Dietary Vitamin D Mitigates Coronavirus-Induced Lung Inflammation and Damage in Mice
et al., Viruses, doi:10.3390/v15122434, Dec 2023
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
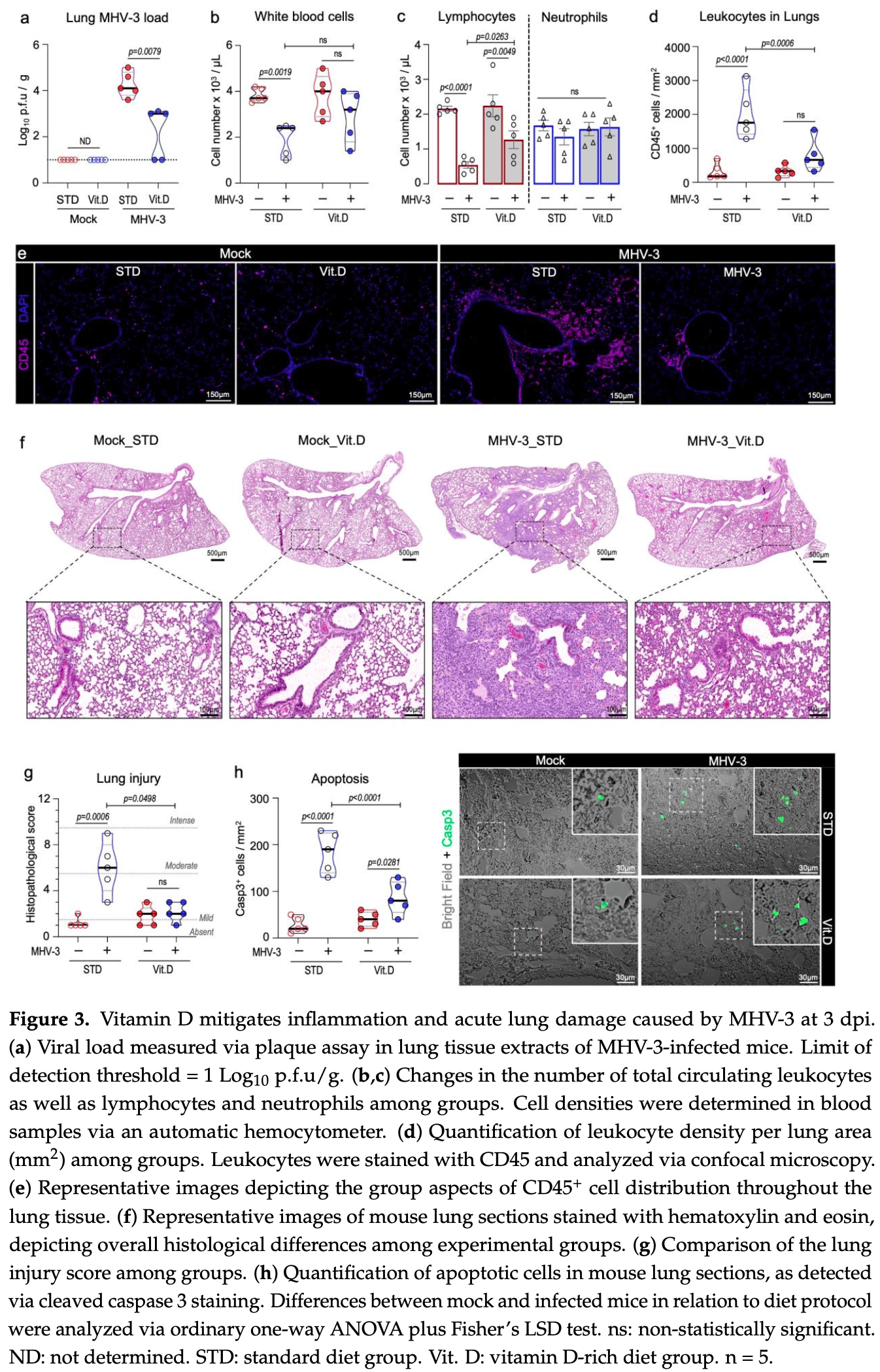
Mouse study showing that vitamin D supplementation mitigates lung inflammation, damage, and lethality induced by the murine coronavirus MHV-3, which serves as a model for studying COVID-19 pathogenesis. Mice fed a vitamin D-rich diet exhibited enhanced resistance to acute respiratory damage and systemic complications from MHV-3 infection, alongside improved survival rates, reduced viral titers, and decreased levels of proinflammatory cytokines TNF, IL-6, IL-1β, and IFNγ. Vitamin D also lessened MHV-3-induced extrapulmonary liver damage. The protective effects are potentially mediated through augmentation of type I interferon antiviral responses. These preclinical findings suggest vitamin D supplementation could alleviate β-coronavirus-triggered respiratory illness and systemic manifestations.
Authors also report in vitro results showing that calcitriol significantly reduces replication of SARS-CoV-2, MHV-3, and MHV-A59 in infected cells. Treatment with 5-10 μM calcitriol lowered viral titers 1.3-2.3 log10 pfu/mL for SARS-CoV-2 and 1.3-2.1 log10 pfu/mL for the murine coronaviruses. These antiviral effects were accompanied by less virus-induced cell damage.
29 preclinical studies support the efficacy of vitamin D for COVID-19:
Vitamin D has been identified by the European Food Safety Authority (EFSA) as having sufficient evidence for a causal relationship between intake and optimal immune system function27-30.
Vitamin D inhibits SARS-CoV-2 replication in vitro17,24, mitigates lung inflammation, damage, and lethality in mice with an MHV-3 model for β-CoV respiratory infections17,24, reduces SARS-CoV-2 replication in nasal epithelial cells via increased type I interferon expression20, downregulates proinflammatory cytokines IL-1β and TNF-α in SARS-CoV-2 spike protein-stimulated cells16, attenuates nucleocapsid protein-induced hyperinflammation by inactivating the NLRP3 inflammasome through the VDR-BRCC3 signaling pathway21, may be neuroprotective by protecting the blood-brain barrier, reducing neuroinflammation, and via immunomodulatory effects31, may mitigate hyperinflammation and cytokine storm by upregulating TLR10 expression which downregulates proinflammatory cytokines13, downregulates ACE2 and TMPRSS2 in human trophoblasts and minimizes spike protein-induced inflammation19, may minimize cytokine storm by dampening excessive cytokine production2, may suppress viral entry and replication via LL-37 induction11,12, and minimizes platelet aggregation mediated by SARS-CoV-2 spike protein via inhibiting integrin αIIbβ3 outside-in signaling15.
Cholecalciferol and calcifediol directly bind two allosteric pockets on the SARS-CoV-2 Spike RBD, bias the trimer toward a closed state, weaken ACE2 engagement, and reduce viral entry in cell models1.
Calcitriol may destabilize the Spike protein architecture and inhibit IL-17R dimerization, blocking viral entry and mitigating hyperinflammatory cytokine storm32.
Vitamin D improves regulatory immune cell levels and control of proinflammatory cytokines in severe COVID-1933.
Calcifediol inhibits SARS-CoV-2 papain-like protease (PLpro), a critical enzyme for viral replication14.
Symptomatic COVID-19 is associated with a lower frequency of natural killer (NK) cells and vitamin D has been shown to improve NK cell activity34,35.
1.
García-Marín et al., Exploring SARS-CoV-2 Spike RBD Pockets as Targets for Generic Drugs: A Combined Computational, Biophysical, and Biological Approach, ACS Omega, doi:10.1021/acsomega.5c05175.
2.
Alzahrani, A., A new investigation into the molecular mechanism of cholecalciferol towards reducing cytokines storm, Octahedron Drug Research, doi:10.21608/odr.2024.308273.1043.
3.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
4.
Morales-Bayuelo et al., New findings on ligand series used as SARS-CoV-2 virus inhibitors within the frameworks of molecular docking, molecular quantum similarity and chemical reactivity indices, F1000Research, doi:10.12688/f1000research.123550.3.
5.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
6.
Pandya et al., Unravelling Vitamin B12 as a potential inhibitor against SARS-CoV-2: A computational approach, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2022.100951.
7.
