Low 25(OH)D Level Is Associated with Severe Course and Poor Prognosis in COVID-19
et al., Nutrients, doi:10.3390/nu13093021, Aug 2021
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
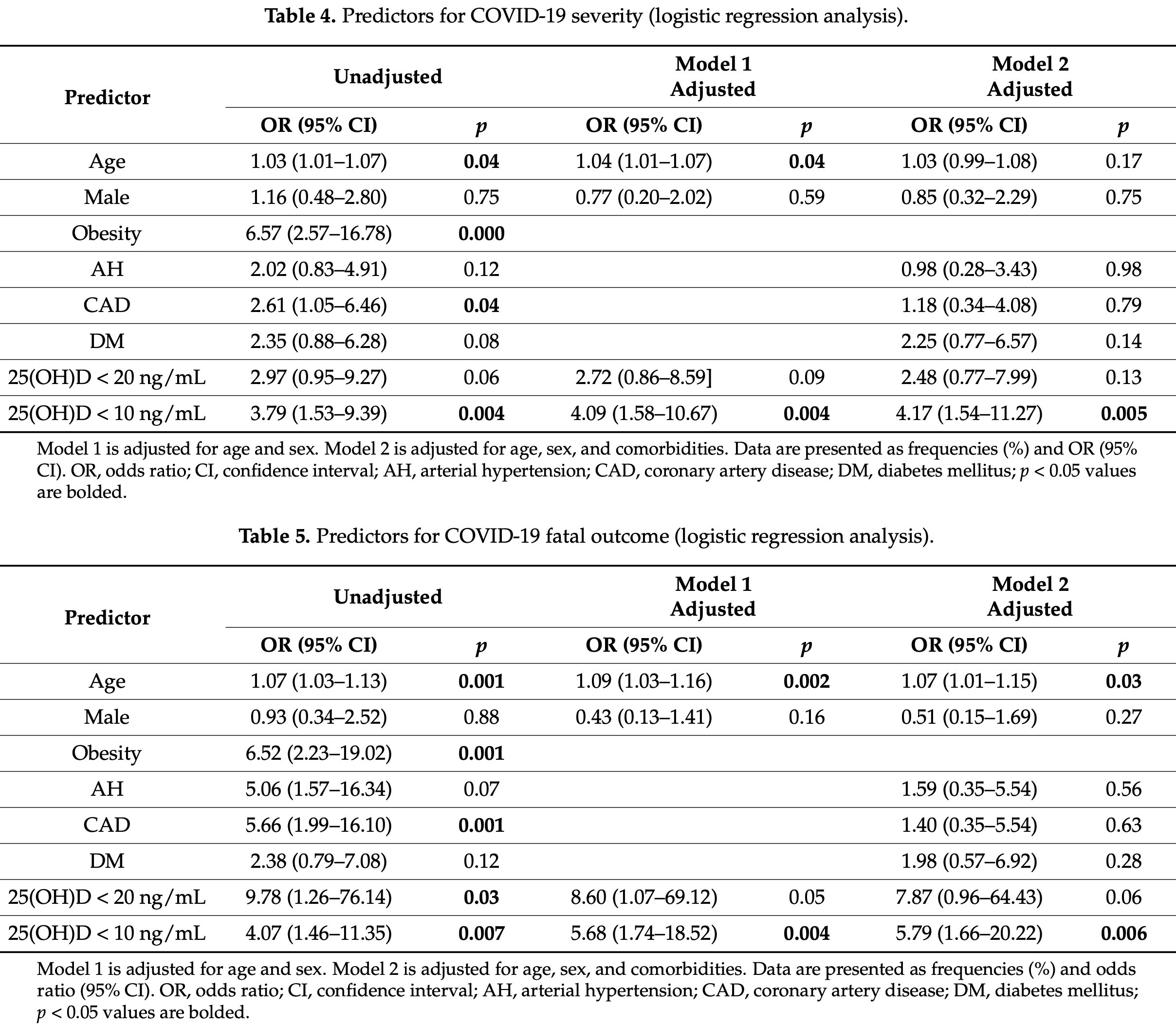
Retrospective 161 hospitalized patients in Russia, showing COVID-19 severity and mortality associated with vitamin D deficiency. Patients in this study may overlap with those in an earlier smaller study from some of the same authors.
