MHV-3 model for β-CoV respiratory infections17,24, reduces SARS-CoV-2 replication in nasal epithelial cells via increased type I interferon expression20, downregulates proinflammatory cytokines IL-1β and TNF-α in SARS-CoV-2 spike protein-stimulated cells16, attenuates nucleocapsid protein-induced hyperinflammation by inactivating the NLRP3 inflammasome through the VDR-BRCC3 signaling pathway21, may be neuroprotective by protecting the blood-brain barrier, reducing neuroinflammation, and via immunomodulatory effects31, may mitigate hyperinflammation and cytokine storm by upregulating TLR10 expression which downregulates proinflammatory cytokines13, downregulates ACE2 and TMPRSS2 in human trophoblasts and minimizes spike protein-induced inflammation19, may minimize cytokine storm by dampening excessive cytokine production2, may suppress viral entry and replication via LL-37 induction11,12, and minimizes platelet aggregation mediated by SARS-CoV-2 spike protein via inhibiting integrin αIIbβ3 outside-in signaling15.
Cholecalciferol and calcifediol directly bind two allosteric pockets on the SARS-CoV-2 Spike RBD, bias the trimer toward a closed state, weaken ACE2 engagement, and reduce viral entry in cell models1.
Calcitriol may destabilize the Spike protein architecture and inhibit IL-17R dimerization, blocking viral entry and mitigating hyperinflammatory cytokine storm32.
Vitamin D improves regulatory immune cell levels and control of proinflammatory cytokines in severe COVID-1933.
Calcifediol inhibits SARS-CoV-2 papain-like protease (PLpro), a critical enzyme for viral replication14.
Symptomatic COVID-19 is associated with a lower frequency of natural killer (NK) cells and vitamin D has been shown to improve NK cell activity34,35.
1.
García-Marín et al., Exploring SARS-CoV-2 Spike RBD Pockets as Targets for Generic Drugs: A Combined Computational, Biophysical, and Biological Approach, ACS Omega, doi:10.1021/acsomega.5c05175.
2.
Alzahrani, A., A new investigation into the molecular mechanism of cholecalciferol towards reducing cytokines storm, Octahedron Drug Research, doi:10.21608/odr.2024.308273.1043.
3.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
4.
Morales-Bayuelo et al., New findings on ligand series used as SARS-CoV-2 virus inhibitors within the frameworks of molecular docking, molecular quantum similarity and chemical reactivity indices, F1000Research, doi:10.12688/f1000research.123550.3.
5.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
6.
Pandya et al., Unravelling Vitamin B12 as a potential inhibitor against SARS-CoV-2: A computational approach, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2022.100951.
7.
Mansouri et al., The impact of calcitriol and estradiol on the SARS-CoV-2 biological activity: a molecular modeling approach, Scientific Reports, doi:10.1038/s41598-022-04778-y.
8.
Song et al., Vitamin D3 and its hydroxyderivatives as promising drugs against COVID-19: a computational study, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1964601.
9.
Qayyum et al., Vitamin D and lumisterol novel metabolites can inhibit SARS-CoV-2 replication machinery enzymes, Endocrinology and Metabolism, doi:10.1152/ajpendo.00174.2021.
10.
Al-Mazaideh et al., Vitamin D is a New Promising Inhibitor to the Main Protease (Mpro) of COVID-19 by Molecular Docking, Journal of Pharmaceutical Research International, doi:10.9734/jpri/2021/v33i29B31603.
11.
Roth et al., Vitamin D-inducible antimicrobial peptide LL-37 binds SARS-CoV-2 Spike and accessory proteins ORF7a and ORF8, Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2025.1671738.
12.
Vercellino et al., Influence of Sex and 1,25α Dihydroxyvitamin D3 on SARS-CoV-2 Infection and Viral Entry, Pathogens, doi:10.3390/pathogens14080765.
13.
Knez et al., TLR10 overexpression modulates immune response in A549 lung epithelial cells challenged with SARS-CoV-2 S and N proteins, Frontiers in Immunology, doi:10.3389/fimmu.2024.1490478.
14.
Chen et al., In Vitro Characterization of Inhibition Function of Calcifediol to the Protease Activity of SARS-COV-2 PLpro, Journal of Medical Virology, doi:10.1002/jmv.70085.
15.
Wang et al., 1,25‐Dihydroxyvitamin D3 attenuates platelet aggregation potentiated by SARS‐CoV‐2 spike protein via inhibiting integrin αIIbβ3 outside‐in signaling, Cell Biochemistry and Function, doi:10.1002/cbf.4039.
16.
Alcalá-Santiago et al., Disentangling the Immunomodulatory Effects of Vitamin D on the SARS-CoV-2 Virus by In Vitro Approaches, The 14th European Nutrition Conference FENS 2023, doi:10.3390/proceedings2023091415.
17.
Campolina-Silva et al., Dietary Vitamin D Mitigates Coronavirus-Induced Lung Inflammation and Damage in Mice, Viruses, doi:10.3390/v15122434.
18.
Moatasim et al., Potent Antiviral Activity of Vitamin B12 against Severe Acute Respiratory Syndrome Coronavirus 2, Middle East Respiratory Syndrome Coronavirus, and Human Coronavirus 229E, Microorganisms, doi:10.3390/microorganisms11112777.
19.
Vargas-Castro et al., Calcitriol prevents SARS-CoV spike-induced inflammation in human trophoblasts through downregulating ACE2 and TMPRSS2 expression, The Journal of Steroid Biochemistry and Molecular Biology, doi:10.1016/j.jsbmb.2024.106625.
20.
Sposito et al., Age differential CD13 and interferon expression in airway epithelia affect SARS-CoV-2 infection - effects of vitamin D, Mucosal Immunology, doi:10.1016/j.mucimm.2023.08.002.
21.
Chen (B) et al., Vitamin D3 attenuates SARS‐CoV‐2 nucleocapsid protein‐caused hyperinflammation by inactivating the NLRP3 inflammasome through the VDR‐BRCC3 signaling pathway in vitro and in vivo, MedComm, doi:10.1002/mco2.318.
22.
Rybakovsky et al., Calcitriol modifies tight junctions, improves barrier function, and reduces TNF‐α‐induced barrier leak in the human lung‐derived epithelial cell culture model, 16HBE 14o‐, Physiological Reports, doi:10.14814/phy2.15592.
23.
DiGuilio et al., The multiphasic TNF-α-induced compromise of Calu-3 airway epithelial barrier function, Experimental Lung Research, doi:10.1080/01902148.2023.2193637.
24.
Pickard et al., Discovery of re-purposed drugs that slow SARS-CoV-2 replication in human cells, PLOS Pathogens, doi:10.1371/journal.ppat.1009840.
25.
Mok et al., Calcitriol, the active form of vitamin D, is a promising candidate for COVID-19 prophylaxis, bioRxiv, doi:10.1101/2020.06.21.162396.
26.
Fernandes de Souza et al., Lung Inflammation Induced by Inactivated SARS-CoV-2 in C57BL/6 Female Mice Is Controlled by Intranasal Instillation of Vitamin D, Cells, doi:10.3390/cells12071092.
27.
Galmés et al., Suboptimal Consumption of Relevant Immune System Micronutrients Is Associated with a Worse Impact of COVID-19 in Spanish Populations, Nutrients, doi:10.3390/nu14112254.
28.
Galmés (B) et al., Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework, Nutrients, doi:10.3390/nu12092738.
29.
EFSA, Scientific Opinion on the substantiation of a health claim related to vitamin D and contribution to the normal function of the immune system pursuant to Article 14 of Regulation (EC) No 1924/2006, EFSA Journal, doi:10.2903/j.efsa.2015.4096.