Mansouri et al., The impact of calcitriol and estradiol on the SARS-CoV-2 biological activity: a molecular modeling approach, Scientific Reports, doi:10.1038/s41598-022-04778-y.
8.
Song et al., Vitamin D3 and its hydroxyderivatives as promising drugs against COVID-19: a computational study, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1964601.
9.
Qayyum et al., Vitamin D and lumisterol novel metabolites can inhibit SARS-CoV-2 replication machinery enzymes, Endocrinology and Metabolism, doi:10.1152/ajpendo.00174.2021.
10.
Al-Mazaideh et al., Vitamin D is a New Promising Inhibitor to the Main Protease (Mpro) of COVID-19 by Molecular Docking, Journal of Pharmaceutical Research International, doi:10.9734/jpri/2021/v33i29B31603.
11.
Roth et al., Vitamin D-inducible antimicrobial peptide LL-37 binds SARS-CoV-2 Spike and accessory proteins ORF7a and ORF8, Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2025.1671738.
12.
Vercellino et al., Influence of Sex and 1,25α Dihydroxyvitamin D3 on SARS-CoV-2 Infection and Viral Entry, Pathogens, doi:10.3390/pathogens14080765.
13.
Knez et al., TLR10 overexpression modulates immune response in A549 lung epithelial cells challenged with SARS-CoV-2 S and N proteins, Frontiers in Immunology, doi:10.3389/fimmu.2024.1490478.
14.
Chen et al., In Vitro Characterization of Inhibition Function of Calcifediol to the Protease Activity of SARS-COV-2 PLpro, Journal of Medical Virology, doi:10.1002/jmv.70085.
15.
Wang et al., 1,25‐Dihydroxyvitamin D3 attenuates platelet aggregation potentiated by SARS‐CoV‐2 spike protein via inhibiting integrin αIIbβ3 outside‐in signaling, Cell Biochemistry and Function, doi:10.1002/cbf.4039.
16.
Alcalá-Santiago et al., Disentangling the Immunomodulatory Effects of Vitamin D on the SARS-CoV-2 Virus by In Vitro Approaches, The 14th European Nutrition Conference FENS 2023, doi:10.3390/proceedings2023091415.
17.
Campolina-Silva et al., Dietary Vitamin D Mitigates Coronavirus-Induced Lung Inflammation and Damage in Mice, Viruses, doi:10.3390/v15122434.
18.
Moatasim et al., Potent Antiviral Activity of Vitamin B12 against Severe Acute Respiratory Syndrome Coronavirus 2, Middle East Respiratory Syndrome Coronavirus, and Human Coronavirus 229E, Microorganisms, doi:10.3390/microorganisms11112777.
19.
Vargas-Castro et al., Calcitriol prevents SARS-CoV spike-induced inflammation in human trophoblasts through downregulating ACE2 and TMPRSS2 expression, The Journal of Steroid Biochemistry and Molecular Biology, doi:10.1016/j.jsbmb.2024.106625.
20.
Sposito et al., Age differential CD13 and interferon expression in airway epithelia affect SARS-CoV-2 infection - effects of vitamin D, Mucosal Immunology, doi:10.1016/j.mucimm.2023.08.002.
21.
Chen (B) et al., Vitamin D3 attenuates SARS‐CoV‐2 nucleocapsid protein‐caused hyperinflammation by inactivating the NLRP3 inflammasome through the VDR‐BRCC3 signaling pathway in vitro and in vivo, MedComm, doi:10.1002/mco2.318.
22.
Rybakovsky et al., Calcitriol modifies tight junctions, improves barrier function, and reduces TNF‐α‐induced barrier leak in the human lung‐derived epithelial cell culture model, 16HBE 14o‐, Physiological Reports, doi:10.14814/phy2.15592.
23.
DiGuilio et al., The multiphasic TNF-α-induced compromise of Calu-3 airway epithelial barrier function, Experimental Lung Research, doi:10.1080/01902148.2023.2193637.
24.
Pickard et al., Discovery of re-purposed drugs that slow SARS-CoV-2 replication in human cells, PLOS Pathogens, doi:10.1371/journal.ppat.1009840.
25.
Mok et al., Calcitriol, the active form of vitamin D, is a promising candidate for COVID-19 prophylaxis, bioRxiv, doi:10.1101/2020.06.21.162396.
26.
Fernandes de Souza et al., Lung Inflammation Induced by Inactivated SARS-CoV-2 in C57BL/6 Female Mice Is Controlled by Intranasal Instillation of Vitamin D, Cells, doi:10.3390/cells12071092.
27.
Galmés et al., Suboptimal Consumption of Relevant Immune System Micronutrients Is Associated with a Worse Impact of COVID-19 in Spanish Populations, Nutrients, doi:10.3390/nu14112254.
28.
Galmés (B) et al., Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework, Nutrients, doi:10.3390/nu12092738.
29.
EFSA, Scientific Opinion on the substantiation of a health claim related to vitamin D and contribution to the normal function of the immune system pursuant to Article 14 of Regulation (EC) No 1924/2006, EFSA Journal, doi:10.2903/j.efsa.2015.4096.
30.
EFSA (B), Scientific Opinion on the substantiation of health claims related to vitamin D and normal function of the immune system and inflammatory response (ID 154, 159), maintenance of normal muscle function (ID 155) and maintenance of normal cardiovascular function (ID 159) pursuant to Article 13(1) of Regulation (E, EFSA Journal, doi:10.2903/j.efsa.2010.1468.
31.
Gotelli et al., Understanding the immune-endocrine effects of vitamin D in SARS-CoV-2 infection: a role in protecting against neurodamage?, Neuroimmunomodulation, doi:10.1159/000533286.
32.
Fadel et al., Targeting asparagine and cysteine in SARS-CoV-2 variants and human pro-inflammatory mediators to alleviate COVID-19 severity; a cross-section and in-silico study, Scientific Reports, doi:10.1038/s41598-025-19359-y.
33.
Saheb Sharif-Askari et al., Increased blood immune regulatory cells in severe COVID-19 with autoantibodies to type I interferons, Scientific Reports, doi:10.1038/s41598-023-43675-w.
Campolina-Silva et al., 15 Dec 2023, peer-reviewed, 13 authors.
Contact: gcampolina@crchudequebec.ulaval.ca (corresponding author), anaclaudiaandrade29@gmail.com, larissesbl.adv@gmail.com, iandemeirachaves@hotmail.com, cleida@icb.ufmg.br, ldeolive2@gmail.com, mmtex.ufmg@gmail.com, glauber@ymail.com, vivianvcosta@ufmg.br.
Dietary Vitamin D Mitigates Coronavirus-Induced Lung Inflammation and Damage in Mice
Viruses, doi:10.3390/v15122434
The COVID-19 pandemic caused by the SARS-CoV-2 (β-CoV) betacoronavirus has posed a significant threat to global health. Despite the availability of vaccines, the virus continues to spread, and there is a need for alternative strategies to alleviate its impact. Vitamin D, a secosteroid hormone best known for its role in bone health, exhibits immunomodulatory effects in certain viral infections. Here, we have shown that bioactive vitamin D (calcitriol) limits in vitro replication of SARS-CoV-2 and murine coronaviruses MHV-3 and MHV-A59. Comparative studies involving wild-type mice intranasally infected with MHV-3, a model for studying β-CoV respiratory infections, confirmed the protective effect of vitamin D in vivo. Accordingly, mice fed a standard diet rapidly succumbed to MHV-3 infection, whereas those on a vitamin D-rich diet (10,000 IU of Vitamin D 3 /kg) displayed increased resistance to acute respiratory damage and systemic complications. Consistent with these findings, the vitamin D-supplemented group exhibited lower viral titers in their lungs and reduced levels of TNF, IL-6, IL-1β, and IFN-γ, alongside an enhanced type I interferon response. Altogether, our findings suggest vitamin D supplementation ameliorates β-CoV-triggered respiratory illness and systemic complications in mice, likely via modulation of the host's immune response to the virus.
by MHV-3. While these findings open promising avenues for further exploration and emphasize the importance of continued research in this area, it is imperative for future studies to broaden the scope of this study by encompassing in vivo infection with SARS-CoV-2. This imperative step will provide a more comprehensive understanding of vitamin D's prospects in addressing COVID-19. As result, we argue that our findings should not be construed in the context of the current clinical landscape of studies on vitamin D and COVID-19. Instead, they may serve as a springboard for future preclinical research aimed at exploring the intricate relationship between Vitamin D and β-CoV infections.