This is the 94th of 228 COVID-19 sufficiency studies for vitamin D, which collectively show higher levels reduce risk with p<0.0000000001.
|
risk of death, 77.8% lower, RR 0.22, p = 0.006, high D levels 8 of 96 (8.3%), low D levels 10 of 37 (27.0%), NNT 5.3, adjusted per study, inverted to make RR<1 favor high D levels, odds ratio converted to relative risk, >10ng/mL, logistic regression model 2.
|
|
risk of death, 84.8% lower, RR 0.15, p = 0.06, high D levels 1 of 43 (2.3%), low D levels 17 of 90 (18.9%), NNT 6.0, adjusted per study, inverted to make RR<1 favor high D levels, odds ratio converted to relative risk, >20ng/mL, logistic regression model 2.
|
|
risk of severe case, 67.3% lower, RR 0.33, p = 0.005, high D levels 12 of 96 (12.5%), low D levels 13 of 37 (35.1%), NNT 4.4, adjusted per study, inverted to make RR<1 favor high D levels, odds ratio converted to relative risk, >10ng/mL, logistic regression model 2.
|
|
risk of severe case, 53.2% lower, RR 0.47, p = 0.13, high D levels 4 of 43 (9.3%), low D levels 21 of 90 (23.3%), NNT 7.1, adjusted per study, inverted to make RR<1 favor high D levels, odds ratio converted to relative risk, >20ng/mL, logistic regression model 2.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Karonova et al., 29 Aug 2021, retrospective, Russia, peer-reviewed, 8 authors, study period April 2020 - December 2020.
Low 25(OH)D Level Is Associated with Severe Course and Poor Prognosis in COVID-19
Nutrients, doi:10.3390/nu13093021
We evaluated associations between serum 25-hydroxyvitamin D [25(OH)D] level and severity of new coronavirus infection (COVID-19) in hospitalized patients. We assessed serum 25(OH)D level in 133 patients aged 21-93 years. Twenty-five (19%) patients had severe disease, 108 patients (81%) had moderate disease, and 18 (14%) patients died. 25(OH)D level ranged from 3.0 to 97.0 ng/mL (median, 13.5 [25%; 75%, 9.6; 23.3] ng/mL). Vitamin D deficiency was diagnosed in 90 patients, including 37 with severe deficiency. In patients with severe course of disease, 25(OH)D level was lower (median, 9.7 [25%; 75%, 6.0; 14.9] ng/mL), and vitamin D deficiency was more common than in patients with moderate course (median, 14.6 [25%; 75%, 10.6; 24.4] ng/mL, p = 0.003). In patients who died, 25(OH)D was 9.6 [25%; 75%, 6.0; 11.5] ng/mL, compared with 14.8 [25%; 75%, 10.1; 24.3] ng/mL in discharged patients (p = 0.001). Severe vitamin D deficiency was associated with increased risk of COVID-19 severity and fatal outcome. The threshold for 25(OH)D level associated with increased risk of severe course was 11.7 ng/mL. Approximately the same 25(OH)D level, 10.9 ng/mL, was associated with increased risk of mortality. Thus, most COVID-19 patients have vitamin D deficiency; severe vitamin D deficiency is associated with increased risk of COVID-19 severity and fatal outcome.
References
Adams, Ren, Liu, Chun, Lagishetty et al., Vitamin d-directed rheostatic regulation of monocyte antibacterial responses, J. Immunol, doi:10.4049/jimmunol.0803736
Baeke, Korf, Overbergh, Van Etten, Verstuyf et al., Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2010.03.037
Beard, Bearden, Striker, Vitamin D and the anti-viral state, J. Clin. Virol, doi:10.1016/j.jcv.2010.12.006
Campbell, Wu-Young, Lee, Rapid response to Elisabeth Mahase E: Covid-19: What treatments are being investigated?, BMJ, doi:10.1136/bmj.m1252
Cannell, Vieth, Umhau, Holick, Grant et al., Epidemic influenza and vitamin D, Epidemiol. Infect, doi:10.1017/S0950268806007175
Cantorna, Snyder, Lin, Yang, Vitamin D and 1,25(OH)2D regulation of T cells, Nutrients
Carter, Baranauskas, Fly, Considerations for obesity, vitamin D, and physical activity amid the COVID-19 pandemic, Obesity, doi:10.1002/oby.22838
Chua, Zheng, Obesity and COVID-19: The clash of two pandemics, Obes. Res. Clin. Pract, doi:10.1016/j.orcp.2020.06.003
Cohen-Lahav, Shany, Tobvin, Chaimovitz, Douvdevani, Vitamin D decreases NFkappaB activity by increasing IkappaBalpha levels, Nephrol. Dial. Transplant, doi:10.1093/ndt/gfi254
Cutolo, Paolino, Smith, Evidences for a protective role of vitamin D in COVID-19, RMD Open, doi:10.1136/rmdopen-2020-001454
D'avolio, Avataneo, Manca, Cusato, De Nicolo et al., 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2, Nutrients, doi:10.3390/nu12051359
Dediego, Nieto-Torres, Regla-Nava, Jimenez-Guardeno, Fernandez-Delgado et al., Inhibition of NF-kappaB-mediated inflammation in severe acute respiratory syndrome coronavirusinfected mice increases survival, J. Virol, doi:10.1128/JVI.02576-13
