Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial
et al., Nutrients, doi:10.3390/nu13072170, Jun 2021
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
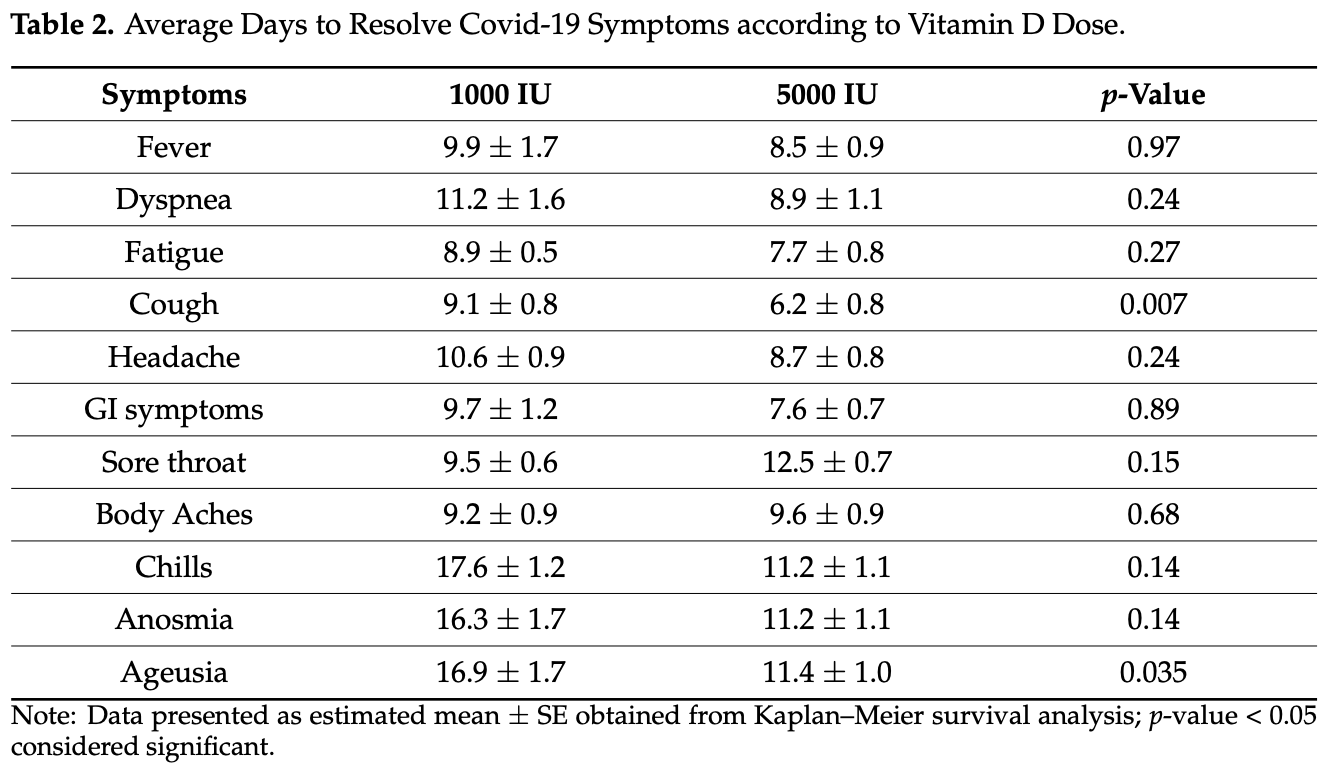
Small RCT of 69 hospitalized patients comparing 1,000IU and 5,000IU daily cholecalciferol, showing faster recovery with the higher dose (statistically significant only for cough and ageusia).
Cholecalciferol was used in this study.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 45% [34‑54%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
This is the 5th of 40 COVID-19 RCTs for vitamin D, which collectively show efficacy with p=0.0000001.
This is the 41st of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
|
risk of death, 192.1% higher, RR 2.92, p = 1.00, treatment 1 of 38 (2.6%), control 0 of 35 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 38.9% lower, RR 0.61, p = 0.66, treatment 2 of 36 (5.6%), control 3 of 33 (9.1%), NNT 28.
|
|
time to discharge, 14.3% lower, relative time 0.86, p = 0.14, treatment 36, control 33.
|
|
recovery time, 14.1% lower, relative time 0.86, p = 0.97, treatment 36, control 33, fever.
|
|
recovery time, 20.5% lower, relative time 0.79, p = 0.24, treatment 36, control 33, dyspnea.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sabico et al., 24 Jun 2021, Randomized Controlled Trial, Saudi Arabia, peer-reviewed, 12 authors, study period 29 July, 2020 - 22 November, 2020.
Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial
Nutrients, doi:10.3390/nu13072170
Objective: Vitamin D deficiency has been associated with an increased risk of COVID-19 severity. This multi-center randomized clinical trial aims to determine the effects of 5000 IU versus 1000 IU daily oral vitamin D3 supplementation in the recovery of symptoms and other clinical parameters among mild to moderate COVID-19 patients with sub-optimal vitamin D status. Study Design and Setting: A total of 69 reverse transcriptase polymerase chain reaction (RT-PCR) SARS-CoV-2 positive adults who were hospitalized for mild to moderate COVID-19 disease were allocated to receive once daily for 2 weeks either 5000 IU oral vitamin D3 (n = 36, 21 males; 15 females) or 1000 IU oral vitamin D3 (standard control) (n = 33, 13 males; 20 females). Anthropometrics were measured and blood samples were taken pre-and post-supplementation. Fasting blood glucose, lipids, serum 25(OH)D, and inflammatory markers were measured. COVID-19 symptoms were noted on admission and monitored until full recovery. Results: Vitamin D supplementation for 2 weeks caused a significant increase in serum 25(OH)D levels in the 5000 IU group only (adjusted p = 0.003). Within-group comparisons also showed a significant decrease in BMI and IL-6 levels overtime in both groups (p-values < 0.05) but was not clinically significant in between-group comparisons. Kaplan-Meier survival analysis revealed that the 5000 IU group had a significantly shorter time to recovery (days) than the 1000 IU group in resolving cough, even after adjusting for age, sex, baseline BMI, and D-dimer (6.2 ± 0.8 versus 9.1 ± 0.8; p = 0.039), and ageusia (loss of taste) (11.4 ± 1.0 versus 16.9 ± 1.7; p = 0.035). Conclusion: A 5000 IU daily oral vitamin D3 supplementation for 2 weeks reduces the time to recovery for cough and gustatory sensory loss among patients with sub-optimal vitamin D status and mild to moderate COVID-19 symptoms. The use of 5000 IU vitamin D3 as an adjuvant therapy for COVID-19 patients with suboptimal vitamin D status, even for a short duration, is recommended.
Supplementary Materials: The following are available online at https://www.mdpi.com/article/10 .3390/nu13072170/s1, Table S1 : Baseline Serological Characteristics of Groups.
References
Al-Daghri, Amer, Alotaibi, Aldisi, Enani et al., Vitamin D status of Arab Gulf residents screened for SARS-CoV-2 and its association with COVID-19 infection: A multi-centre case-control study, J. Transl. Med, doi:10.1186/s12967-021-02838-x
Al-Saleh, Sulimani, Sabico, Raef, Fouda et al., Guidelines for Osteoporosis in Saudi Arabia: Recommendations from the Saudi Osteoporosis Society, Ann. Saudi Med, doi:10.5144/0256-4947.2015.1
Alguwaihes, Al-Sofiani, Megdad, Albader, Alsari et al., Diabetes and Covid-19 among hospitalized patients in Saudi Arabia: A single-centre retrospective study, Cardiovasc. Diabetol, doi:10.1186/s12933-020-01184-4
Alguwaihes, Sabico, Hasanato, Al-Sofiani, Megdad et al., Severe vitamin D deficiency is not related to SARS-CoV-2 infection but may increase mortality risk in hospitalized adults: A retrospective case-control study in an Arab Gulf country, Aging Clin. Exp. Res
Annweiler, Corvaisier, Gautier, Dubee, Legrand et al., Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study, Nutrients, doi:10.3390/nu12113377
Bassatne, Basbous, Chakhtoura, El Zein, Rahme et al., The link between COVID-19 and Vitamin D (VIVID): A systematic review and meta-analysis, Metabolism, doi:10.1016/j.metabol.2021.154753
Bergman, Norlin, Hansen, Bjorkhem-Bergman, Vitamin D supplementation improves well-being in patients with frequent respiratory tract infections: A post hoc analysis of a randomized, placebo-controlled trial, BMC Res. Notes, doi:10.1186/s13104-015-1504-2
Bjelakovic, Gluud, Nikolova, Whitfield, Wetterslev et al., Vitamin D Supplementation for Prevention of Mortality in Adults, Cochrane Database Syst. Rev, doi:10.1002/14651858.CD007470.pub3
Castillo, Entrenas Costa, Vaquero Barrios, Alcala Diaz, Lopez Miranda et al., Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J. Steroid. Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105751
Di Filippo, De Lorenzo, D'amico, Sofia, Roveri et al., COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: A post-hoc analysis of a prospective cohort study, Clin. Nutr, doi:10.1016/j.clnu.2020.10.043
Di Filippo, Doga, Frara, Giustina, Hypocalcemia in COVID-19: Prevalence, clinical significance and therapeutic implications, Rev. Endocr. Metab. Disord, doi:10.1007/s11154-021-09655-z