Supplementary Materials: The following supporting information can be downloaded at: https:// www.mdpi.com/article/10.3390/v15122434/s1, Figure S1 and Raw data_Figures 1-6. Informed Consent Statement: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest.
References
Agrawal, Gupta, Agrawal, Vitamin D Supplementation Reduces Airway Hyperresponsiveness and Allergic Airway Inflammation in a Murine Model, Clin. Exp. Allergy, doi:10.1111/cea.12102
Alon, Sportiello, Kozlovski, Kumar, Reilly et al., Leukocyte Trafficking to the Lungs and beyond: Lessons from Influenza for COVID-19, Nat. Rev. Immunol, doi:10.1038/s41577-020-00470-2
Amrein, Scherkl, Hoffmann, Neuwersch-Sommeregger, Köstenberger et al., Vitamin D deficiency 2.0: An update on the current status worldwide, Eur. J. Clin. Nutr, doi:10.1038/s41430-020-0558-y
Andrade, Campolina-Silva, Queiroz-Junior, De Oliveira, Lacerda et al., A Biosafety Level 2 Mouse Model for Studying Betacoronavirus-Induced Acute Lung Damage and Systemic Manifestations, J. Virol, doi:10.1128/jvi.01276-21
Araujo, Machado, Amgarten, Malta, De Araujo et al., SARS-CoV-2 Isolation from the First Reported Patients in Brazil and Establishment of a Coordinated Task Network, Mem. Inst. Oswaldo Cruz, doi:10.1590/0074-02760200342
Arora, Patel, Nicol, Field, Restori et al., Vitamin D and the Ability to Produce 1,25(OH)2D Are Critical for Protection from Viral Infection of the Lungs, Nutrients, doi:10.3390/nu14153061
Bastard, Rosen, Zhang, Michailidis, Hoffmann et al., Autoantibodies against Type I IFNs in Patients with Life-Threatening COVID-19, Science, doi:10.1126/science.abd4585
Bikle, Christakos, New Aspects of Vitamin D Metabolism and Action-Addressing the Skin as Source and Target, Nat. Rev. Endocrinol, doi:10.1038/s41574-019-0312-5
Blanco-Melo, Nilsson-Payant, Liu, Uhl, Hoagland et al., Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19, Cell, doi:10.1016/j.cell.2020.04.026
Butler-Laporte, Nakanishi, Mooser, Morrison, Abdullah et al., Vitamin D and COVID-19 Susceptibility and Severity in the COVID-19 Host Genetics Initiative: A Mendelian Randomization Study, PLoS Med, doi:10.1371/journal.pmed.1003605
Campolina-Silva, Maria, Mahecha, Oliveira, Reduced Vitamin D (VDR) Expression and Plasma Vitamin D Levels Are Associated with Aging-Related Prostate Lesions, Prostate, doi:10.1002/pros.23498
Castillo, Costa, Barrios, Díaz, Miranda et al., Effect of Calcifediol Treatment and Best Available Therapy versus Best Available Therapy on Intensive Care Unit Admission and Mortality among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical Study, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105751
Cervantes-Barragán, Kalinke, Züst, König, Reizis et al., Type I IFN-Mediated Protection of Macrophages and Dendritic Cells Secures Control of Murine Coronavirus Infection, J. Immunol, doi:10.4049/jimmunol.182.2.1099
Charoenngam, Holick, Immunologic Effects of Vitamin on Human Health and Disease, Nutrients, doi:10.3390/nu12072097
Chen, Sung, Chuang, Lai, Lee et al., Vitamin D3 Decreases TNF-α-Induced Inflammation in Lung Epithelial Cells through a Reduction in Mitochondrial Fission and Mitophagy, Cell Biol. Toxicol, doi:10.1007/s10565-021-09629-6
Chu, Chan, -W.; Yuen, Shuai, Yuan et al., Comparative Tropism, Replication Kinetics, and Cell Damage Profiling of SARS-CoV-2 and SARS-CoV with Implications for Clinical Manifestations, Transmissibility, and Laboratory Studies of COVID-19: An Observational Study, Lancet Microbe, doi:10.1016/S2666-5247(20)30004-5
Coussens, Wilkinson, Hanifa, Nikolayevskyy, Elkington et al., Vitamin D Accelerates Resolution of Inflammatory Responses during Tuberculosis Treatment, Proc. Natl. Acad. Sci, doi:10.1073/pnas.1200072109
De Albuquerque, Baig, Ma, Zhang, He et al., MurineHepatitis Virus Strain 1 Produces a Clinically Relevant Model of Severe Acute Respiratory Syndrome in A/J Mice, J. Virol, doi:10.1128/JVI.00747-06
Dimitrov, Barbier, Ismailova, Wang, Dmowski et al., Vitamin D-Regulated Gene Expression Profiles: Species-Specificity and Cell-Specific Effects on Metabolism and Immunity, Endocrinology, doi:10.1210/endocr/bqaa218
Ding, Ning, Liu, Lai, Leibowitz et al., Fulminant Hepatic Failure in Murine Hepatitis Virus Strain 3 Infection: Tissue-Specific Expression of a Novel Fgl2 Prothrombinase, J. Virol, doi:10.1128/JVI.72.4.3504a-3504a.1998
Dissanayake, De Silva, Sumanatilleke, De Silva, Gamage et al., Prognostic and Therapeutic Role of Vitamin D in COVID-19: Systematic Review and Meta-Analysis, J. Clin. Endocrinol. Metab, doi:10.1210/clinem/dgab892
Fischer, Hall, Agrawal, Vitamin D Supplementation Reduces Induction of Epithelial-Mesenchymal Transition in Allergen Sensitized and Challenged Mice, PLoS ONE, doi:10.1371/journal.pone.0149180
Garcia, De Moraes, Rodrigues, Gilioli, De Oliveira-Filho et al., Coding-Complete Genome Sequence of Murine Hepatitis Virus, Microbiol. Resour. Announc, doi:10.1128/MRA.00248-21
Gombart, Borregaard, Koeffler, Human Cathelicidin Antimicrobial Peptide (CAMP) Gene Is a Direct Target of the Vitamin D Receptor and Is Strongly Up-regulated in Myeloid Cells by 1,25-dihydroxyvitamin D 3, FASEB J, doi:10.1096/fj.04-3284com
Gong, Worley, Carver, Goldstein, Deng, Neutrophils Drive Pulmonary Vascular Leakage in MHV-1 Infection of Susceptible A/J Mice, Front. Immunol, doi:10.3389/fimmu.2022.1089064
Hadjadj, Yatim, Barnabei, Corneau, Boussier, Impaired Type I Interferon Activity and Inflammatory Responses in Severe COVID-19 Patients, Science, doi:10.1126/science.abc6027
Hafezi, Saheb Sharif-Askari, Saheb Sharif-Askari, Ali Hussain Alsayed, Alsafar et al., Vitamin D Enhances Type I IFN Signaling in COVID-19 Patients, Sci. Rep, doi:10.1038/s41598-022-22307-9
Hirai, Ohtsuka, Ikeda, Taniguchi, Blau et al., Role of Mouse Hepatitis Virus (MHV) Receptor Murine CEACAM1 in the Resistance of Mice to MHV Infection: Studies of Mice with Chimeric MCEACAM1a and MCEACAM1b, J. Virol, doi:10.1128/JVI.02680-09
Jolliffe, Camargo, Sluyter, Aglipay, Aloia et al., Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587(21)00051-6
Jolliffe, Holt, Greenig, Talaei, Perdek et al., Effect of a Test-and-Treat Approach to Vitamin D Supplementation on Risk of All Cause Acute Respiratory Tract Infection and COVID-19: Phase 3 Randomised Controlled Trial (CORONAVIT), BMJ, doi:10.1136/bmj-2022-071230
Karki, Sharma, Tuladhar, Williams, Zalduondo et al., Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes, Cell, doi:10.1016/j.cell.2020.11.025
Kim, Park, Cho, Yoon, Song et al., Low Serum 25-Hydroxyvitamin D Level: An Independent Risk Factor for Tuberculosis?, Clin. Nutr, doi:10.1016/j.clnu.2013.11.014
King, Sprent, Dual Nature of Type I Interferons in SARS-CoV-2-Induced Inflammation, Trends Immunol, doi:10.1016/j.it.2021.02.003
Körner, Majjouti, Alejandre Alcazar, Mahabir, Of Mice and Men: The Coronavirus Mhv and Mouse Models as a Translational Approach to Understand Sars-Cov-2, Viruses, doi:10.3390/v12080880
Leow, Simpson, Cursons, Karalus, Hancox et al., Innate Immunity and Outcomes in Community Acquired Pneumonia, Respirology, doi:10.1111/j.1440-1843.2011.01924.x
Li, Pei, Chen, Song, Zhang et al., Substantial Undocumented Infection Facilitates the Rapid Dissemination of Novel Coronavirus (SARS-CoV-2), Science, doi:10.1126/science.abb3221