Fakhoury, Kvietys, Shakir, Shams, Grant et al., Lung-centric inflammation of COVID-19: Potential modulation by vitamin D, Nutrients, doi:10.3390/nu13072216
Ghasemian, Shamshirian, Heydari, Malekan, Alizadeh-Navaei et al., The role of vitamin D in the age of COVID-19: A systematic review and meta-analysis, Int. J. Clin. Pract, doi:10.1111/ijcp.14675
Ginde, Liu, Camargo, Jr, Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004, Arch. Intern. Med, doi:10.1001/archinternmed.2008.604
Grant, Lahore, Mcdonnell, Baggerly, Franch et al., Evidence That Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths, Nutrients, doi:10.3390/nu12040988
Han, Jones, Tangpricha, Brown, Brown et al., High Dose Vitamin D Administration in Ventilated Intensive Care Unit Patients: A Pilot Double Blind Randomized Controlled Trial, J. Clin. Transl. Endocrinol, doi:10.1016/j.jcte.2016.04.004
Hewison, Freeman, Hughes, Evans, Bland et al., Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells, J. Immunol, doi:10.4049/jimmunol.170.11.5382
Holick, Binkley, Bischoff-Ferrari, Gordon, Hanley et al., Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline, J. Clin. Endocrinol. Metab, doi:10.1210/jc.2011-0385
Hope-Simpson, The role of season in the epidemiology of influenza, J. Hyg, doi:10.1017/S0022172400068728
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Hussain, Bhowmik, Do Vale Moreira, COVID-19 and diabetes: Knowledge in progress, Diabetes Res. Clin. Pract, doi:10.1016/j.diabres.2020.108142
Infante, Buoso, Pieri, Lupisella, Nuccetelli et al., Low vitamin D status at admission as a risk factor for poor survival in hospitalized patients with COVID-19: An italian retrospective study, J. Am. Coll. Nutr, doi:10.1080/07315724.2021.1877580
Jeffery, Burke, Mura, Zheng, Qureshi et al., 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3, J. Immunol, doi:10.4049/jimmunol.0803217
Karonova, Andreeva, Nikitina, Belyaeva, Mokhova et al., Prevalence of Vitamin D deficiency in the North-West region of Russia: A cross-sectional study, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2016.03.026
Karonova, Vashukova, Gusev, Golovatuk, Grineva, Vitamin D deficiency as a factor for immunity stimulation and lower risk of acute respiratory infections and COVID-19, Arter. Hypertens, doi:10.18705/1607-419X-2020-26-3-295-303
Kumar, Arora, Sharma, Anikhindi, Bansal et al., Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis, Diabetes Metab. Syndr. Clin. Res. Rev, doi:10.1016/j.dsx.2020.04.044
Laaksi, Vitamin D and respiratory infection in adults, Proc. Nutr. Soc, doi:10.1017/S0029665111003351
Lavie, Sanchis-Gomar, Henry, Lippi, COVID-19 and obesity: Links and risks, Expert Rev. Endocrinol. Metab, doi:10.1080/17446651.2020.1767589
Lemire, Adams, Kermani-Arab, Bakke, Sakai et al., 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro, J. Immunol
Liu, Stenger, Li, Wenzel, Tan et al., Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response, Science, doi:10.1126/science.1123933
Martineau, Jolliffe, Hooper, Greenberg, Aloia et al., Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data, BMJ, doi:10.1136/bmj.i6583
Mcelvaney, Mcevoy, Mcelvaney, Carroll, Murphy et al., Characterization of the Inflammatory Response to Severe COVID-19 Illness, Am. J. Respir. Crit. Care Med, doi:10.1164/rccm.202005-1583OC
Merad, Martin, Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages, Nat. Rev. Immunol, doi:10.1038/s41577-020-0331-4
Mercola, Grant, Wagner, Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity, Nutrients, doi:10.3390/nu12113361
Park, Kwon, Choi, Kang, Choe et al., Virus Isolation from the First Patient with SARS-CoV-2 in Korea, J. Korean Med. Sci, doi:10.3346/jkms.2020.35.e84
Pham, Rahman, Majidi, Waterhouse, Neale, Acute Respiratory Tract Infection and 25-Hydroxyvitamin D Concentration: A Systematic Review and Meta-Analysis, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph16173020
Pigarova, Rozhinskaya, Belaya, Dzeranova, Karonova et al., Russian Association of endocrinologists recommendations for diagnosis, treatment and prevention of vitamin D deficiency in adults, Probl. Endocrinol, doi:10.14341/probl201662460-84
Rondanelli, Miccono, Lamburghini, Avanzato, Riva et al., Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, Zinc, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved during an Episode of Common Colds-Practical Advice on Dosages and on the Time to Take These Nutrients/Botanicals in order to Prevent or Treat Common Colds, Evid. Based Complement. Altern. Med, doi:10.1155/2018/5813095
Sulli, Gotelli, Casabella, Paolino, Pizzorni et al., Vitamin D and lung outcomes in elderly COVID-19 patients, Nutrients, doi:10.3390/nu13030717
