Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and covid-19: phase 3 randomised controlled trial (CORONAVIT)
et al., BMJ, doi:10.1136/bmj-2022-071230, CORONAVIT, NCT04579640, Mar 2022 (preprint)
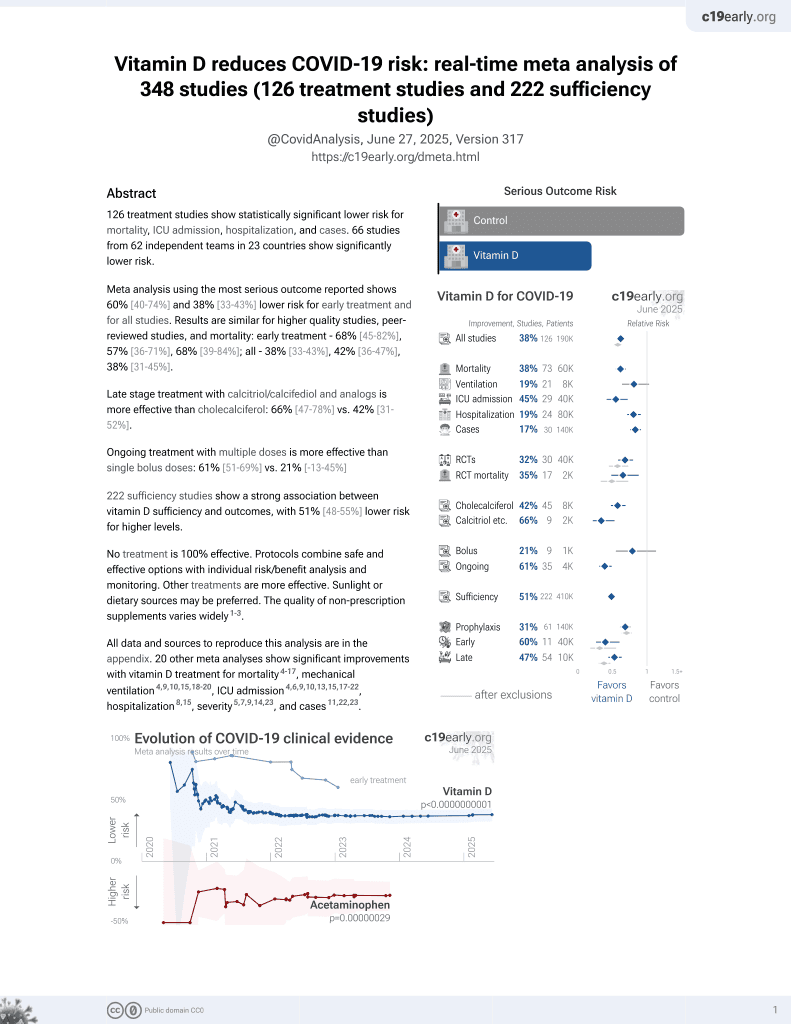
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 5,979 low risk patients (0 COVID-19 deaths) in the UK,
showing no significant differences with vitamin D prophylaxis. CORONAVIT.
NCT04579640 (history). For more discussion see1,2.
51% of confirmed COVID-19 cases were hospitalized in the
control arm which is 7 times the median rate in other studies reporting both
cases and hospitalization as of Sep 2022 (7.2%), suggesting possible issues
with the data or major differences between the study population and the
general population.
Authors do not provide exact start/end dates (month only) or
specify when infections occurred, however based on cases in the UK, most
infections may have been closer to the start of the trial when vitamin D
levels may still have been relatively low. Reportedly, authors do not plan to
analyze this issue, and have declined to allow one of the funders access to
the data.
3 present an RCT showing conflicting
results, 78% lower cases with vitamin D prophylaxis. In comparison,3 used a higher dose, the participants had much higher
exposure to SARS-CoV-2 patients, and the study was prior to vaccination. In
this study, 89% of participants had received a vaccine dose by the end of the
study period, and the period overlapped with increasing solar UVB.
For other limitations and concerns see4.
This is the 14th of 40 COVID-19 RCTs for vitamin D, which collectively show efficacy with p=0.0000001.