Liu, Liu, Xiang, Pu, Xiong et al., Neutrophil-to-Lymphocyte Ratio Predicts Critical Illness Patients with 2019 Coronavirus Disease in the Early Stage, J. Transl. Med, doi:10.1186/s12967-020-02374-0
Lucas, Wong, Klein, Castro, Silva et al., Longitudinal Analyses Reveal Immunological Misfiring in Severe COVID-19, Nature, doi:10.1038/s41586-020-2588-y
Maestro, Molnár, Carlberg, Vitamin D and Its Synthetic Analogs, J. Med. Chem, doi:10.1021/acs.jmedchem.9b00208
Maj, Trynda, Maj, Gębura, Bogunia-Kubik et al., Differential Response of Lung Cancer Cell Lines to Vitamin D Derivatives Depending on EGFR, KRAS, P53 Mutation Status and VDR Polymorphism, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2019.105431
Malireddi, Bynigeri, Mall, Connelly, Pruett-Miller et al., Inflammatory Cell Death, PANoptosis, Screen Identifies Host Factors in Coronavirus Innate Immune Response as Therapeutic Targets, Commun. Biol, doi:10.1038/s42003-023-05414-9
Marim, Silveira, Lima, Zamboni, A Method for Generation of Bone Marrow-Derived Macrophages from Cryopreserved Mouse Bone Marrow Cells, PLoS ONE, doi:10.1371/journal.pone.0015263
Meng, Li, Liu, Xiao, Tang et al., The role of vitamin D in the prevention and treatment of SARS-CoV-2 infection: A meta-analysis of randomized controlled trials, Clin. Nutr, doi:10.1016/j.clnu.2023.09.008
Mok, Ng, Ahidjo, Aw, Chen et al., Evaluation of In Vitro and In Vivo Antiviral Activities of Vitamin D for SARS-CoV-2 and Variants, Pharmaceutics, doi:10.3390/pharmaceutics15030925
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Randomized Clinical Trial, JAMA-J. Am. Med. Assoc, doi:10.1001/jama.2020.26848
Oh, Riek, Darwech, Funai, Shao et al., Deletion of Macrophage Vitamin D Receptor Promotes Insulin Resistance and Monocyte Cholesterol Transport to Accelerate Atherosclerosis in Mice, Cell Rep, doi:10.1016/j.celrep.2015.02.043
Otto, Rathkolb, Oestereicher, Lengger, Moerth et al., Clinical Chemistry Reference Intervals for C57BL/6J, C57BL/6N, and C3HeB/FeJ Mice (Mus Musculus), J. Am. Assoc. Lab. Anim. Sci
Pan, Shen, Yu, Ge, Chen et al., SARS-CoV-2 N Protein Promotes NLRP3 Inflammasome Activation to Induce Hyperinflammation, Nat. Commun, doi:10.1038/s41467-021-25015-6
Pelaia, Tinello, Vatrella, De Sarro, Pelaia, Lung under Attack by COVID-19-Induced Cytokine Storm: Pathogenic Mechanisms and Therapeutic Impications, Ther. Adv. Respir. Dis, doi:10.1177/1753466620933508
Petrelli, Luciani, Perego, Dognini, Colombelli et al., Therapeutic and prognostic role of vitamin D for COVID-19 infection: A systematic review and meta-analysis of 43 observational studies, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2021.105883
Qiao, Lau, Xie, Poon, Chan et al., SARS-CoV-2 infection induces inflammatory bone loss in golden Syrian hamsters, Nat. Commun, doi:10.1038/s41467-022-30195-w
Queiroz-Junior, Santos, Gonçalves, Brito, Barrioni et al., Acute Coronavirus Infection Triggers a TNF-Dependent Osteoporotic Phenotype in Mice, Life Sci, doi:10.1016/j.lfs.2023.121750
Ray, Personius, Dungan, Dhillon, Hershberger, Vitamin D3 Intake Modulates Diaphragm but Not Peripheral Muscle Force in Young Mice, J. Appl. Physiol, doi:10.1152/japplphysiol.00643.2015
Sarhan, Warda, Ibrahim, Schaalan, Fathy, Evaluation of Infliximab/Tocilizumab versus Tocilizumab among COVID-19 Patients with Cytokine Storm Syndrome, Sci. Rep, doi:10.1038/s41598-023-33484-6
Sharma, Peng, Qing, Hilliard, Kim et al., Distinct Roles of Type I and Type III Interferons during a Native Murine β Coronavirus Lung Infection, J. Virol, doi:10.1128/JVI.01241-21
Taylor, Nadeau, Abbasi, Lachance, Nguyen et al., The Ultimate QPCR Experiment: Producing Publication Quality, Reproducible Data the First Time, Trends Biotechnol, doi:10.1016/j.tibtech.2018.12.002
Tebben, Singh, Kumar, Vitamin D-Mediated Hypercalcemia: Mechanisms, Diagnosis, and Treatment, Endocr. Rev, doi:10.1210/er.2016-1070
Toro, Arevalo, Pereira-Gómez, Sabater, Zizzi et al., Coronavirus Pathogenesis in Mice Explains the SARS-CoV-2 Multi-Organ Spread by Red Blood Cells Hitch-Hiking, medRxiv, doi:10.1101/2023.03.29.23287591
Verone-Boyle, Shoemaker, Attwood, Morrison, Makowski et al., Diet-Derived 25-Hydroxyvitamin D3 Activates Vitamin D Receptor Target Gene Expression and Suppresses EGFR Mutant Non-Small Cell Lung Cancer Growth in Vitro and in Vivo, Oncotarget, doi:10.18632/oncotarget.6493
Vieira-Alves, Alves, Souza, Melo, Coimbra Campos et al., TNF/INOS/NO Pathway Mediates Host Susceptibility to Endothelial-Dependent Circulatory Failure and Death Induced by Betacoronavirus Infection, Clin. Sci, doi:10.1042/CS20220663
Villasis-Keever, López-Alarcón, Miranda-Novales, Zurita-Cruz, Barrada-Vázquez et al., Efficacy and Safety of Vitamin D Supplementation to Prevent COVID-19 in Frontline Healthcare Workers. A Randomized Clinical Trial, Arch. Med. Res, doi:10.1016/j.arcmed.2022.04.003
Wang, Nestel, Bourdeau, Nagai, Wang et al., Cutting Edge: 1,25-Dihydroxyvitamin D3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression, J. Immunol, doi:10.4049/jimmunol.173.5.2909
Wang, Wang, Li, Chen, Han et al., Human Cathelicidin Inhibits SARS-CoV-2 Infection: Killing Two Birds with One Stone, ACS Infect. Dis, doi:10.1021/acsinfecdis.1c00096
White, Emerging Roles of Vitamin D-Induced Antimicrobial Peptides in Antiviral Innate Immunity, Nutrients, doi:10.3390/nu14020284
Xu, Li, Cao, Wu, Chen, Tumor Necrosis Factor α (TNF-α) Receptor-I Is Required for TNF-α-Mediated Fulminant Virus Hepatitis Caused by Murine Hepatitis Virus Strain-3 Infection, Immunol. Lett, doi:10.1016/j.imlet.2013.11.008
Yang, Du, Chen, Zhao, Yang et al., Coronavirus MHV-A59 Infects the Lung and Causes Severe Pneumonia in C57BL/6 Mice, Virol. Sin, doi:10.1007/s12250-014-3530-y
Zeng, Xie, Feng, Xu, Han et al., Specific Inhibition of the NLRP3 Inflammasome Suppresses Immune Overactivation and Alleviates COVID-19 like Pathology in Mice, eBioMedicine, doi:10.1016/j.ebiom.2021.103803
Zhang, Li, Yang, Wang, Effect of vitamin D supplementation on COVID-19 patients: A systematic review and meta-analysis, Front. Nutr, doi:10.3389/fnut.2023.1131103
Zhang, Liu, Moncada-Velez, Chen, Ogishi et al., Inborn Errors of Type I IFN Immunity in Patients with Life-Threatening COVID-19, Science, doi:10.1126/science.abd4570
Zheng, Williams, Subbarao Malireddi, Karki, Banoth et al., Impaired NLRP3 Inflammasome Activation/Pyroptosis Leads to Robust Inflammatory Cell Death via Caspase-8/RIPK3 during Coronavirus Infection, J. Biol. Chem, doi:10.1074/jbc.RA120.015036
Zhu, Zhu, Gu, Zhan, Chen et al., Association Between Vitamin D and Influenza: Meta-Analysis and Systematic Review of Randomized Controlled Trials, Front. Nutr, doi:10.3389/fnut.2021.799709
DOI record:
{
"DOI": "10.3390/v15122434",
"ISSN": [
"1999-4915"
],
"URL": "http://dx.doi.org/10.3390/v15122434",