Vankadari, Wilce, Emerging WuHan (COVID-19) coronavirus: Glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26, Emerg. Microbes Infect, doi:10.1080/22221751.2020.1739565
Vasarhelyi, Satori, Olajos, Szabo, Beko, Low vitamin D levels among patients at Semmelweis University: Retrospective analysis during a one-year period, Orv. Hetil, doi:10.1556/OH.2011.29187
Wang, Hu, Hu, Zhu, Liu et al., Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA, doi:10.1001/jama.2020.1585
White, Nafilyan, Coronavirus (COVID-19) Related Deaths by Ethnic Group
White, Regulation of intracrine production of 1,25-dihydroxyvitamin D and its role in innate immune defense against infection, Arch. Biochem. Biophys, doi:10.1016/j.abb.2011.11.006
Yang, Zhang, Xu, Effect of Vitamin D on ACE2 and Vitamin D receptor expression in rats with LPS-induced acute lung injury, Chin. J. Emerg. Med, doi:10.3760/cma.j.issn.1671-0282.2016.12.016
Zemb, Bergman, Camargo, Jr, Cavalier et al., Vitamin D deficiency and COVID-19 pandemic, Glob. Antimicrob. Resist, doi:10.1016/j.jgar.2020.05.006
Zhonghua, The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China, CMA.J, doi:10.3760/cma.j.issn.0254-6450.2020.02.003
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study, Lancet
Zneng, Chen, Yao, Huang, Tan et al., Dynamic changes in the immune response correlate with disease severity and outcomes during infection with SARS-CoV-2, Infect. Dis. Ther, doi:10.1007/s40121-021-00458-y
DOI record:
{
"DOI": "10.3390/nu13093021",
"ISSN": [
"2072-6643"
],
"URL": "http://dx.doi.org/10.3390/nu13093021",
"abstract": "<jats:p>We evaluated associations between serum 25-hydroxyvitamin D [25(OH)D] level and severity of new coronavirus infection (COVID-19) in hospitalized patients. We assessed serum 25(OH)D level in 133 patients aged 21–93 years. Twenty-five (19%) patients had severe disease, 108 patients (81%) had moderate disease, and 18 (14%) patients died. 25(OH)D level ranged from 3.0 to 97.0 ng/mL (median, 13.5 [25%; 75%, 9.6; 23.3] ng/mL). Vitamin D deficiency was diagnosed in 90 patients, including 37 with severe deficiency. In patients with severe course of disease, 25(OH)D level was lower (median, 9.7 [25%; 75%, 6.0; 14.9] ng/mL), and vitamin D deficiency was more common than in patients with moderate course (median, 14.6 [25%; 75%, 10.6; 24.4] ng/mL, p = 0.003). In patients who died, 25(OH)D was 9.6 [25%; 75%, 6.0; 11.5] ng/mL, compared with 14.8 [25%; 75%, 10.1; 24.3] ng/mL in discharged patients (p = 0.001). Severe vitamin D deficiency was associated with increased risk of COVID-19 severity and fatal outcome. The threshold for 25(OH)D level associated with increased risk of severe course was 11.7 ng/mL. Approximately the same 25(OH)D level, 10.9 ng/mL, was associated with increased risk of mortality. Thus, most COVID-19 patients have vitamin D deficiency; severe vitamin D deficiency is associated with increased risk of COVID-19 severity and fatal outcome.</jats:p>",
"alternative-id": [
"nu13093021"
],
"author": [
{
"affiliation": [],
"family": "Karonova",
"given": "Tatiana L.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-4878-6909",
"affiliation": [],
"authenticated-orcid": false,
"family": "Andreeva",
"given": "Alena T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Golovatuk",
"given": "Ksenia A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bykova",
"given": "Ekaterina S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3300-1280",
"affiliation": [],
"authenticated-orcid": false,
"family": "Simanenkova",
"given": "Anna V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vashukova",
"given": "Maria A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1439-3285",
"affiliation": [],
"authenticated-orcid": false,
"family": "Grant",
"given": "William B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shlyakhto",
"given": "Evgeny V.",
"sequence": "additional"
}
],
"container-title": "Nutrients",
"container-title-short": "Nutrients",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
8,
31
]
],
"date-time": "2021-08-31T08:07:28Z",
"timestamp": 1630397248000
},
"deposited": {
"date-parts": [
[
2023,
1,
8
]
],
"date-time": "2023-01-08T09:43:15Z",
"timestamp": 1673170995000
},
"indexed": {
"date-parts": [
[
2024,
3,
6
]
],
"date-time": "2024-03-06T06:24:44Z",
"timestamp": 1709706284967
},
"is-referenced-by-count": 19,
"issue": "9",
"issued": {
"date-parts": [
[
2021,
8,
29
]
]
},
"journal-issue": {
"issue": "9",
"published-online": {
"date-parts": [
[
2021,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