30.
EFSA (B), Scientific Opinion on the substantiation of health claims related to vitamin D and normal function of the immune system and inflammatory response (ID 154, 159), maintenance of normal muscle function (ID 155) and maintenance of normal cardiovascular function (ID 159) pursuant to Article 13(1) of Regulation (E, EFSA Journal, doi:10.2903/j.efsa.2010.1468.
31.
Gotelli et al., Understanding the immune-endocrine effects of vitamin D in SARS-CoV-2 infection: a role in protecting against neurodamage?, Neuroimmunomodulation, doi:10.1159/000533286.
32.
Fadel et al., Targeting asparagine and cysteine in SARS-CoV-2 variants and human pro-inflammatory mediators to alleviate COVID-19 severity; a cross-section and in-silico study, Scientific Reports, doi:10.1038/s41598-025-19359-y.
33.
Saheb Sharif-Askari et al., Increased blood immune regulatory cells in severe COVID-19 with autoantibodies to type I interferons, Scientific Reports, doi:10.1038/s41598-023-43675-w.
Fernandes de Souza et al., 6 Apr 2023, USA, peer-reviewed, 13 authors.
Contact: wdansouza@hotmail.com (corresponding author), denisefonseca@usp.br.
Lung Inflammation Induced by Inactivated SARS-CoV-2 in C57BL/6 Female Mice Is Controlled by Intranasal Instillation of Vitamin D
Cells, doi:10.3390/cells12071092
The COVID-19 pandemic was triggered by the coronavirus SARS-CoV-2, whose peak occurred in the years 2020 and 2021. The main target of this virus is the lung, and the infection is associated with an accentuated inflammatory process involving mainly the innate arm of the immune system. Here, we described the induction of a pulmonary inflammatory process triggered by the intranasal (IN) instillation of UV-inactivated SARS-CoV-2 in C57BL/6 female mice, and then the evaluation of the ability of vitamin D (VitD) to control this process. The assays used to estimate the severity of lung involvement included the total and differential number of cells in the bronchoalveolar lavage fluid (BALF), histopathological analysis, quantification of T cell subsets, and inflammatory mediators by RT-PCR, cytokine quantification in lung homogenates, and flow cytometric analysis of cells recovered from lung parenchyma. The IN instillation of inactivated SARS-CoV-2 triggered a pulmonary inflammatory process, consisting of various cell types and mediators, resembling the typical inflammation found in transgenic mice infected with SARS-CoV-2. This inflammatory process was significantly decreased by the IN delivery of VitD, but not by its IP administration, suggesting that this hormone could have a therapeutic potential in COVID-19 if locally applied. To our knowledge, the local delivery of VitD to downmodulate lung inflammation in COVID-19 is an original proposition.
Supplementary Materials: The following supporting information can be downloaded at: https:// www.mdpi.com/article/10.3390/cells12071092/s1.
Conflicts of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adamczak, The Role of Toll-Like Receptors and Vitamin D in Cardiovascular Diseases-A Review, Int. J. Mol. Sci, doi:10.3390/ijms18112252
Albornoz, Amarilla, Modhiran, Parker, Li et al., SARS-CoV-2 Drives NLRP3 Inflammasome Activation in Human Microglia through Spike Protein, Mol. Psychiatry, doi:10.1038/s41380-022-01831-0
Alexander, Tinkov, Strand, Alehagen, Skalny et al., Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance against Progressive COVID-19, Nutrients, doi:10.3390/nu12082358
Alunno, Najm, Mariette, De Marco, Emmel et al., Immunomodulatory Therapies for the Treatment of SARS-CoV-2 Infection: An Update of the Systematic Literature Review to Inform EULAR Points to Consider, RMD Open, doi:10.1136/rmdopen-2021-001899
Barnes, Adrover, Baxter-Stoltzfus, Borczuk, Cools-Lartigue et al., Targeting Potential Drivers of COVID-19: Neutrophil Extracellular Traps, J. Exp. Med, doi:10.1084/jem.20200652
Bednash, Kagan, Englert, Farkas, Tyurina et al., Syrian Hamsters as a Model of Lung Injury with SARS-CoV-2 Infection: Pathologic, Physiologic, and Detailed Molecular Profiling, Transl. Res, doi:10.1016/j.trsl.2021.10.007
Bi, Hong, Que, He, Ren et al., Inactivated SARS-CoV-2 Induces Acute Respiratory Distress Syndrome in Human ACE2-Transgenic Mice, Signal Transduct. Target, doi:10.1038/s41392-021-00851-6
Bispo-Dos-Santos, Barbosa, Granja, Martini, Oliveira et al., Ultraviolet Germicidal Irradiation Is Effective against SARS-CoV-2 in Contaminated Makeup Powder and Lipstick, J. Photochem. Photobiol, doi:10.1016/j.jpap.2021.100072
Bösmüller, Matter, Fend, Tzankov, The Pulmonary Pathology of COVID-19, Virchows Arch, doi:10.1007/s00428-021-03053-1
Carta, Penco, Lavieri, Martini, Dinarello et al., Cell Stress Increases ATP Release in NLRP3 Inflammasome-Mediated Autoinflammatory Diseases, Resulting in Cytokine Imbalance, doi:10.1073/pnas.1424741112
Castillo, Entrenas Costa, Vaquero Barrios, Alcalá Díaz, López Miranda et al., Effect of Calcifediol Treatment and Best Available Therapy versus Best Available Therapy on Intensive Care Unit Admission and Mortality among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical Study, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105751
Cavalcante-Silva, Carvalho, De Almeida Lima, Galvão, Da Silva et al., Neutrophils and COVID-19: The Road so Far, Int. Immunopharmacol, doi:10.1016/j.intimp.2020.107233
Cho, Zhang, Ko, Shin, Lee et al., Intranasal Treatment with 1, 25-Dihydroxyvitamin D3 Alleviates Allergic Rhinitis Symptoms in a Mouse Model, Allergy Asthma Immunol. Res, doi:10.4168/aair.2019.11.2.267
Coimbra, Borin, Fontoura, Gravina, Nagai et al., Identification of Compounds with Antiviral Activity Against SARS-CoV-2 in the MMV Pathogen Box Using a Phenotypic High-Throughput Screening Assay, Front.Virol, doi:10.3389/fviro.2022.854363
Darif, Hammi, Kihel, El Idrissi Saik, Guessous et al., The Pro-Inflammatory Cytokines in COVID-19 Pathogenesis: What Goes Wrong?, Microb. Pathog, doi:10.1016/j.micpath.2021.104799
De Oliveira, Mimura, De Campos Fraga-Silva, Ishikawa, Fernandes et al., Calcitriol Prevents Neuroinflammation and Reduces Blood-Brain Barrier Disruption and Local Macrophage/Microglia Activation, Front. Pharmacol, doi:10.3389/fphar.2020.00161
Deluca, Prahl, Plum, 1,25-Dihydroxyvitamin D Is Not Responsible for Toxicity Caused by Vitamin D or 25-Hydroxyvitamin D, Arch. Biochem. Biophys, doi:10.1016/j.abb.2010.10.012
Diamond, Kanneganti, Innate Immunity: The First Line of Defense against SARS-CoV-2, Nat. Immunol, doi:10.1038/s41590-021-01091-0
Enkhjargal, Mcbride, Manaenko, Reis, Sakai et al., Intranasal Administration of Vitamin D Attenuates Blood-Brain Barrier Disruption through Endogenous Upregulation of Osteopontin and Activation of CD44/P-Gp Glycosylation Signaling after Subarachnoid Hemorrhage in Rats, J. Cereb. Blood Flow Metab, doi:10.1177/0271678X16671147
Feng, Meng, Qi, Athari, Chen, Study Effect of Vitamin D on the Immunopathology Responses of the Bronchi in Murine Model of Asthma. Iran, J. Allergy Asthma Immunol, doi:10.18502/ijaai.v20i5.7399