"abstract": "<jats:p>The COVID-19 pandemic caused by the SARS-CoV-2 (β-CoV) betacoronavirus has posed a significant threat to global health. Despite the availability of vaccines, the virus continues to spread, and there is a need for alternative strategies to alleviate its impact. Vitamin D, a secosteroid hormone best known for its role in bone health, exhibits immunomodulatory effects in certain viral infections. Here, we have shown that bioactive vitamin D (calcitriol) limits in vitro replication of SARS-CoV-2 and murine coronaviruses MHV-3 and MHV-A59. Comparative studies involving wild-type mice intranasally infected with MHV-3, a model for studying β-CoV respiratory infections, confirmed the protective effect of vitamin D in vivo. Accordingly, mice fed a standard diet rapidly succumbed to MHV-3 infection, whereas those on a vitamin D-rich diet (10,000 IU of Vitamin D3/kg) displayed increased resistance to acute respiratory damage and systemic complications. Consistent with these findings, the vitamin D-supplemented group exhibited lower viral titers in their lungs and reduced levels of TNF, IL-6, IL-1β, and IFN-γ, alongside an enhanced type I interferon response. Altogether, our findings suggest vitamin D supplementation ameliorates β-CoV-triggered respiratory illness and systemic complications in mice, likely via modulation of the host’s immune response to the virus.</jats:p>",
"alternative-id": [
"v15122434"
],
"author": [
{
"affiliation": [
{
"name": "Department of Morphology, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
},
{
"name": "Department of Biochemistry and Immunology, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
},
{
"name": "CHU de Québec Research Center (CHUL), Université Laval, Quebec, QC G1V 4G2, Canada"
}
],
"family": "Campolina-Silva",
"given": "Gabriel",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Morphology, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
},
{
"name": "Department of Biochemistry and Immunology, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
},
{
"name": "CHU de Québec Research Center (CHUL), Université Laval, Quebec, QC G1V 4G2, Canada"
}
],
"family": "Andrade",
"given": "Ana Cláudia dos Santos Pereira",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Morphology, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
}
],
"family": "Couto",
"given": "Manoela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Physiology and Biophysics, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
}
],
"family": "Bittencourt-Silva",
"given": "Paloma G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Morphology, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
}
],
"family": "Queiroz-Junior",
"given": "Celso M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Morphology, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
}
],
"family": "Lacerda",
"given": "Larisse de Souza B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Morphology, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
}
],
"family": "Chaves",
"given": "Ian de Meira",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biochemistry and Immunology, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
}
],
"family": "de Oliveira",
"given": "Leonardo C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Genetics, Ecology and Evolution, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
}
],
"family": "Marim",
"given": "Fernanda Martins",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9610-7846",
"affiliation": [
{
"name": "Department of Morphology, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
}
],
"authenticated-orcid": false,
"family": "Oliveira",
"given": "Cleida A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Physiology and Biophysics, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
}
],
"family": "da Silva",
"given": "Glauber S. F.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6944-3008",
"affiliation": [
{
"name": "Department of Biochemistry and Immunology, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
}
],
"authenticated-orcid": false,
"family": "Teixeira",
"given": "Mauro Martins",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Morphology, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
},
{
"name": "Department of Biochemistry and Immunology, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Belo Horizonte 30270-901, MG, Brazil"
}
],
"family": "Costa",
"given": "Vivian Vasconcelos",
"sequence": "additional"
}
],
"container-title": "Viruses",
"container-title-short": "Viruses",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
12,
15
]
],
"date-time": "2023-12-15T11:53:16Z",
"timestamp": 1702641196000
},
"deposited": {
"date-parts": [
[
2023,
12,
15
]
],
"date-time": "2023-12-15T12:06:54Z",
"timestamp": 1702642014000
},
"funder": [
{
"name": "National Institute of Science and Technology in Dengue and Host-microorganism Interaction"
},
{
"award": [
"465425/2014-3"
],
"name": "Brazilian National Science Council"
},
{
"award": [
"25036/2014-3"
],
"name": "Minas Gerais Foundation for Science"
},
{
"award": [
"RED-00202-22 29568-1",
"APQ-02281-18",
"APQ-02618-23"
],
"name": "Rede de Pesquisa em Imunobiológicos e Biofármacos para terapias avançadas e inovadoras"
},
{
"award": [
"88881.507175/2020-01"
],
"name": "Coordenação de Aperfeiçoamento de Pessoal de Nível Superior"
}
],
"indexed": {
"date-parts": [
[
2023,
12,
16
]
],
"date-time": "2023-12-16T00:48:06Z",
"timestamp": 1702687686310
},
"is-referenced-by-count": 0,
"issue": "12",
"issued": {
"date-parts": [
[
2023,
12,
15
]
]
},
"journal-issue": {
"issue": "12",
"published-online": {
"date-parts": [
[
2023,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
15
]
],
"date-time": "2023-12-15T00:00:00Z",
"timestamp": 1702598400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4915/15/12/2434/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "2434",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
12,
15
]
]
},
"published-online": {
"date-parts": [
[
2023,
12,
15
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1038/s41574-019-0312-5",
"article-title": "New Aspects of Vitamin D Metabolism and Action—Addressing the Skin as Source and Target",
"author": "Bikle",
"doi-asserted-by": "crossref",
"first-page": "234",
"journal-title": "Nat. Rev. Endocrinol.",
"key": "ref_1",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1210/endocr/bqaa218",
"article-title": "Vitamin D-Regulated Gene Expression Profiles: Species-Specificity and Cell-Specific Effects on Metabolism and Immunity",
"author": "Dimitrov",
"doi-asserted-by": "crossref",
"first-page": "bqaa218",
"journal-title": "Endocrinology",
"key": "ref_2",
"volume": "162",
"year": "2021"
},
{
"DOI": "10.4049/jimmunol.173.5.2909",
"article-title": "Cutting Edge: 1,25-Dihydroxyvitamin D3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "2909",
"journal-title": "J. Immunol.",
"key": "ref_3",
"volume": "173",
"year": "2004"
},
{
"DOI": "10.1096/fj.04-3284com",
"article-title": "Human Cathelicidin Antimicrobial Peptide (CAMP) Gene Is a Direct Target of the Vitamin D Receptor and Is Strongly Up-regulated in Myeloid Cells by 1,25-dihydroxyvitamin D 3",
"author": "Gombart",
"doi-asserted-by": "crossref",
"first-page": "1067",
"journal-title": "FASEB J.",
"key": "ref_4",
"volume": "19",
"year": "2005"
},
{
"DOI": "10.3390/nu14020284",
"doi-asserted-by": "crossref",
"key": "ref_5",
"unstructured": "White, J.H. (2022). Emerging Roles of Vitamin D-Induced Antimicrobial Peptides in Antiviral Innate Immunity. Nutrients, 14."