8,
29
]
],
"date-time": "2021-08-29T00:00:00Z",
"timestamp": 1630195200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2072-6643/13/9/3021/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "3021",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
8,
29
]
]
},
"published-online": {
"date-parts": [
[
2021,
8,
29
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.18705/1607-419X-2020-26-3-295-303",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1016/j.jgar.2020.05.006",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1016/j.jsbmb.2010.03.037",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.4049/jimmunol.170.11.5382",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1001/archinternmed.2008.604",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1155/2018/5813095",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1016/j.abb.2011.11.006",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1126/science.1123933",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.4049/jimmunol.0803736",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1017/S0029665111003351",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1136/bmj.m1252",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.4049/jimmunol.134.5.3032",
"article-title": "1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro",
"author": "Lemire",
"doi-asserted-by": "crossref",
"first-page": "3032",
"journal-title": "J. Immunol.",
"key": "ref12",
"volume": "134",
"year": "1985"
},
{
"DOI": "10.3390/nu7043011",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.4049/jimmunol.0803217",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1136/rmdopen-2020-001454",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.3760/cma.j.issn.1671-0282.2016.12.016",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1080/22221751.2020.1739565",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1556/OH.2011.29187",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.3760/cma.j.issn.0254-6450.2020.02.003",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"key": "ref21"
},
{
"DOI": "10.1017/S0022172400068728",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1017/S0950268806007175",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1080/17446651.2020.1767589",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1016/j.diabres.2020.108142",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.3390/nu12040988",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.3390/nu12051359",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1016/j.jsbmb.2016.03.026",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1210/jc.2011-0385",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.14341/probl201662460-84",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1136/bmj.i6583",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.3390/ijerph16173020",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1001/jama.2020.1585",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1080/07315724.2021.1877580",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1016/j.orcp.2020.06.003",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.1002/oby.22838",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1016/j.dsx.2020.04.044",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1111/ijcp.14675",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1164/rccm.202005-1583OC",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1007/s40121-021-00458-y",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1038/s41577-020-0331-4",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.3346/jkms.2020.35.e84",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.3390/nu13030717",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.3390/nu13072216",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.3390/nu12113361",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1016/j.jcv.2010.12.006",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.1093/ndt/gfi254",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1128/JVI.02576-13",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1016/j.jcte.2016.04.004",
"doi-asserted-by": "publisher",
"key": "ref50"
}
],
"reference-count": 50,
"references-count": 50,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2072-6643/13/9/3021"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Food Science",
"Nutrition and Dietetics"
],
"subtitle": [],
"title": "Low 25(OH)D Level Is Associated with Severe Course and Poor Prognosis in COVID-19",
"type": "journal-article",
"volume": "13"
}