This is the 76th of 136 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments5.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
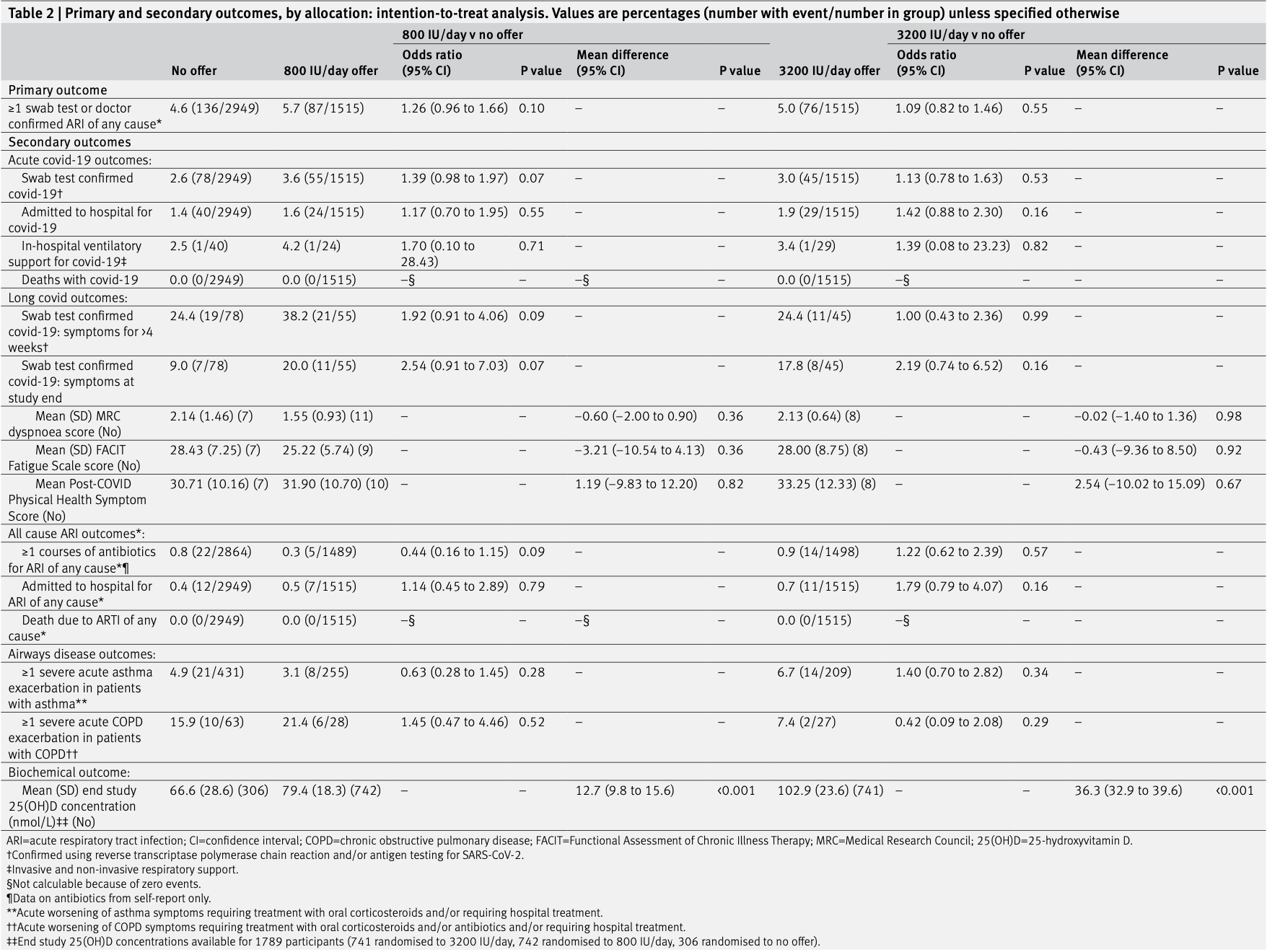
risk of mechanical ventilation, 94.7% higher, RR 1.95, p = 1.00, treatment 1 of 1,515 (0.1%), control 1 of 2,949 (0.0%), 3200IU/day.