Forschner, Buchholtz, Stockfleth, Current State of Vitiligo Therapy? Evidence-Based Analysis of the Literature, JDDG J. Dtsch. Dermatol. Ges, doi:10.1111/j.1610-0387.2007.06280.x
Gardiman, Bianchetto-Aguilera, Gasperini, Tiberio, Scandola et al., SARS-CoV-2-Associated SsRNAs Activate Human Neutrophils in a TLR8-Dependent Fashion, Cells, doi:10.3390/cells11233785
Gavriatopoulou, Korompoki, Fotiou, Ntanasis-Stathopoulos, Psaltopoulou et al., Organ-Specific Manifestations of COVID-19 Infection, Clin. Exp. Med, doi:10.1007/s10238-020-00648-x
Hansdottir, Monick, Vitamin D Effects on Lung Immunity and Respiratory Diseases
Hou, Xiao, Tang, Xie, Diversity of Macrophages in Lung Homeostasis and Diseases, Front. Immunol, doi:10.3389/fimmu.2021.753940
Hu, Huang, Yin, The Cytokine Storm and COVID-19, J. Med. Virol, doi:10.1002/jmv.26232
Häusler, Torke, Weber, High-Dose Vitamin D-Mediated Hypercalcemia as a Potential Risk Factor in Central Nervous System Demyelinating Disease, Front. Immunol, doi:10.3389/fimmu.2020.00301
Karki, Sharma, Tuladhar, Williams, Zalduondo et al., Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes, Cell, doi:10.1016/j.cell.2020.11.025
Karonova, Andreeva, Golovatuk, Bykova, Simanenkova et al., Low 25(OH)D Level Is Associated with Severe Course and Poor Prognosis in COVID-19, Nutrients, doi:10.3390/nu13093021
Khreefa, Barbier, Koksal, Love, Del Valle, Pathogenesis and Mechanisms of SARS-CoV-2 Infection in the Intestine, Liver, and Pancreas, Cells, doi:10.3390/cells12020262
Kieffer, Topical Vitamin D Analogs, Dermatol. Nurs
Kobusiak-Prokopowicz, Fułek, Fułek, Kaaz, Mysiak et al., Pulmonary, and Neuropsychiatric Short-and Long-Term Complications of COVID-19, Cells
Krasemann, Haferkamp, Pfefferle, Woo, Heinrich et al., The Blood-Brain Barrier Is Dysregulated in COVID-19 and Serves as a CNS Entry Route for SARS-CoV-2, Stem Cell Rep, doi:10.1016/j.stemcr.2021.12.011
Labiris, Dolovich, Pulmonary Drug Delivery. Part I: Physiological Factors Affecting Therapeutic Effectiveness of Aerosolized Medications: Physiological Factors Affecting the Effectiveness of Inhaled Drugs, Br. J. Clin. Pharmacol, doi:10.1046/j.1365-2125.2003.01892.x
Liang, Li, Li, Yang, Li et al., Role of Neutrophil Chemoattractant CXCL5 in SARS-CoV-2 Infection-Induced Lung Inflammatory Innate Immune Response in an in vivo. HACE2 Transfection Mouse Model, Zool. Res, doi:10.24272/j.issn.2095-8137.2020.118
Liao, Liu, Yuan, Wen, Xu et al., Single-Cell Landscape of Bronchoalveolar Immune Cells in Patients with COVID-19, Nat. Med, doi:10.1038/s41591-020-0901-9
Lou, Duan, Qin, Teng, Gan et al., Advances in Oral Drug Delivery Systems: Challenges and Opportunities, Pharmaceutics, doi:10.3390/pharmaceutics15020484
Machhi, Herskovitz, Senan, Dutta, Nath et al., The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections, J. Neuroimmune Pharmacol, doi:10.1007/s11481-020-09944-5
Majumder, Minko, Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19, AAPS J, doi:10.1208/s12248-020-00532-2
Marcinkowska, Brown, Editorial: Vitamin D and COVID-19: New Mechanistic and Therapeutic Insights, Front. Pharmacol, doi:10.3389/fphar.2022.882046
Marcinowska-Suchowierska, Kupisz-Urba Ńska, Łukaszkiewicz, Płudowski, Jones, Vitamin D Toxicity-A Clinical Perspective, Front. Endocrinol, doi:10.3389/fendo.2018.00550
Mariani, Antonietti, Tajer, Ferder, Inserra et al., High-Dose Vitamin D versus Placebo to Prevent Complications in COVID-19 Patients: Multicentre Randomized Controlled Clinical Trial, PLoS ONE, doi:10.1371/journal.pone.0267918
Marongiu, Valache, Facchini, Granucci, How Dendritic Cells Sense and Respond to Viral Infections, Clin. Sci, doi:10.1042/CS20210577
Meizlish, Pine, Bishai, Goshua, Nadelmann et al., A Neutrophil Activation Signature Predicts Critical Illness and Mortality in COVID-19, Blood Adv
Mercola, Grant, Wagner, Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity, Nutrients, doi:10.3390/nu12113361
Meyer, Raghu, Bronchoalveolar Lavage for the Evaluation of Interstitial Lung Disease: Is It Clinically Useful?, Eur. Respir. J, doi:10.1183/09031936.00069509
Mimura, De Campos Fraga-Silva, De Oliveira, Ishikawa, Borim et al., Preclinical Therapy with Vitamin D3 in Experimental Encephalomyelitis: Efficacy and Comparison with Paricalcitol, Int. J. Mol. Sci, doi:10.3390/ijms22041914
Murai, Fernandes, Antonangelo, Gualano, Pereira, Effect of a Single High-Dose Vitamin D3 on the Length of Hospital Stay of Severely 25-Hydroxyvitamin D-Deficient Patients with COVID-19, Clinics, doi:10.6061/clinics/2021/e3549
Ohaegbulam, Swalih, Patel, Smith, Perrin, Vitamin D Supplementation in COVID-19 Patients: A Clinical Case Series, Am. J. Ther, doi:10.1097/MJT.0000000000001222
Pan, Shen, Yu, Ge, Chen et al., SARS-CoV-2 N Protein Promotes NLRP3 Inflammasome Activation to Induce Hyperinflammation, Nat. Commun, doi:10.1038/s41467-021-25015-6
Parackova, Zentsova, Bloomfield, Vrabcova, Smetanova et al., Disharmonic Inflammatory Signatures in COVID-19: Augmented Neutrophils' but Impaired Monocytes' and Dendritic Cells' Responsiveness, Cells, doi:10.3390/cells9102206
Puthia, Tanner, Petruk, Schmidtchen, Experimental Model of Pulmonary Inflammation Induced by SARS-CoV-2 Spike Protein and Endotoxin, ACS Pharmacol. Transl. Sci, doi:10.1021/acsptsci.1c00219
Radujkovic, Hippchen, Tiwari-Heckler, Dreher, Boxberger et al., Vitamin D Deficiency and Outcome of COVID-19 Patients, Nutrients, doi:10.3390/nu12092757
Rao, Chen, Wu, Xiao, Zhang et al., Vitamin D Receptor Inhibits NLRP3 Activation by Impeding Its BRCC3-Mediated Deubiquitination, Front. Immunol, doi:10.3389/fimmu.2019.02783
Reusch, De Domenico, Bonaguro, Schulte-Schrepping, Baßler et al., Neutrophils in COVID-19, Front. Immunol, doi:10.3389/fimmu.2021.652470
Roberts, Deming, Paddock, Cheng, Yount et al., A Mouse-Adapted SARS-Coronavirus Causes Disease and Mortality in BALB/c Mice, PLoS Pathog, doi:10.1371/journal.ppat.0030005
Rodrigues, De Sá, Ishimoto, Becerra, Oliveira et al., Inflammasomes Are Activated in Response to SARS-CoV-2 Infection and Are Associated with COVID-19 Severity in Patients, J. Exp. Med, doi:10.1084/jem.20201707
Rossol, Pierer, Raulien, Quandt, Meusch et al., Extracellular Ca 2+ Is a Danger Signal Activating the NLRP3 Inflammasome through G Protein-Coupled Calcium Sensing Receptors, Nat. Commun, doi:10.1038/ncomms2339
Sabico, Enani, Sheshah, Aljohani, Aldisi et al., Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate COVID-19: A Randomized Clinical Trial, Nutrients, doi:10.3390/nu13072170
Samadizadeh, Masoudi, Rastegar, Salimi, Shahbaz et al., COVID-19: Why Does Disease Severity Vary among Individuals?, Respir. Med, doi:10.1016/j.rmed.2021.106356
Sassi, Tamone, D'amelio, Vitamin, Nutrient, Hormone, and Immunomodulator, Nutrients, doi:10.3390/nu10111656
Schrumpf, Van Der Does, Hiemstra, Impact of the Local Inflammatory Environment on Mucosal Vitamin D Metabolism and Signaling in Chronic Inflammatory Lung Diseases, Front. Immunol, doi:10.3389/fimmu.2020.01433
Serré, Mathyssen, Ajime, Heigl, Verlinden et al., Local Nebulization of 1α,25(OH)2D3 Attenuates LPS-Induced Acute Lung Inflammation, Respir. Res