Di Filippo, Frara, Giustina, The emerging osteo-metabolic phenotype of COVID-19: Clinical and pathophysiological aspects, Nat. Rev. Endocrinol, doi:10.1038/s41574-021-00516-y
Do, Kwon, Park, Lee, Effects of vitamin D on expression of Toll-like receptors of monocytes from patients with Behcet's disease, Rheumatology, doi:10.1093/rheumatology/ken109
Dong, Du, Gardner, An Interactive Web-Based Dashboard to Track COVID-19 in Real Time, Lancet Infect. Dis, doi:10.1016/S1473-3099(20)30120-1
Geiger, Khan, Murugan, Boison, Possible Role of Adenosine in COVID-19 Pathogenesis and Therapeutic Opportunities, Front. Pharmacol, doi:10.3389/fphar.2020.594487
Goncalves De Carvalho, Ribeiro, Aging, low-grade systemic inflammation and vitamin D: A mini-review, Eur. J. Clin Nutr, doi:10.1038/ejcn.2016.177
Jakovac, COVID-19 and vitamin D-Is there a link and an opportunity for intervention?, Am. J. Physiol. Endocrinol. Metab, doi:10.1152/ajpendo.00138.2020
Jolliffe, Greenberg, Hooper, Griffiths, Camargo et al., Vitamin D supplementation to prevent asthma exacerbations: A systematic review and meta-analysis of individual participant data, Lancet Respir. Med, doi:10.1016/S2213-2600(17)30306-5
Khan, Chen, Geiger, Role of Endolysosomes in Severe Acute Respiratory Syndrome Coronavirus-2 Infection and Coronavirus Disease 2019 Pathogenesis: Implications for Potential Treatments, Front. Pharmacol, doi:10.3389/fphar.2020.595888
Lancet, COVID-19 Vaccines: No Time for Complacency, Lancet, doi:10.1016/S0140-6736(20)32472-7
Ma, Zhou, Heianza, Qi, Habitual Use of Vitamin D Supplements and Risk of Coronavirus Disease 2019 (COVID-19) Infection: A Prospective Study in UK Biobank, Am. J. Clin. Nutr, doi:10.1093/ajcn/nqaa381
Martha, Wibowo, Pranata, Hypocalcemia is associated with severe COVID-19: A systematic review and meta-analysis, Diabetes Metab. Syndr, doi:10.1016/j.dsx.2021.01.003
Martineau, Jolliffe, Hooper, Greenberg, Aloia et al., Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data, BMJ, doi:10.1136/bmj.i6583
Meng, Jiang, Ji, Role of neurotrophin in the taste system following gustatory nerve injury, Metab. Brain. Dis, doi:10.1007/s11011-014-9626-0
Mercola, Grant, Wagner, Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity, Nutrients, doi:10.3390/nu12113361
Minakshi, Padhan, Rani, Khan, Ahmad et al., The SARS Coronavirus 3a protein causes endoplasmic reticulum stress and induces ligand-independent downregulation of the type 1 interferon receptor, PLoS ONE
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.26848
Musavi, Abazari, Barartabar, Kalaki-Jouybari, Hemmati-Dinarvand et al., The Benefits of Vitamin D in the COVID-19 Pandemic: Biochemical and Immunological Mechanisms, Arch. Physiol. Biochem, doi:10.1080/13813455.2020.1826530
Ohaegbulam, Swalih, Patel, Smith, Perrin, Vitamin D Supplementation in COVID-19 Patients: A Clinical Case Series, Am. J. Ther, doi:10.1097/MJT.0000000000001222
Ojaimi, Skinner, Strauss, Sundararajan, Woolley et al., Vitamin D deficiency impacts on expression of toll-like receptor-2 and cytokine profile: A pilot study, J. Transl. Med, doi:10.1186/1479-5876-11-176
Oran, Topol, Prevalence of Asymptomatic SARS-CoV-2 Infection, Ann. Intern. Med, doi:10.7326/M20-3012
Pironi, Sasdelli, Ravaioli, Baracco, Battaiola et al., Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease, Clin. Nutr, doi:10.1016/j.clnu.2020.08.021
Radujkovic, Hippchen, Tiwari-Heckler, Dreher, Boxberger et al., Vitamin D Deficiency and Outcome of COVID-19 Patients, Nutrients, doi:10.3390/nu12092757
Rawson, Huang, Symposium overview: Impact of oronasal inflammation on taste and smell, Ann. N. Y. Acad. Sci, doi:10.1111/j.1749-6632.2009.04489.x
Rizzoli, Vitamin D supplementation: Upper limit for safety revisited?, Aging Clin. Exp. Res, doi:10.1007/s40520-020-01678-x
Saleh, Beshyah, Hussein, Almadani, Hassoun et al., Diagnosis and management of vitamin D deficiency in the Gulf Cooperative Council (GCC) countries: An expert consensus summary statement from the GCC vitamin D advisory board, Arch. Osteoporos, doi:10.1007/s11657-020-0709-8
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19), JAMA, doi:10.1001/jama.2020.6019
Sarhan, Elrifai, Serum Level of Vitamin D as a Predictor for Severity and Outcome of Pneumonia, Clin. Nutr, doi:10.1016/j.clnu.2020.10.035
Schulz, Altman, Moher, Consort, statement: Updated guidelines for reporting parallel group randomised trials, J. Pharmacol. Pharmacother, doi:10.4103/0976-500X.72352
Simpson, Van Der Mei, Stewart, Blizzard, Tettey et al., Weekly cholecalciferol supplementation results in significant reductions in infection risk among the vitamin D deficient: Results from the CIPRIS pilot RCT, BMC Nutr