|
|
risk of mechanical ventilation, 94.7% higher, RR 1.95, p = 1.00, treatment 1 of 1,515 (0.1%), control 1 of 2,949 (0.0%), 800IU/day.
|
|
risk of hospitalization, 41.1% higher, RR 1.41, p = 0.16, treatment 29 of 1,515 (1.9%), control 40 of 2,949 (1.4%), 3200IU/day.
|
|
risk of hospitalization, 16.8% higher, RR 1.17, p = 0.60, treatment 24 of 1,515 (1.6%), control 40 of 2,949 (1.4%), 800IU/day.
|
|
risk of case, 8.8% higher, RR 1.09, p = 0.55, treatment 76 of 1,515 (5.0%), control 136 of 2,949 (4.6%), 3200IU/day.
|
|
risk of case, 24.5% higher, RR 1.25, p = 0.11, treatment 87 of 1,515 (5.7%), control 136 of 2,949 (4.6%), 800IU/day.
|
|
risk of case, 12.3% higher, RR 1.12, p = 0.56, treatment 45 of 1,515 (3.0%), control 78 of 2,949 (2.6%), confirmed, 3200IU/day.
|
|
risk of case, 37.3% higher, RR 1.37, p = 0.08, treatment 55 of 1,515 (3.6%), control 78 of 2,949 (2.6%), confirmed, 800IU/day.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
3.
Villasis-Keever et al., Efficacy and Safety of Vitamin D Supplementation to Prevent COVID-19 in Frontline Healthcare Workers. A Randomized Clinical Trial, Archives of Medical Research, doi:10.1016/j.arcmed.2022.04.003.
Jolliffe et al., 23 Mar 2022, Randomized Controlled Trial, United Kingdom, peer-reviewed, median age 60.2, 25 authors, study period December 2020 - June 2021, dosage 3,200IU daily, daily, trial NCT04579640 (history) (CORONAVIT).
Contact: d.a.jolliffe@qmul.ac.uk, a.martineau@qmul.ac.uk.
Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and covid-19: phase 3 randomised controlled trial (CORONAVIT)
BMJ, doi:10.1136/bmj-2022-071230
Objective To determine the effect of population level implementation of a test-and-treat approach to correction of suboptimal vitamin D status (25-hydroxyvitamin D (25(OH)D) <75 nmol/L) on risk of all cause acute respiratory tract infection and covid 19.
Design Phase 3 open label randomised controlled trial. setting United Kingdom. ParticiPants 6200 people aged ≥16 years who were not taking vitamin D supplements at baseline.
interventiOns Offer of a postal finger prick test of blood 25(OH)D concentration with provision of a six month supply of lower dose vitamin D (800 IU/day, n=1550) or higher dose vitamin D (3200 IU/day, n=1550) to those with blood 25(OH)D concentration <75 nmol/L, compared with no offer of testing or supplementation (n=3100). Follow-up was for six months.
Main OutcOMe Measures The primary outcome was the proportion of participants with at least one swab test or doctor confirmed acute respiratory tract infection of any cause. A secondary outcome was the proportion of participants with swab test confirmed covid-19. Logistic regression was used to calculate odds ratios and associated 95% confidence intervals. The primary analysis was conducted by intention to treat.
results Of 3100 participants offered a vitamin D test, 2958 (95.4%) accepted and 2674 (86.3%) had 25(OH) D concentrations <75 nmol/L and received vitamin D supplements (n=1328 lower dose, n=1346 higher dose). Compared with 136/2949 (4.6%) participants in the no offer group, at least one acute respiratory tract infection of any cause occurred in 87/1515 (5.7%) in the lower dose group (odds ratio 1.26, 95% confidence interval 0.96 to 1.66) and 76/1515 (5.0%) in the higher dose group (1.09, 0.82 to 1.46). Compared with 78/2949 (2.6%) participants in the no offer group, 55/1515 (3.6%) developed covid-19 in the lower dose group (1.39, 0.98 to 1.97) and 45/1515 (3.0%) in the higher dose group (1.13, 0.78 to 1.63). cOnclusiOns Among people aged 16 years and older with a high baseline prevalence of suboptimal vitamin D status, implementation of a population level test-and-treat approach to vitamin D supplementation was not associated with a reduction in risk of all cause acute respiratory tract infection or covid-19. trial registratiOn ClinicalTrials.gov NCT04579640.