},
{
"DOI": "10.1073/pnas.1200072109",
"article-title": "Vitamin D Accelerates Resolution of Inflammatory Responses during Tuberculosis Treatment",
"author": "Coussens",
"doi-asserted-by": "crossref",
"first-page": "15449",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_6",
"volume": "109",
"year": "2012"
},
{
"DOI": "10.1038/s41598-022-22307-9",
"article-title": "Vitamin D Enhances Type I IFN Signaling in COVID-19 Patients",
"author": "Hafezi",
"doi-asserted-by": "crossref",
"first-page": "17778",
"journal-title": "Sci. Rep.",
"key": "ref_7",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3390/nu12072097",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "Charoenngam, N., and Holick, M.F. (2020). Immunologic Effects of Vitamin d on Human Health and Disease. Nutrients, 12."
},
{
"DOI": "10.3389/fnut.2021.799709",
"article-title": "Association Between Vitamin D and Influenza: Meta-Analysis and Systematic Review of Randomized Controlled Trials",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "799709",
"journal-title": "Front. Nutr.",
"key": "ref_9",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1111/j.1440-1843.2011.01924.x",
"article-title": "Vitamin D, Innate Immunity and Outcomes in Community Acquired Pneumonia",
"author": "Leow",
"doi-asserted-by": "crossref",
"first-page": "611",
"journal-title": "Respirology",
"key": "ref_10",
"volume": "16",
"year": "2011"
},
{
"DOI": "10.1016/j.clnu.2013.11.014",
"article-title": "Low Serum 25-Hydroxyvitamin D Level: An Independent Risk Factor for Tuberculosis?",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "1081",
"journal-title": "Clin. Nutr.",
"key": "ref_11",
"volume": "33",
"year": "2014"
},
{
"DOI": "10.1016/S2213-8587(21)00051-6",
"article-title": "Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials",
"author": "Jolliffe",
"doi-asserted-by": "crossref",
"first-page": "276",
"journal-title": "Lancet Diabetes Endocrinol.",
"key": "ref_12",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.jsbmb.2021.105883",
"doi-asserted-by": "crossref",
"key": "ref_13",
"unstructured": "Petrelli, F., Luciani, A., Perego, G., Dognini, G., Colombelli, P.L., and Ghidini, A. (2021). Therapeutic and prognostic role of vitamin D for COVID-19 infection: A systematic review and meta-analysis of 43 observational studies. J. Steroid Biochem. Mol. Biol., 211."
},
{
"DOI": "10.1371/journal.pmed.1003605",
"doi-asserted-by": "crossref",
"key": "ref_14",
"unstructured": "Butler-Laporte, G., Nakanishi, T., Mooser, V., Morrison, D.R., Abdullah, T., Adeleye, O., Mamlouk, N., Kimchi, N., Afrasiabi, Z., and Rezk, N. (2021). Vitamin D and COVID-19 Susceptibility and Severity in the COVID-19 Host Genetics Initiative: A Mendelian Randomization Study. PLoS Med., 18."
},
{
"DOI": "10.1016/j.arcmed.2022.04.003",
"article-title": "Efficacy and Safety of Vitamin D Supplementation to Prevent COVID-19 in Frontline Healthcare Workers. A Randomized Clinical Trial",
"doi-asserted-by": "crossref",
"first-page": "423",
"journal-title": "Arch. Med. Res.",
"key": "ref_15",
"volume": "53",
"year": "2022"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"doi-asserted-by": "crossref",
"key": "ref_16",
"unstructured": "Castillo, M.E., Costa, L.M.E., Barrios, J.M.V., Díaz, J.F.A., Miranda, J.L., Bouillon, R., and Gomez, J.M.Q. (2020). Effect of Calcifediol Treatment and Best Available Therapy versus Best Available Therapy on Intensive Care Unit Admission and Mortality among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical Study. J. Steroid Biochem. Mol. Biol., 203."
},
{
"DOI": "10.1001/jama.2020.26848",
"article-title": "Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Randomized Clinical Trial",
"author": "Murai",
"doi-asserted-by": "crossref",
"first-page": "1053",
"journal-title": "JAMA—J. Am. Med. Assoc.",
"key": "ref_17",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1136/bmj-2022-071230",
"article-title": "Effect of a Test-and-Treat Approach to Vitamin D Supplementation on Risk of All Cause Acute Respiratory Tract Infection and COVID-19: Phase 3 Randomised Controlled Trial (CORONAVIT)",
"author": "Jolliffe",
"doi-asserted-by": "crossref",
"first-page": "e071230",
"journal-title": "BMJ",
"key": "ref_18",
"volume": "378",
"year": "2022"
},
{
"DOI": "10.1016/j.clnu.2023.09.008",
"article-title": "The role of vitamin D in the prevention and treatment of SARS-CoV-2 infection: A meta-analysis of randomized controlled trials",
"author": "Meng",
"doi-asserted-by": "crossref",
"first-page": "2198",
"journal-title": "Clin. Nutr.",
"key": "ref_19",
"volume": "42",
"year": "2023"
},
{
"DOI": "10.3389/fnut.2023.1131103",
"article-title": "Effect of vitamin D supplementation on COVID-19 patients: A systematic review and meta-analysis",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "1131103",
"journal-title": "Front. Nutr.",
"key": "ref_20",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1021/acsinfecdis.1c00096",
"article-title": "Human Cathelicidin Inhibits SARS-CoV-2 Infection: Killing Two Birds with One Stone",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1545",
"journal-title": "ACS Infect. Dis.",
"key": "ref_21",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1101/2022.06.29.498158",
"doi-asserted-by": "crossref",
"key": "ref_22",
"unstructured": "Arora, J., Patel, D.R., Nicol, M.J., Field, C.J., Restori, K.H., Wang, J., Froelich, N.E., Katkere, B., Terwilliger, J.A., and Weaver, V. (2022). Vitamin D and the Ability to Produce 1,25(OH)2D Are Critical for Protection from Viral Infection of the Lungs. Nutrients, 14."
},
{
"DOI": "10.3390/pharmaceutics15030925",
"doi-asserted-by": "crossref",
"key": "ref_23",
"unstructured": "Mok, C.K., Ng, Y.L., Ahidjo, B.A., Aw, Z.Q., Chen, H., Wong, Y.H., Lee, R.C.H., Loe, M.W.C., Liu, J., and Tan, K.S. (2023). Evaluation of In Vitro and In Vivo Antiviral Activities of Vitamin D for SARS-CoV-2 and Variants. Pharmaceutics, 15."
},
{
"DOI": "10.1128/jvi.01276-21",
"article-title": "A Biosafety Level 2 Mouse Model for Studying Betacoronavirus-Induced Acute Lung Damage and Systemic Manifestations",
"author": "Andrade",
"doi-asserted-by": "crossref",
"first-page": "e0127621",
"journal-title": "J. Virol.",
"key": "ref_24",
"volume": "95",
"year": "2021"
},
{
"DOI": "10.1016/j.lfs.2023.121750",
"article-title": "Acute Coronavirus Infection Triggers a TNF-Dependent Osteoporotic Phenotype in Mice",
"author": "Santos",
"doi-asserted-by": "crossref",
"first-page": "121750",
"journal-title": "Life Sci.",
"key": "ref_25",
"volume": "324",
"year": "2023"
},
{
"DOI": "10.1042/CS20220663",
"article-title": "TNF/INOS/NO Pathway Mediates Host Susceptibility to Endothelial-Dependent Circulatory Failure and Death Induced by Betacoronavirus Infection",
"author": "Alves",
"doi-asserted-by": "crossref",
"first-page": "543",
"journal-title": "Clin. Sci.",
"key": "ref_26",
"volume": "137",
"year": "2023"
},
{
"DOI": "10.1128/MRA.00248-21",
"article-title": "Coding-Complete Genome Sequence of Murine Hepatitis Virus",
"author": "Garcia",
"doi-asserted-by": "crossref",
"first-page": "10",
"journal-title": "Microbiol. Resour. Announc.",
"key": "ref_27",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1590/0074-02760200342",
"article-title": "SARS-CoV-2 Isolation from the First Reported Patients in Brazil and Establishment of a Coordinated Task Network",
"author": "Araujo",
"doi-asserted-by": "crossref",
"first-page": "e200342",
"journal-title": "Mem. Inst. Oswaldo Cruz",
"key": "ref_28",
"volume": "115",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0015263",
"doi-asserted-by": "crossref",
"key": "ref_29",
"unstructured": "Marim, F.M., Silveira, T.N., Lima, D.S., and Zamboni, D.S. (2010). A Method for Generation of Bone Marrow-Derived Macrophages from Cryopreserved Mouse Bone Marrow Cells. PLoS ONE, 5."