Tomar, Anders, Desai, Mulay, Neutrophils and Neutrophil Extracellular Traps Drive Necroinflammation in COVID-19, Cells, doi:10.3390/cells9061383
Van Hoecke, Job, Saelens, Roose, Bronchoalveolar Lavage of Murine Lungs to Analyze Inflammatory Cell Infiltration, J. Vis. Exp, doi:10.3791/55398
Wang, Xiong, Tsang, Schätzlein, Uchegbu, Nose-to-Brain Delivery, J. Pharmacol. Exp, doi:10.1124/jpet.119.258152
Winheim, Rinke, Lutz, Reischer, Leutbecher et al., Impaired Function and Delayed Regeneration of Dendritic Cells in COVID-19, PLoS Pathog, doi:10.1371/journal.ppat.1009742
Winkler, Bailey, Kafai, Nair, Mccune et al., SARS-CoV-2 Infection of Human ACE2-Transgenic Mice Causes Severe Lung Inflammation and Impaired Function, Nat. Immunol, doi:10.1038/s41590-020-0778-2
Wölfel, Corman, Guggemos, Seilmaier, Zange et al., Virological Assessment of Hospitalized Patients with COVID-2019, Nature, doi:10.1038/s41586-020-2196-x
Xia, Tang, Wang, Lai, Xu et al., SARS-CoV-2 N Protein Induces Acute Lung Injury in Mice via NF-κB Activation, Front. Immunol, doi:10.3389/fimmu.2021.791753
Xu, Baylink, Chen, Reeves, Xiao et al., The Importance of Vitamin d Metabolism as a Potential Prophylactic, Immunoregulatory and Neuroprotective Treatment for COVID-19, J. Transl. Med, doi:10.1186/s12967-020-02488-5
Zeitelhofer, Adzemovic, Gomez-Cabrero, Bergman, Hochmeister et al., Functional Genomics Analysis of Vitamin D Effects on CD4 + T Cells in Vivo in Experimental Autoimmune Encephalomyelitis, doi:10.1073/pnas.1615783114
Zeng, Xie, Feng, Xu, Han et al., Specific Inhibition of the NLRP3 Inflammasome Suppresses Immune Overactivation and Alleviates COVID-19 like Pathology in Mice, eBioMedicine, doi:10.1016/j.ebiom.2021.103803
Zhao, Di, Xu, The NLRP3 Inflammasome and COVID-19: Activation, Pathogenesis and Therapeutic Strategies, Cytokine Growth Factor Rev, doi:10.1016/j.cytogfr.2021.06.002
Zhou, Yang, Chi, Dong, Lv et al., Comorbidities and the Risk of Severe or Fatal Outcomes Associated with Coronavirus Disease 2019: A Systematic Review and Meta-Analysis, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.07.029
Şimşek Yavuz, Komşuo Glu Çelikyurt, ˙I. An Update of Anti-Viral Treatment of COVID-19, Turk. J. Med. Sci
DOI record:
{
"DOI": "10.3390/cells12071092",
"ISSN": [
"2073-4409"
],
"URL": "http://dx.doi.org/10.3390/cells12071092",
"abstract": "<jats:p>The COVID-19 pandemic was triggered by the coronavirus SARS-CoV-2, whose peak occurred in the years 2020 and 2021. The main target of this virus is the lung, and the infection is associated with an accentuated inflammatory process involving mainly the innate arm of the immune system. Here, we described the induction of a pulmonary inflammatory process triggered by the intranasal (IN) instillation of UV-inactivated SARS-CoV-2 in C57BL/6 female mice, and then the evaluation of the ability of vitamin D (VitD) to control this process. The assays used to estimate the severity of lung involvement included the total and differential number of cells in the bronchoalveolar lavage fluid (BALF), histopathological analysis, quantification of T cell subsets, and inflammatory mediators by RT-PCR, cytokine quantification in lung homogenates, and flow cytometric analysis of cells recovered from lung parenchyma. The IN instillation of inactivated SARS-CoV-2 triggered a pulmonary inflammatory process, consisting of various cell types and mediators, resembling the typical inflammation found in transgenic mice infected with SARS-CoV-2. This inflammatory process was significantly decreased by the IN delivery of VitD, but not by its IP administration, suggesting that this hormone could have a therapeutic potential in COVID-19 if locally applied. To our knowledge, the local delivery of VitD to downmodulate lung inflammation in COVID-19 is an original proposition.</jats:p>",
"alternative-id": [
"cells12071092"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-9610-694X",
"affiliation": [
{
"name": "Department of Chemical and Biological Sciences, Institute of Biosciences, São Paulo State University (UNESP), Botucatu 18618-689, SP, Brazil"
}
],
"authenticated-orcid": false,
"family": "Fernandes de Souza",
"given": "William Danilo",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Chemical and Biological Sciences, Institute of Biosciences, São Paulo State University (UNESP), Botucatu 18618-689, SP, Brazil"
}
],
"family": "Zorzella-Pezavento",
"given": "Sofia Fernanda Gonçalves",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Mucosal Immunology, Department of Immunology, Institute of Biomedical Sciences, University of São Paulo (USP), São Paulo 05508-000, SP, Brazil"
}
],
"family": "Ayupe",
"given": "Marina Caçador",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Mucosal Immunology, Department of Immunology, Institute of Biomedical Sciences, University of São Paulo (USP), São Paulo 05508-000, SP, Brazil"
}
],
"family": "Salgado",
"given": "Caio Loureiro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Mucosal Immunology, Department of Immunology, Institute of Biomedical Sciences, University of São Paulo (USP), São Paulo 05508-000, SP, Brazil"
}
],
"family": "Oliveira",
"given": "Bernardo de Castro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Mucosal Immunology, Department of Immunology, Institute of Biomedical Sciences, University of São Paulo (USP), São Paulo 05508-000, SP, Brazil"
}
],
"family": "Moreira",
"given": "Francielly",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3398-6035",
"affiliation": [
{
"name": "Laboratory of Mucosal Immunology, Department of Immunology, Institute of Biomedical Sciences, University of São Paulo (USP), São Paulo 05508-000, SP, Brazil"
}
],
"authenticated-orcid": false,
"family": "da Silva",
"given": "Guilherme William",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5105-6659",
"affiliation": [
{
"name": "Laboratory of Emerging Viruses, Department of Genetics, Evolution, Microbiology and Immunology, Institute of Biology, University of Campinas (UNICAMP), Campinas 13083-862, SP, Brazil"
}
],
"authenticated-orcid": false,
"family": "Muraro",
"given": "Stefanie Primon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Emerging Viruses, Department of Genetics, Evolution, Microbiology and Immunology, Institute of Biology, University of Campinas (UNICAMP), Campinas 13083-862, SP, Brazil"
}
],
"family": "de Souza",
"given": "Gabriela Fabiano",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4996-3153",
"affiliation": [
{
"name": "Laboratory of Emerging Viruses, Department of Genetics, Evolution, Microbiology and Immunology, Institute of Biology, University of Campinas (UNICAMP), Campinas 13083-862, SP, Brazil"
}
],
"authenticated-orcid": false,
"family": "Proença-Módena",
"given": "José Luiz",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9153-1485",
"affiliation": [
{
"name": "Department of Chemical and Biological Sciences, Institute of Biosciences, São Paulo State University (UNESP), Botucatu 18618-689, SP, Brazil"