Contributors: ARM, DAJ, and CR designed the study, with input from PP, JS, DF, RAL, GAD, FK, CJG, JN, AS, SEF, AGR, and SOS. DAJ, HH, NP, SM, MT, and ARM managed the trial. AN, NLB, and RG performed the laboratory assays. DAJ, MG, MT, GV, and CO managed and analysed the data. DAJ and ARM contributed equally and are the guarantors. All the authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. ARM wrote the first draft of the paper. All authors contributed to the interpretation of the results, review and approval of the manuscript, and the decision to submit it for publication. There were no agreements concerning confidentiality of the data between the sponsor and the authors or the institutions named in the credit lines. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. Funding:
References
Bergman, Norlin, Hansen, Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study, BMJ Open, doi:10.1136/bmjopen-2012-001663
Bhatnagar, Wickramasinghe, Wilkins, Townsend, Trends in the epidemiology of cardiovascular disease in the UK, Heart, doi:10.1136/heartjnl-2016-309573
Bishop, Ismailova, Dimeloe, Hewison, White, Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory, JBMR Plus, doi:10.1002/jbm4.10405
Breiman, Random Forests, Mach Learn, doi:10.1023/A:1010933404324
Butler-Laporte, Nakanishi, Mooser, Vitamin D and COVID-19 susceptibility and severity in the COVID-19 Host Genetics Initiative: A Mendelian randomization study, PLoS Med, doi:10.1371/journal.pmed.1003605
Camargo, Sluyter, Stewart, Effect of monthly high-dose vitamin D supplementation on acute respiratory infections in older adults: A randomized controlled trial, Clin Infect Dis, doi:10.1093/cid/ciz801
Chauss, Freiwald, Mcgregor, Autocrine vitamin D signaling switches off pro-inflammatory programs of T H 1 cells, Nat Immunol, doi:10.1038/s41590-021-01080-3
Chiodini, Gatti, Soranna, Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes, Front Public Health, doi:10.3389/fpubh.2021.736665
Dawson-Hughes, Heaney, Holick, Lips, Meunier et al., Estimates of optimal vitamin D status, Osteoporos Int, doi:10.1007/s00198-005-1867-7
Dissanayake, Silva, Sumanatilleke, Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia, J Clin Endocrinol Metab, doi:10.1210/clinem/dgab892on13
Dror, Eisenbach, Taiber, Vaccine hesitancy: the next challenge in the fight against COVID-19, Eur J Epidemiol, doi:10.1007/s10654-020-00671-y
Dunnett, A multiple comparison procedure for comparing several treatments with a control, J Am Stat Assoc, doi:10.1080/01621459.1955.10501294
Fletcher, Standardised questionnaire on respiratory symptoms: a statement prepared and approved by the MRC Committee on the Aetiology of Chronic Bronchitis (MRC breathlessness score), BMJ
Ganmaa, Uyanga, Zhou, Vitamin D Supplements for Prevention of Tuberculosis Infection and Disease, N Engl J Med, doi:10.1056/NEJMoa1915176
Grayling, Wason, A web application for the design of multi-arm clinical trials, BMC Cancer, doi:10.1186/s12885-020-6525-0
Greiller, Martineau, Modulation of the immune response to respiratory viruses by vitamin D, Nutrients, doi:10.3390/nu7064240
Ha, The 25-hydroxyvitamin D threshold for better health, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2006.12.016
Holt, Relton, Talaei, Cohort Profile: Longitudinal population-based study of COVID-19 in UK adults, doi:10.1101/2022.06.20.22276205
Holt, Talaei, Greenig, Risk factors for developing COVID-19: a population-based longitudinal study (COVIDENCE UK), Thorax, doi:10.1136/thoraxjnl-2021-217487
Howarth, Munro, Theodorou, Mills, Trends in healthcare utilisation during COVID-19: a longitudinal study from the UK, BMJ Open, doi:10.1136/bmjopen-2020-048151
Ilahi, Armas, Heaney, Pharmacokinetics of a single, large dose of cholecalciferol, Am J Clin Nutr, doi:10.1093/ajcn/87.3.688