},
{
"DOI": "10.1002/pros.23498",
"article-title": "Reduced Vitamin D Receptor (VDR) Expression and Plasma Vitamin D Levels Are Associated with Aging-Related Prostate Lesions",
"author": "Maria",
"doi-asserted-by": "crossref",
"first-page": "532",
"journal-title": "Prostate",
"key": "ref_30",
"volume": "78",
"year": "2018"
},
{
"DOI": "10.1016/j.tibtech.2018.12.002",
"article-title": "The Ultimate QPCR Experiment: Producing Publication Quality, Reproducible Data the First Time",
"author": "Taylor",
"doi-asserted-by": "crossref",
"first-page": "761",
"journal-title": "Trends Biotechnol.",
"key": "ref_31",
"volume": "37",
"year": "2019"
},
{
"DOI": "10.1016/j.celrep.2015.02.043",
"article-title": "Deletion of Macrophage Vitamin D Receptor Promotes Insulin Resistance and Monocyte Cholesterol Transport to Accelerate Atherosclerosis in Mice",
"author": "Oh",
"doi-asserted-by": "crossref",
"first-page": "1872",
"journal-title": "Cell Rep.",
"key": "ref_32",
"volume": "10",
"year": "2015"
},
{
"DOI": "10.1016/S2666-5247(20)30004-5",
"article-title": "Comparative Tropism, Replication Kinetics, and Cell Damage Profiling of SARS-CoV-2 and SARS-CoV with Implications for Clinical Manifestations, Transmissibility, and Laboratory Studies of COVID-19: An Observational Study",
"author": "Chu",
"doi-asserted-by": "crossref",
"first-page": "e14",
"journal-title": "Lancet Microbe",
"key": "ref_33",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1016/j.jsbmb.2019.105431",
"doi-asserted-by": "crossref",
"key": "ref_34",
"unstructured": "Maj, E., Trynda, J., Maj, B., Gębura, K., Bogunia-Kubik, K., Chodyński, M., Kutner, A., and Wietrzyk, J. (2019). Differential Response of Lung Cancer Cell Lines to Vitamin D Derivatives Depending on EGFR, KRAS, P53 Mutation Status and VDR Polymorphism. J. Steroid Biochem. Mol. Biol., 193."
},
{
"DOI": "10.1210/er.2016-1070",
"article-title": "Vitamin D-Mediated Hypercalcemia: Mechanisms, Diagnosis, and Treatment",
"author": "Tebben",
"doi-asserted-by": "crossref",
"first-page": "521",
"journal-title": "Endocr. Rev.",
"key": "ref_35",
"volume": "37",
"year": "2016"
},
{
"article-title": "Clinical Chemistry Reference Intervals for C57BL/6J, C57BL/6N, and C3HeB/FeJ Mice (Mus Musculus)",
"author": "Otto",
"first-page": "375",
"journal-title": "J. Am. Assoc. Lab. Anim. Sci.",
"key": "ref_36",
"volume": "55",
"year": "2016"
},
{
"DOI": "10.1038/s41577-020-00470-2",
"article-title": "Leukocyte Trafficking to the Lungs and beyond: Lessons from Influenza for COVID-19",
"author": "Alon",
"doi-asserted-by": "crossref",
"first-page": "49",
"journal-title": "Nat. Rev. Immunol.",
"key": "ref_37",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1007/s12250-014-3530-y",
"article-title": "Coronavirus MHV-A59 Infects the Lung and Causes Severe Pneumonia in C57BL/6 Mice",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "393",
"journal-title": "Virol. Sin.",
"key": "ref_38",
"volume": "29",
"year": "2014"
},
{
"DOI": "10.1128/JVI.00747-06",
"article-title": "MurineHepatitis Virus Strain 1 Produces a Clinically Relevant Model of Severe Acute Respiratory Syndrome in A/J Mice",
"author": "Baig",
"doi-asserted-by": "crossref",
"first-page": "10382",
"journal-title": "J. Virol.",
"key": "ref_39",
"volume": "80",
"year": "2006"
},
{
"DOI": "10.3389/fimmu.2022.1089064",
"article-title": "Neutrophils Drive Pulmonary Vascular Leakage in MHV-1 Infection of Susceptible A/J Mice",
"author": "Gong",
"doi-asserted-by": "crossref",
"first-page": "1089064",
"journal-title": "Front. Immunol.",
"key": "ref_40",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1101/2023.03.29.23287591",
"doi-asserted-by": "crossref",
"key": "ref_41",
"unstructured": "Toro, A., Arevalo, A.P., Pereira-Gómez, M., Sabater, A., Zizzi, E.A., Pascual, G., Lage-Vickers, S., Porfido, J.L., Achinelli, I., and Seniuk, R. (2023). Coronavirus Pathogenesis in Mice Explains the SARS-CoV-2 Multi-Organ Spread by Red Blood Cells Hitch-Hiking. medRxiv."
},
{
"DOI": "10.1186/s12967-020-02374-0",
"article-title": "Neutrophil-to-Lymphocyte Ratio Predicts Critical Illness Patients with 2019 Coronavirus Disease in the Early Stage",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "206",
"journal-title": "J. Transl. Med.",
"key": "ref_42",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1016/j.imlet.2013.11.008",
"article-title": "Tumor Necrosis Factor α (TNF-α) Receptor-I Is Required for TNF-α-Mediated Fulminant Virus Hepatitis Caused by Murine Hepatitis Virus Strain-3 Infection",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "25",
"journal-title": "Immunol. Lett.",
"key": "ref_43",
"volume": "158",
"year": "2014"
},
{
"DOI": "10.1016/j.cell.2020.11.025",
"article-title": "Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes",
"author": "Karki",
"doi-asserted-by": "crossref",
"first-page": "149",
"journal-title": "Cell",
"key": "ref_44",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1038/s41598-023-33484-6",
"article-title": "Evaluation of Infliximab/Tocilizumab versus Tocilizumab among COVID-19 Patients with Cytokine Storm Syndrome",
"author": "Sarhan",
"doi-asserted-by": "crossref",
"first-page": "6456",
"journal-title": "Sci. Rep.",
"key": "ref_45",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1128/JVI.02680-09",
"article-title": "Role of Mouse Hepatitis Virus (MHV) Receptor Murine CEACAM1 in the Resistance of Mice to MHV Infection: Studies of Mice with Chimeric MCEACAM1a and MCEACAM1b",
"author": "Hirai",
"doi-asserted-by": "crossref",
"first-page": "6654",
"journal-title": "J. Virol.",
"key": "ref_46",
"volume": "84",
"year": "2010"
},
{
"DOI": "10.1038/s42003-023-05414-9",
"doi-asserted-by": "crossref",
"key": "ref_47",
"unstructured": "Malireddi, R.K.S., Bynigeri, R.R., Mall, R., Connelly, J.P., Pruett-Miller, S.M., and Kanneganti, T.D. (2023). Inflammatory Cell Death, PANoptosis, Screen Identifies Host Factors in Coronavirus Innate Immune Response as Therapeutic Targets. Commun. Biol., 6."