}
],
"authenticated-orcid": false,
"family": "Araujo Junior",
"given": "Joao Pessoa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Mucosal Immunology, Department of Immunology, Institute of Biomedical Sciences, University of São Paulo (USP), São Paulo 05508-000, SP, Brazil"
}
],
"family": "Fonseca",
"given": "Denise Morais da",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Chemical and Biological Sciences, Institute of Biosciences, São Paulo State University (UNESP), Botucatu 18618-689, SP, Brazil"
}
],
"family": "Sartori",
"given": "Alexandrina",
"sequence": "additional"
}
],
"container-title": "Cells",
"container-title-short": "Cells",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
4,
6
]
],
"date-time": "2023-04-06T07:59:55Z",
"timestamp": 1680767995000
},
"deposited": {
"date-parts": [
[
2023,
4,
6
]
],
"date-time": "2023-04-06T08:38:51Z",
"timestamp": 1680770331000
},
"funder": [
{
"name": "JBS S.A."
},
{
"name": "scholarships of the Coordination for the Improvement of Higher Education Personnel"
},
{
"award": [
"88882.495054/2020-01"
],
"name": "WDFS master’s scholarship"
},
{
"award": [
"2020/04558-0"
],
"name": "São Paulo Research Support Foundation"
},
{
"award": [
"313429/2020-0",
"305628/2020-8",
"307269/2017-5"
],
"name": "CNPq scholarship"
},
{
"award": [
"2021/06881-5",
"2019/12691-4",
"2019/13916-0",
"2019/07771-9",
"2021/12768-7"
],
"name": "FAPESP scholarships"
}
],
"indexed": {
"date-parts": [
[
2023,
4,
7
]
],
"date-time": "2023-04-07T04:59:01Z",
"timestamp": 1680843541453
},
"is-referenced-by-count": 0,
"issue": "7",
"issued": {
"date-parts": [
[
2023,
4,
6
]
]
},
"journal-issue": {
"issue": "7",
"published-online": {
"date-parts": [
[
2023,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
4,
6
]
],
"date-time": "2023-04-06T00:00:00Z",
"timestamp": 1680739200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2073-4409/12/7/1092/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1092",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
4,
6
]
]
},
"published-online": {
"date-parts": [
[
2023,
4,
6
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1016/j.rmed.2021.106356",
"article-title": "COVID-19: Why Does Disease Severity Vary among Individuals?",
"author": "Samadizadeh",
"doi-asserted-by": "crossref",
"first-page": "106356",
"journal-title": "Respir. Med.",
"key": "ref_1",
"volume": "180",
"year": "2021"
},
{
"DOI": "10.1007/s11481-020-09944-5",
"article-title": "The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections",
"author": "Machhi",
"doi-asserted-by": "crossref",
"first-page": "359",
"journal-title": "J. Neuroimmune Pharmacol.",
"key": "ref_2",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.3390/cells12020262",
"doi-asserted-by": "crossref",
"key": "ref_3",
"unstructured": "Khreefa, Z., Barbier, M.T., Koksal, A.R., Love, G., and Del Valle, L. (2023). Pathogenesis and Mechanisms of SARS-CoV-2 Infection in the Intestine, Liver, and Pancreas. Cells, 12."
},
{
"DOI": "10.1007/s10238-020-00648-x",
"article-title": "Organ-Specific Manifestations of COVID-19 Infection",
"author": "Gavriatopoulou",
"doi-asserted-by": "crossref",
"first-page": "493",
"journal-title": "Clin. Exp. Med.",
"key": "ref_4",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.07.029",
"article-title": "Comorbidities and the Risk of Severe or Fatal Outcomes Associated with Coronavirus Disease 2019: A Systematic Review and Meta-Analysis",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "47",
"journal-title": "Int. J. Infect. Dis.",
"key": "ref_5",
"volume": "99",
"year": "2020"
},
{
"DOI": "10.3390/cells11233882",
"doi-asserted-by": "crossref",
"key": "ref_6",
"unstructured": "Kobusiak-Prokopowicz, M., Fułek, K., Fułek, M., Kaaz, K., Mysiak, A., Kurpas, D., Beszłej, J.A., Brzecka, A., and Leszek, J. (2022). Cardiovascular, Pulmonary, and Neuropsychiatric Short- and Long-Term Complications of COVID-19. Cells, 11."
},
{
"DOI": "10.1038/s41590-021-01091-0",
"article-title": "Innate Immunity: The First Line of Defense against SARS-CoV-2",
"author": "Diamond",
"doi-asserted-by": "crossref",
"first-page": "165",
"journal-title": "Nat. Immunol.",
"key": "ref_7",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1002/jmv.26232",
"article-title": "The Cytokine Storm and COVID-19",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "250",
"journal-title": "J. Med. Virol.",
"key": "ref_8",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.11.025",
"article-title": "Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes",
"author": "Karki",
"doi-asserted-by": "crossref",
"first-page": "149",
"journal-title": "Cell",
"key": "ref_9",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1016/j.trsl.2021.10.007",
"article-title": "Syrian Hamsters as a Model of Lung Injury with SARS-CoV-2 Infection: Pathologic, Physiologic, and Detailed Molecular Profiling",
"author": "Bednash",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Transl. Res.",
"key": "ref_10",
"volume": "240",
"year": "2022"
},
{
"DOI": "10.1038/s41590-020-0778-2",
"article-title": "SARS-CoV-2 Infection of Human ACE2-Transgenic Mice Causes Severe Lung Inflammation and Impaired Function",
"author": "Winkler",
"doi-asserted-by": "crossref",
"first-page": "1327",
"journal-title": "Nat. Immunol.",
"key": "ref_11",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2021.791753",
"article-title": "SARS-CoV-2 N Protein Induces Acute Lung Injury in Mice via NF-ĸB Activation",
"author": "Xia",
"doi-asserted-by": "crossref",
"first-page": "791753",
"journal-title": "Front. Immunol.",
"key": "ref_12",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1021/acsptsci.1c00219",
"article-title": "Experimental Model of Pulmonary Inflammation Induced by SARS-CoV-2 Spike Protein and Endotoxin",
"author": "Puthia",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "ACS Pharmacol. Transl. Sci.",
"key": "ref_13",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1136/rmdopen-2021-001899",
"article-title": "Immunomodulatory Therapies for the Treatment of SARS-CoV-2 Infection: An Update of the Systematic Literature Review to Inform EULAR Points to Consider",
"author": "Alunno",
"doi-asserted-by": "crossref",
"first-page": "e001899",
"journal-title": "RMD Open",
"key": "ref_14",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1208/s12248-020-00532-2",
"article-title": "Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19",
"author": "Majumder",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "AAPS J.",
"key": "ref_15",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.3906/sag-2106-250",
"article-title": "An Update of Anti-Viral Treatment of COVID-19",
"doi-asserted-by": "crossref",
"first-page": "3372",
"journal-title": "Turk. J. Med. Sci.",
"key": "ref_16",
"volume": "51",
"year": "2021"
},
{
"DOI": "10.3390/nu12113361",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Mercola, J., Grant, W.B., and Wagner, C.L. (2020). Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity. Nutrients, 12."