Vdscp Vitamin, Assays, Certifications from
Waldron, Ashby, Cornes, Bechervaise, Razavi et al., A Negative Acute Phase Reactant, J. Clin. Pathol, doi:10.1136/jclinpath-2012-201301
Wang, Zhou, Brand, Huang, Inflammation and taste disorders: Mechanisms in taste buds, Ann. N. Y. Acad. Sci, doi:10.1111/j.1749-6632.2009.04480.x
Wrapp, Wang, Corbett, Goldsmith, Hsieh et al., Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science, doi:10.1126/science.abb2507
Xu, Baylink, Chen, Reeves, Xiao et al., The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19, J. Transl. Med, doi:10.1186/s12967-020-02488-5
Zhou, Frey, Yang, Viral calciomics: Interplays between Ca 2+ and virus, Cell Calcium, doi:10.1016/j.ceca.2009.05.005
DOI record:
{
"DOI": "10.3390/nu13072170",
"ISSN": [
"2072-6643"
],
"URL": "http://dx.doi.org/10.3390/nu13072170",
"abstract": "<jats:p>Objective: Vitamin D deficiency has been associated with an increased risk of COVID-19 severity. This multi-center randomized clinical trial aims to determine the effects of 5000 IU versus 1000 IU daily oral vitamin D3 supplementation in the recovery of symptoms and other clinical parameters among mild to moderate COVID-19 patients with sub-optimal vitamin D status. Study Design and Setting: A total of 69 reverse transcriptase polymerase chain reaction (RT-PCR) SARS-CoV-2 positive adults who were hospitalized for mild to moderate COVID-19 disease were allocated to receive once daily for 2 weeks either 5000 IU oral vitamin D3 (n = 36, 21 males; 15 females) or 1000 IU oral vitamin D3 (standard control) (n = 33, 13 males; 20 females). Anthropometrics were measured and blood samples were taken pre- and post-supplementation. Fasting blood glucose, lipids, serum 25(OH)D, and inflammatory markers were measured. COVID-19 symptoms were noted on admission and monitored until full recovery. Results: Vitamin D supplementation for 2 weeks caused a significant increase in serum 25(OH)D levels in the 5000 IU group only (adjusted p = 0.003). Within-group comparisons also showed a significant decrease in BMI and IL-6 levels overtime in both groups (p-values < 0.05) but was not clinically significant in between-group comparisons. Kaplan–Meier survival analysis revealed that the 5000 IU group had a significantly shorter time to recovery (days) than the 1000 IU group in resolving cough, even after adjusting for age, sex, baseline BMI, and D-dimer (6.2 ± 0.8 versus 9.1 ± 0.8; p = 0.039), and ageusia (loss of taste) (11.4 ± 1.0 versus 16.9 ± 1.7; p = 0.035). Conclusion: A 5000 IU daily oral vitamin D3 supplementation for 2 weeks reduces the time to recovery for cough and gustatory sensory loss among patients with sub-optimal vitamin D status and mild to moderate COVID-19 symptoms. The use of 5000 IU vitamin D3 as an adjuvant therapy for COVID-19 patients with suboptimal vitamin D status, even for a short duration, is recommended.</jats:p>",
"alternative-id": [
"nu13072170"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5248-2350",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sabico",
"given": "Shaun",
"sequence": "first"
},
{
"affiliation": [],
"family": "Enani",
"given": "Mushira A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sheshah",
"given": "Eman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aljohani",
"given": "Naji J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aldisi",
"given": "Dara A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alotaibi",
"given": "Naif H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alshingetti",
"given": "Naemah",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2864-1649",
"affiliation": [],
"authenticated-orcid": false,
"family": "Alomar",
"given": "Suliman Y.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alnaami",
"given": "Abdullah M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8657-0682",
"affiliation": [],
"authenticated-orcid": false,
"family": "Amer",
"given": "Osama E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hussain",
"given": "Syed D.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5472-1725",
"affiliation": [],
"authenticated-orcid": false,
"family": "Al-Daghri",
"given": "Nasser M.",
"sequence": "additional"
}
],
"container-title": "Nutrients",
"container-title-short": "Nutrients",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
6,
24
]
],
"date-time": "2021-06-24T15:01:38Z",
"timestamp": 1624546898000
},
"deposited": {
"date-parts": [
[
2021,
6,
24
]
],
"date-time": "2021-06-24T15:52:47Z",
"timestamp": 1624549967000
},
"funder": [
{
"DOI": "10.13039/501100011665",
"award": [
"NA"
],