},
{
"DOI": "10.1128/JVI.72.4.3504a-3504a.1998",
"article-title": "Fulminant Hepatic Failure in Murine Hepatitis Virus Strain 3 Infection: Tissue-Specific Expression of a Novel Fgl2 Prothrombinase",
"author": "Ding",
"doi-asserted-by": "crossref",
"first-page": "3504",
"journal-title": "J. Virol.",
"key": "ref_48",
"volume": "72",
"year": "1998"
},
{
"DOI": "10.1210/clinem/dgab892",
"article-title": "Prognostic and Therapeutic Role of Vitamin D in COVID-19: Systematic Review and Meta-Analysis",
"author": "Dissanayake",
"doi-asserted-by": "crossref",
"first-page": "1484",
"journal-title": "J. Clin. Endocrinol. Metab.",
"key": "ref_49",
"volume": "107",
"year": "2022"
},
{
"DOI": "10.1111/cea.12102",
"article-title": "Vitamin D Supplementation Reduces Airway Hyperresponsiveness and Allergic Airway Inflammation in a Murine Model",
"author": "Agrawal",
"doi-asserted-by": "crossref",
"first-page": "672",
"journal-title": "Clin. Exp. Allergy",
"key": "ref_50",
"volume": "43",
"year": "2013"
},
{
"DOI": "10.1371/journal.pone.0149180",
"doi-asserted-by": "crossref",
"key": "ref_51",
"unstructured": "Fischer, K.D., Hall, S.C., and Agrawal, D.K. (2016). Vitamin D Supplementation Reduces Induction of Epithelial-Mesenchymal Transition in Allergen Sensitized and Challenged Mice. PLoS ONE, 11."
},
{
"DOI": "10.1007/s10565-021-09629-6",
"article-title": "Vitamin D3 Decreases TNF-α-Induced Inflammation in Lung Epithelial Cells through a Reduction in Mitochondrial Fission and Mitophagy",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "427",
"journal-title": "Cell Biol. Toxicol.",
"key": "ref_52",
"volume": "38",
"year": "2022"
},
{
"DOI": "10.1152/japplphysiol.00643.2015",
"article-title": "Vitamin D3 Intake Modulates Diaphragm but Not Peripheral Muscle Force in Young Mice",
"author": "Ray",
"doi-asserted-by": "crossref",
"first-page": "1124",
"journal-title": "J. Appl. Physiol.",
"key": "ref_53",
"volume": "120",
"year": "2016"
},
{
"DOI": "10.18632/oncotarget.6493",
"article-title": "Diet-Derived 25-Hydroxyvitamin D3 Activates Vitamin D Receptor Target Gene Expression and Suppresses EGFR Mutant Non-Small Cell Lung Cancer Growth in Vitro and in Vivo",
"author": "Shoemaker",
"doi-asserted-by": "crossref",
"first-page": "995",
"journal-title": "Oncotarget",
"key": "ref_54",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1021/acs.jmedchem.9b00208",
"article-title": "Vitamin D and Its Synthetic Analogs",
"author": "Maestro",
"doi-asserted-by": "crossref",
"first-page": "6854",
"journal-title": "J. Med. Chem.",
"key": "ref_55",
"volume": "62",
"year": "2019"
},
{
"DOI": "10.4049/jimmunol.182.2.1099",
"article-title": "Type I IFN-Mediated Protection of Macrophages and Dendritic Cells Secures Control of Murine Coronavirus Infection",
"author": "Kalinke",
"doi-asserted-by": "crossref",
"first-page": "1099",
"journal-title": "J. Immunol.",
"key": "ref_56",
"volume": "182",
"year": "2009"
},
{
"DOI": "10.1128/JVI.01241-21",
"article-title": "Distinct Roles of Type I and Type III Interferons during a Native Murine β Coronavirus Lung Infection",
"author": "Sharma",
"doi-asserted-by": "crossref",
"first-page": "e0124121",
"journal-title": "J. Virol.",
"key": "ref_57",
"volume": "96",
"year": "2022"
},
{
"DOI": "10.1126/science.abd4585",
"article-title": "Autoantibodies against Type I IFNs in Patients with Life-Threatening COVID-19",
"author": "Bastard",
"doi-asserted-by": "crossref",
"first-page": "eabd4585",
"journal-title": "Science",
"key": "ref_58",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1126/science.abd4570",
"article-title": "Inborn Errors of Type I IFN Immunity in Patients with Life-Threatening COVID-19",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "eabd4570",
"journal-title": "Science",
"key": "ref_59",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.04.026",
"article-title": "Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "1036",
"journal-title": "Cell",
"key": "ref_60",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1126/science.abc6027",
"article-title": "Impaired Type I Interferon Activity and Inflammatory Responses in Severe COVID-19 Patients",
"author": "Hadjadj",
"doi-asserted-by": "crossref",
"first-page": "718",
"journal-title": "Science",
"key": "ref_61",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1126/science.abb3221",
"article-title": "Substantial Undocumented Infection Facilitates the Rapid Dissemination of Novel Coronavirus (SARS-CoV-2)",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "489",
"journal-title": "Science",
"key": "ref_62",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1016/j.it.2021.02.003",
"article-title": "Dual Nature of Type I Interferons in SARS-CoV-2-Induced Inflammation",
"author": "King",
"doi-asserted-by": "crossref",
"first-page": "312",
"journal-title": "Trends Immunol.",
"key": "ref_63",
"volume": "42",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2588-y",
"article-title": "Longitudinal Analyses Reveal Immunological Misfiring in Severe COVID-19",
"author": "Lucas",
"doi-asserted-by": "crossref",
"first-page": "463",
"journal-title": "Nature",
"key": "ref_64",
"volume": "584",
"year": "2021"
},
{
"DOI": "10.1177/1753466620933508",
"article-title": "Lung under Attack by COVID-19-Induced Cytokine Storm: Pathogenic Mechanisms and Therapeutic Impications",
"author": "Pelaia",
"doi-asserted-by": "crossref",
"first-page": "1753466620933508",
"journal-title": "Ther. Adv. Respir. Dis.",
"key": "ref_65",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1074/jbc.RA120.015036",
"article-title": "Impaired NLRP3 Inflammasome Activation/Pyroptosis Leads to Robust Inflammatory Cell Death via Caspase-8/RIPK3 during Coronavirus Infection",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "14040",
"journal-title": "J. Biol. Chem.",
"key": "ref_66",
"volume": "295",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-25015-6",
"article-title": "SARS-CoV-2 N Protein Promotes NLRP3 Inflammasome Activation to Induce Hyperinflammation",
"author": "Pan",
"doi-asserted-by": "crossref",
"first-page": "4664",
"journal-title": "Nat. Commun.",
"key": "ref_67",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2021.103803",
"doi-asserted-by": "crossref",
"key": "ref_68",
"unstructured": "Zeng, J., Xie, X., Feng, X.L., Xu, L., Han, J.B., Yu, D., Zou, Q.C., Liu, Q., Li, X., and Ma, G. (2022). Specific Inhibition of the NLRP3 Inflammasome Suppresses Immune Overactivation and Alleviates COVID-19 like Pathology in Mice. eBioMedicine, 75."
},
{
"DOI": "10.3390/v12080880",
"doi-asserted-by": "crossref",
"key": "ref_69",
"unstructured": "Körner, R.W., Majjouti, M., Alejandre Alcazar, M.A., and Mahabir, E. (2020). Of Mice and Men: The Coronavirus Mhv and Mouse Models as a Translational Approach to Understand Sars-Cov-2. Viruses, 12."
},
{
"DOI": "10.1038/s41430-020-0558-y",
"article-title": "Vitamin D deficiency 2.0: An update on the current status worldwide",
"author": "Amrein",
"doi-asserted-by": "crossref",
"first-page": "1498",
"journal-title": "Eur. J. Clin. Nutr.",
"key": "ref_70",
"volume": "74",
"year": "2020"
},
{
"DOI": "10.1038/s41467-022-30195-w",
"article-title": "SARS-CoV-2 infection induces inflammatory bone loss in golden Syrian hamsters",
"author": "Qiao",
"doi-asserted-by": "crossref",
"first-page": "2539",
"journal-title": "Nat. Commun.",
"key": "ref_71",
"volume": "13",
"year": "2022"
}
],
"reference-count": 71,
"references-count": 71,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4915/15/12/2434"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Infectious Diseases"
],
"subtitle": [],
"title": "Dietary Vitamin D Mitigates Coronavirus-Induced Lung Inflammation and Damage in Mice",
"type": "journal-article",
"volume": "15"
}