},
{
"DOI": "10.3390/nu12082358",
"doi-asserted-by": "crossref",
"key": "ref_18",
"unstructured": "Alexander, J., Tinkov, A., Strand, T.A., Alehagen, U., Skalny, A., and Aaseth, J. (2020). Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance against Progressive COVID-19. Nutrients, 12."
},
{
"DOI": "10.1186/s12967-020-02488-5",
"article-title": "The Importance of Vitamin d Metabolism as a Potential Prophylactic, Immunoregulatory and Neuroprotective Treatment for COVID-19",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "322",
"journal-title": "J. Transl. Med.",
"key": "ref_19",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0267918",
"doi-asserted-by": "crossref",
"key": "ref_20",
"unstructured": "Mariani, J., Antonietti, L., Tajer, C., Ferder, L., Inserra, F., Sanchez Cunto, M., Brosio, D., Ross, F., Zylberman, M., and López, D.E. (2022). High-Dose Vitamin D versus Placebo to Prevent Complications in COVID-19 Patients: Multicentre Randomized Controlled Clinical Trial. PLoS ONE, 17."
},
{
"DOI": "10.6061/clinics/2021/e3549",
"article-title": "Effect of a Single High-Dose Vitamin D3 on the Length of Hospital Stay of Severely 25-Hydroxyvitamin D-Deficient Patients with COVID-19",
"author": "Murai",
"doi-asserted-by": "crossref",
"first-page": "e3549",
"journal-title": "Clinics",
"key": "ref_21",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1097/MJT.0000000000001222",
"article-title": "Vitamin D Supplementation in COVID-19 Patients: A Clinical Case Series",
"author": "Ohaegbulam",
"doi-asserted-by": "crossref",
"first-page": "e485",
"journal-title": "Am. J. Ther.",
"key": "ref_22",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.3390/nu13072170",
"doi-asserted-by": "crossref",
"key": "ref_23",
"unstructured": "Sabico, S., Enani, M.A., Sheshah, E., Aljohani, N.J., Aldisi, D.A., Alotaibi, N.H., Alshingetti, N., Alomar, S.Y., Alnaami, A.M., and Amer, O.E. (2021). Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate COVID-19: A Randomized Clinical Trial. Nutrients, 13."
},
{
"DOI": "10.3390/pharmaceutics15020484",
"doi-asserted-by": "crossref",
"key": "ref_24",
"unstructured": "Lou, J., Duan, H., Qin, Q., Teng, Z., Gan, F., Zhou, X., and Zhou, X. (2023). Advances in Oral Drug Delivery Systems: Challenges and Opportunities. Pharmaceutics, 15."
},
{
"DOI": "10.3389/fphar.2020.00161",
"article-title": "Calcitriol Prevents Neuroinflammation and Reduces Blood-Brain Barrier Disruption and Local Macrophage/Microglia Activation",
"author": "Mimura",
"doi-asserted-by": "crossref",
"first-page": "161",
"journal-title": "Front. Pharmacol.",
"key": "ref_25",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.4168/aair.2019.11.2.267",
"article-title": "Intranasal Treatment with 1, 25-Dihydroxyvitamin D3 Alleviates Allergic Rhinitis Symptoms in a Mouse Model",
"author": "Cho",
"doi-asserted-by": "crossref",
"first-page": "267",
"journal-title": "Allergy Asthma Immunol. Res.",
"key": "ref_26",
"volume": "11",
"year": "2019"
},
{
"article-title": "Study Effect of Vitamin D on the Immunopathology Responses of the Bronchi in Murine Model of Asthma",
"author": "Feng",
"first-page": "509",
"journal-title": "Iran. J. Allergy Asthma Immunol.",
"key": "ref_27",
"volume": "20",
"year": "2021"
},
{
"DOI": "10.1111/j.1610-0387.2007.06280.x",
"article-title": "Current State of Vitiligo Therapy? Evidence-Based Analysis of the Literature",
"author": "Forschner",
"doi-asserted-by": "crossref",
"first-page": "467",
"journal-title": "JDDG J. Dtsch. Dermatol. Ges.",
"key": "ref_28",
"volume": "5",
"year": "2007"
},
{
"key": "ref_29",
"unstructured": "Kieffer, M.A. (2004). Topical Vitamin D Analogs. Dermatol. Nurs., 16."
},
{
"DOI": "10.1177/0271678X16671147",
"article-title": "Intranasal Administration of Vitamin D Attenuates Blood–Brain Barrier Disruption through Endogenous Upregulation of Osteopontin and Activation of CD44/P-Gp Glycosylation Signaling after Subarachnoid Hemorrhage in Rats",
"author": "Enkhjargal",
"doi-asserted-by": "crossref",
"first-page": "2555",
"journal-title": "J. Cereb. Blood Flow Metab.",
"key": "ref_30",
"volume": "37",
"year": "2017"
},
{
"DOI": "10.1124/jpet.119.258152",
"article-title": "Nose-to-Brain Delivery",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "593",
"journal-title": "J. Pharmacol. Exp.",
"key": "ref_31",
"volume": "370",
"year": "2019"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"article-title": "Virological Assessment of Hospitalized Patients with COVID-2019",
"author": "Corman",
"doi-asserted-by": "crossref",
"first-page": "465",
"journal-title": "Nature",
"key": "ref_32",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.3389/fviro.2022.854363",
"article-title": "Identification of Compounds with Antiviral Activity Against SARS-CoV-2 in the MMV Pathogen Box Using a Phenotypic High-Throughput Screening Assay",
"author": "Coimbra",
"doi-asserted-by": "crossref",
"first-page": "854363",
"journal-title": "Front.Virol.",
"key": "ref_33",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.1016/j.jpap.2021.100072",
"article-title": "Ultraviolet Germicidal Irradiation Is Effective against SARS-CoV-2 in Contaminated Makeup Powder and Lipstick",
"author": "Barbosa",
"doi-asserted-by": "crossref",
"first-page": "100072",
"journal-title": "J. Photochem. Photobiol.",
"key": "ref_34",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1371/journal.ppat.0030005",
"doi-asserted-by": "crossref",
"key": "ref_35",
"unstructured": "Roberts, A., Deming, D., Paddock, C.D., Cheng, A., Yount, B., Vogel, L., Herman, B.D., Sheahan, T., Heise, M., and Genrich, G.L. (2007). A Mouse-Adapted SARS-Coronavirus Causes Disease and Mortality in BALB/c Mice. PLoS Pathog., 3."