"doi-asserted-by": "publisher",
"name": "Deanship of Scientific Research, King Saud University"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
7
]
],
"date-time": "2024-05-07T22:13:19Z",
"timestamp": 1715119999356
},
"is-referenced-by-count": 118,
"issue": "7",
"issued": {
"date-parts": [
[
2021,
6,
24
]
]
},
"journal-issue": {
"issue": "7",
"published-online": {
"date-parts": [
[
2021,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
6,
24
]
],
"date-time": "2021-06-24T00:00:00Z",
"timestamp": 1624492800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2072-6643/13/7/2170/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "2170",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
6,
24
]
]
},
"published-online": {
"date-parts": [
[
2021,
6,
24
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1016/S1473-3099(20)30120-1",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1038/s41564-020-0695-z",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.7326/M20-3012",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1001/jama.2020.6019",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1016/S0140-6736(20)32472-7",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1016/S2213-2600(17)30306-5",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1002/14651858.CD007470.pub3",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1016/j.clnu.2020.10.035",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1136/bmj.i6583",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1093/ajcn/nqaa381",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.3390/nu12092757",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.3390/nu12113361",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1186/s12933-020-01184-4",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1126/science.abb2507",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1080/13813455.2020.1826530",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1186/s12967-021-02838-x",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1136/jclinpath-2012-201301",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1038/ejcn.2016.177",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1016/j.metabol.2021.154753",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1007/s40520-021-01831-0",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"key": "ref21"
},
{
"key": "ref22"
},
{
"key": "ref23"
},
{
"key": "ref24"
},
{
"DOI": "10.4103/0976-500X.72352",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"key": "ref26"
},
{
"DOI": "10.5144/0256-4947.2015.1",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1007/s11657-020-0709-8",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1186/2055-0928-1-7",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1007/s40520-020-01678-x",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1097/MJT.0000000000001222",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.3390/nu12113377",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1001/jama.2020.26848",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1186/s13104-015-1504-2",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1371/journal.pone.0008342",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.1152/ajpendo.00138.2020",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.3389/fphar.2020.595888",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.3389/fphar.2020.594487",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1111/j.1749-6632.2009.04489.x",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1111/j.1749-6632.2009.04480.x",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1093/rheumatology/ken109",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1186/1479-5876-11-176",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1186/s12967-020-02488-5",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1007/s11011-014-9626-0",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1016/j.clnu.2020.10.043",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1016/j.clnu.2020.08.021",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.1016/j.ceca.2009.05.005",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1007/s11154-021-09655-z",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1016/j.dsx.2021.01.003",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.1038/s41574-021-00516-y",
"doi-asserted-by": "publisher",
"key": "ref51"
}
],
"reference-count": 51,
"references-count": 51,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2072-6643/13/7/2170"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial",
"type": "journal-article",
"volume": "13"
}