},
{
"DOI": "10.1007/s00428-021-03053-1",
"article-title": "The Pulmonary Pathology of COVID-19",
"author": "Matter",
"doi-asserted-by": "crossref",
"first-page": "137",
"journal-title": "Virchows Arch.",
"key": "ref_36",
"volume": "478",
"year": "2021"
},
{
"DOI": "10.1183/09031936.00069509",
"article-title": "Bronchoalveolar Lavage for the Evaluation of Interstitial Lung Disease: Is It Clinically Useful?",
"author": "Meyer",
"doi-asserted-by": "crossref",
"first-page": "761",
"journal-title": "Eur. Respir. J.",
"key": "ref_37",
"volume": "38",
"year": "2011"
},
{
"article-title": "Bronchoalveolar Lavage of Murine Lungs to Analyze Inflammatory Cell Infiltration",
"author": "Job",
"first-page": "55398",
"journal-title": "J. Vis. Exp.",
"key": "ref_38",
"volume": "123",
"year": "2017"
},
{
"DOI": "10.1038/s41591-020-0901-9",
"article-title": "Single-Cell Landscape of Bronchoalveolar Immune Cells in Patients with COVID-19",
"author": "Liao",
"doi-asserted-by": "crossref",
"first-page": "842",
"journal-title": "Nat. Med.",
"key": "ref_39",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-25015-6",
"article-title": "SARS-CoV-2 N Protein Promotes NLRP3 Inflammasome Activation to Induce Hyperinflammation",
"author": "Pan",
"doi-asserted-by": "crossref",
"first-page": "4664",
"journal-title": "Nat. Commun.",
"key": "ref_40",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41380-022-01831-0",
"doi-asserted-by": "crossref",
"key": "ref_41",
"unstructured": "Albornoz, E.A., Amarilla, A.A., Modhiran, N., Parker, S., Li, X.X., Wijesundara, D.K., Aguado, J., Zamora, A.P., McMillan, C.L.D., and Liang, B. (2022). SARS-CoV-2 Drives NLRP3 Inflammasome Activation in Human Microglia through Spike Protein. Mol. Psychiatry, 1–16."
},
{
"DOI": "10.1084/jem.20201707",
"article-title": "Inflammasomes Are Activated in Response to SARS-CoV-2 Infection and Are Associated with COVID-19 Severity in Patients",
"author": "Rodrigues",
"doi-asserted-by": "crossref",
"first-page": "e20201707",
"journal-title": "J. Exp. Med.",
"key": "ref_42",
"volume": "218",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2021.103803",
"article-title": "Specific Inhibition of the NLRP3 Inflammasome Suppresses Immune Overactivation and Alleviates COVID-19 like Pathology in Mice",
"author": "Zeng",
"doi-asserted-by": "crossref",
"first-page": "103803",
"journal-title": "eBioMedicine",
"key": "ref_43",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1016/j.cytogfr.2021.06.002",
"article-title": "The NLRP3 Inflammasome and COVID-19: Activation, Pathogenesis and Therapeutic Strategies",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "2",
"journal-title": "Cytokine Growth Factor Rev.",
"key": "ref_44",
"volume": "61",
"year": "2021"
},
{
"DOI": "10.1073/pnas.1424741112",
"article-title": "Cell Stress Increases ATP Release in NLRP3 Inflammasome-Mediated Autoinflammatory Diseases, Resulting in Cytokine Imbalance",
"author": "Carta",
"doi-asserted-by": "crossref",
"first-page": "2835",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_45",
"volume": "112",
"year": "2015"
},
{
"DOI": "10.3389/fimmu.2021.753940",
"article-title": "Diversity of Macrophages in Lung Homeostasis and Diseases",
"author": "Hou",
"doi-asserted-by": "crossref",
"first-page": "753940",
"journal-title": "Front. Immunol.",
"key": "ref_46",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2021.652470",
"article-title": "Neutrophils in COVID-19",
"author": "Reusch",
"doi-asserted-by": "crossref",
"first-page": "652470",
"journal-title": "Front. Immunol.",
"key": "ref_47",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1084/jem.20200652",
"article-title": "Targeting Potential Drivers of COVID-19: Neutrophil Extracellular Traps",
"author": "Barnes",
"doi-asserted-by": "crossref",
"first-page": "e20200652",
"journal-title": "J. Exp. Med.",
"key": "ref_48",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.1182/bloodadvances.2020003568",
"article-title": "A Neutrophil Activation Signature Predicts Critical Illness and Mortality in COVID-19",
"author": "Meizlish",
"doi-asserted-by": "crossref",
"first-page": "1164",
"journal-title": "Blood Adv.",
"key": "ref_49",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1016/j.intimp.2020.107233",
"article-title": "Neutrophils and COVID-19: The Road so Far",
"author": "Carvalho",
"doi-asserted-by": "crossref",
"first-page": "107233",
"journal-title": "Int. Immunopharmacol.",
"key": "ref_50",
"volume": "90",
"year": "2021"
},
{
"DOI": "10.3390/cells9102206",
"doi-asserted-by": "crossref",
"key": "ref_51",
"unstructured": "Parackova, Z., Zentsova, I., Bloomfield, M., Vrabcova, P., Smetanova, J., Klocperk, A., Mesežnikov, G., Casas Mendez, L.F., Vymazal, T., and Sediva, A. (2020). Disharmonic Inflammatory Signatures in COVID-19: Augmented Neutrophils’ but Impaired Monocytes’ and Dendritic Cells’ Responsiveness. Cells, 9."
},
{
"DOI": "10.3390/cells9061383",
"doi-asserted-by": "crossref",
"key": "ref_52",
"unstructured": "Tomar, B., Anders, H.-J., Desai, J., and Mulay, S.R. (2020). Neutrophils and Neutrophil Extracellular Traps Drive Necroinflammation in COVID-19. Cells, 9."
},
{
"DOI": "10.3390/cells11233785",
"doi-asserted-by": "crossref",
"key": "ref_53",
"unstructured": "Gardiman, E., Bianchetto-Aguilera, F., Gasperini, S., Tiberio, L., Scandola, M., Lotti, V., Gibellini, D., Salvi, V., Bosisio, D., and Cassatella, M.A. (2022). SARS-CoV-2-Associated SsRNAs Activate Human Neutrophils in a TLR8-Dependent Fashion. Cells, 11."
},
{
"DOI": "10.24272/j.issn.2095-8137.2020.118",
"article-title": "Role of Neutrophil Chemoattractant CXCL5 in SARS-CoV-2 Infection-Induced Lung Inflammatory Innate Immune Response in an in vivo. HACE2 Transfection Mouse Model",
"author": "Liang",
"doi-asserted-by": "crossref",
"first-page": "621",
"journal-title": "Zool. Res.",
"key": "ref_54",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1042/CS20210577",
"article-title": "How Dendritic Cells Sense and Respond to Viral Infections",
"author": "Marongiu",
"doi-asserted-by": "crossref",
"first-page": "2217",
"journal-title": "Clin. Sci.",
"key": "ref_55",
"volume": "135",
"year": "2021"
},
{
"DOI": "10.1101/2021.05.26.445809",
"doi-asserted-by": "crossref",
"key": "ref_56",
"unstructured": "Winheim, E., Rinke, L., Lutz, K., Reischer, A., Leutbecher, A., Wolfram, L., Rausch, L., Kranich, J., Wratil, P.R., and Huber, J.E. (2021). Impaired Function and Delayed Regeneration of Dendritic Cells in COVID-19. PLoS Pathog., 17."
},
{
"DOI": "10.1016/j.micpath.2021.104799",
"article-title": "The Pro-Inflammatory Cytokines in COVID-19 Pathogenesis: What Goes Wrong?",
"author": "Darif",
"doi-asserted-by": "crossref",
"first-page": "104799",
"journal-title": "Microb. Pathog.",
"key": "ref_57",
"volume": "153",
"year": "2021"
},
{
"DOI": "10.1038/s41392-021-00851-6",
"article-title": "Inactivated SARS-CoV-2 Induces Acute Respiratory Distress Syndrome in Human ACE2-Transgenic Mice",
"author": "Bi",
"doi-asserted-by": "crossref",
"first-page": "439",
"journal-title": "Signal Transduct. Target.",
"key": "ref_58",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.3390/nu10111656",
"doi-asserted-by": "crossref",
"key": "ref_59",
"unstructured": "Sassi, F., Tamone, C., and D’Amelio, P. (2018). Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients, 10."
},
{
"DOI": "10.3390/nu13093021",
"doi-asserted-by": "crossref",
"key": "ref_60",
"unstructured": "Karonova, T.L., Andreeva, A.T., Golovatuk, K.A., Bykova, E.S., Simanenkova, A.V., Vashukova, M.A., Grant, W.B., and Shlyakhto, E.V. (2021). Low 25(OH)D Level Is Associated with Severe Course and Poor Prognosis in COVID-19. Nutrients, 13."
},
{
"DOI": "10.3390/ijms22041914",
"doi-asserted-by": "crossref",
"key": "ref_61",
"unstructured": "Mimura, L.A.N., de Campos Fraga-Silva, T.F., de Oliveira, L.R.C., Ishikawa, L.L.W., Borim, P.A., de Moraes Machado, C., Júnior, J.D.A.D.C.E.H., da Fonseca, D.M., and Sartori, A. (2021). Preclinical Therapy with Vitamin D3 in Experimental Encephalomyelitis: Efficacy and Comparison with Paricalcitol. Int. J. Mol. Sci., 22."
},
{
"DOI": "10.3390/nu12092757",
"doi-asserted-by": "crossref",
"key": "ref_62",
"unstructured": "Radujkovic, A., Hippchen, T., Tiwari-Heckler, S., Dreher, S., Boxberger, M., and Merle, U. (2020). Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients, 12."
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"article-title": "“Effect of Calcifediol Treatment and Best Available Therapy versus Best Available Therapy on Intensive Care Unit Admission and Mortality among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical Study”",
"author": "Bouillon",
"doi-asserted-by": "crossref",
"first-page": "105751",
"journal-title": "J. Steroid Biochem. Mol. Biol.",
"key": "ref_63",
"volume": "203",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2022.882046",
"article-title": "Editorial: Vitamin D and COVID-19: New Mechanistic and Therapeutic Insights",
"author": "Marcinkowska",
"doi-asserted-by": "crossref",
"first-page": "882046",
"journal-title": "Front. Pharmacol.",
"key": "ref_64",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/B978-0-12-386960-9.00009-5",
"article-title": "Vitamin D Effects on Lung Immunity and Respiratory Diseases",
"author": "Hansdottir",
"doi-asserted-by": "crossref",
"first-page": "217",
"journal-title": "Vitamins & Hormones",
"key": "ref_65",
"volume": "Volume 86",
"year": "2011"
},
{
"DOI": "10.3390/ijms18112252",
"doi-asserted-by": "crossref",
"key": "ref_66",
"unstructured": "Adamczak, D. (2017). The Role of Toll-Like Receptors and Vitamin D in Cardiovascular Diseases—A Review. Int. J. Mol. Sci., 18."
},
{
"DOI": "10.1046/j.1365-2125.2003.01892.x",
"article-title": "Pulmonary Drug Delivery. Part I: Physiological Factors Affecting Therapeutic Effectiveness of Aerosolized Medications: Physiological Factors Affecting the Effectiveness of Inhaled Drugs",
"author": "Labiris",
"doi-asserted-by": "crossref",
"first-page": "588",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "ref_67",
"volume": "56",
"year": "2003"
},
{
"DOI": "10.1073/pnas.1615783114",
"article-title": "Functional Genomics Analysis of Vitamin D Effects on CD4+ T Cells in Vivo in Experimental Autoimmune Encephalomyelitis",
"author": "Zeitelhofer",
"doi-asserted-by": "crossref",
"first-page": "E1678",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_68",
"volume": "114",
"year": "2017"
},
{
"DOI": "10.3389/fimmu.2019.02783",
"article-title": "Vitamin D Receptor Inhibits NLRP3 Activation by Impeding Its BRCC3-Mediated Deubiquitination",
"author": "Rao",
"doi-asserted-by": "crossref",
"first-page": "2783",
"journal-title": "Front. Immunol.",
"key": "ref_69",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.3389/fimmu.2020.01433",
"article-title": "Impact of the Local Inflammatory Environment on Mucosal Vitamin D Metabolism and Signaling in Chronic Inflammatory Lung Diseases",
"author": "Schrumpf",
"doi-asserted-by": "crossref",
"first-page": "1433",
"journal-title": "Front. Immunol.",
"key": "ref_70",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1186/s12931-022-01997-9",
"article-title": "Local Nebulization of 1α,25(OH)2D3 Attenuates LPS-Induced Acute Lung Inflammation",
"author": "Mathyssen",
"doi-asserted-by": "crossref",
"first-page": "76",
"journal-title": "Respir. Res.",
"key": "ref_71",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1016/j.abb.2010.10.012",
"article-title": "1,25-Dihydroxyvitamin D Is Not Responsible for Toxicity Caused by Vitamin D or 25-Hydroxyvitamin D",
"author": "DeLuca",
"doi-asserted-by": "crossref",
"first-page": "226",
"journal-title": "Arch. Biochem. Biophys.",
"key": "ref_72",
"volume": "505",
"year": "2011"
},
{
"DOI": "10.3389/fimmu.2020.00301",
"article-title": "High-Dose Vitamin D-Mediated Hypercalcemia as a Potential Risk Factor in Central Nervous System Demyelinating Disease",
"author": "Torke",
"doi-asserted-by": "crossref",
"first-page": "301",
"journal-title": "Front. Immunol.",
"key": "ref_73",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3389/fendo.2018.00550",
"article-title": "Vitamin D Toxicity–A Clinical Perspective",
"author": "Jones",
"doi-asserted-by": "crossref",
"first-page": "550",
"journal-title": "Front. Endocrinol.",
"key": "ref_74",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1038/ncomms2339",
"article-title": "Extracellular Ca2+ Is a Danger Signal Activating the NLRP3 Inflammasome through G Protein-Coupled Calcium Sensing Receptors",
"author": "Rossol",
"doi-asserted-by": "crossref",
"first-page": "1329",
"journal-title": "Nat. Commun.",
"key": "ref_75",
"volume": "3",
"year": "2012"
},
{
"DOI": "10.1016/j.stemcr.2021.12.011",
"article-title": "The Blood-Brain Barrier Is Dysregulated in COVID-19 and Serves as a CNS Entry Route for SARS-CoV-2",
"author": "Krasemann",
"doi-asserted-by": "crossref",
"first-page": "307",
"journal-title": "Stem Cell Rep.",
"key": "ref_76",
"volume": "17",
"year": "2022"
}
],
"reference-count": 76,
"references-count": 76,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2073-4409/12/7/1092"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Lung Inflammation Induced by Inactivated SARS-CoV-2 in C57BL/6 Female Mice Is Controlled by Intranasal Instillation of Vitamin D",
"type": "journal-article",
"volume": "12"
}