Jolliffe, Cajr, Sluyter, Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587(21)00051-6
Liu, Meigs, Pittas, Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study, Am J Clin Nutr, doi:10.3945/ajcn.2009.28441
Louca, Murray, Klaser, Modest effects of dietary supplements during the COVID-19 pandemic: insights from 445 850 users of the COVID-19 Symptom Study app, BMJ Nutr Prev Health, doi:10.1136/bmjnph-2021-000250
Ma, Zhou, Heianza, Qi, Habitual use of vitamin D supplements and risk of coronavirus disease 2019 (COVID-19) infection: a prospective study in UK Biobank, Am J Clin Nutr, doi:10.1093/ajcn/nqaa381
Martineau, Hanifa, Witt, Double-blind randomised controlled trial of vitamin D3 supplementation for the prevention of acute respiratory infection in older adults and their carers (ViDiFlu), Thorax, doi:10.1136/thoraxjnl-2015-206996
Martineau, Jolliffe, Hooper, Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, BMJ, doi:10.1136/bmj.i6583
Merzon, Tworowski, Gorohovski, Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study, FEBS J, doi:10.1111/febs.15495
Moynihan, Sanders, Michaleff, Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review, BMJ Open, doi:10.1136/bmjopen-2020-045343
Pham, Waterhouse, Baxter, The effect of vitamin D supplementation on acute respiratory tract infection in older Australian adults: an analysis of data from the D-Health Trial, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587(20)30380-6
Raisi-Estabragh, Mccracken, Bethell, Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK Biobank, J Public Health (Oxf), doi:10.1093/pubmed/fdaa095
Redlberger-Fritz, Kundi, Aberle, Puchhammer-Stöckl, Significant impact of nationwide SARS-CoV-2 lockdown measures on the circulation of other respiratory virus infections in Austria, J Clin Virol, doi:10.1016/j.jcv.2021.104795
Relton, Torgerson, 'cathain, Nicholl, Rethinking pragmatic randomised controlled trials: introducing the "cohort multiple randomised controlled trial" design, BMJ, doi:10.1136/bmj.c1066
Roth, Lütke, Meinberger, LL-37 fights SARS-CoV-2: The Vitamin D-Inducible Peptide LL-37 Inhibits Binding of SARS-CoV-2 Spike Protein to its Cellular Receptor Angiotensin Converting Enzyme 2 In Vitro, BioRxiv, doi:10.1101/2020.12.02.408153
Shea, Berg, Self-administration of vitamin D supplements in the general public may be associated with high 25-hydroxyvitamin D concentrations, Ann Clin Biochem, doi:10.1177/0004563216662073
Talaei, Faustini, Holt, Determinants of pre-vaccination antibody responses to SARS-CoV-2: a population-based longitudinal study (COVIDENCE UK), BMC Med, doi:10.1186/s12916-022-02286-4
Taylor, Trivedi, Patel, Post-COVID symptoms reported at asynchronous virtual review and stratified follow-up after COVID-19 pneumonia, Clin Med (Lond), doi:10.7861/clinmed.2021-0037
Vickerstaff, Omar, Ambler, Methods to adjust for multiple comparisons in the analysis and sample size calculation of randomised controlled trials with multiple primary outcomes, BMC Med Res Methodol, doi:10.1186/s12874-019-0754-4
Vieth, What is the optimal vitamin D status for health?, Prog Biophys Mol Biol, doi:10.1016/j.pbiomolbio.2006.02.003
Villasis-Keever, López-Alarcón, Miranda-Novales, Efficacy and Safety of Vitamin D Supplementation to Prevent COVID-19 in Frontline Healthcare Workers. A Randomized Clinical Trial, Arch Med Res, doi:10.1016/j.arcmed.2022.04.003
Williams, Burgers, SARS-CoV-2 evolution and vaccines: cause for concern?, Lancet Respir Med, doi:10.1016/S2213-2600(21)00075-8
Williamson, Tydeman, Miners, Acute and long-term impacts of COVID-19 on economic vulnerability: a populationbased longitudinal study, doi:10.1101/2022.03.03.22271835
Yellen, Cella, Webster, Blendowski, Kaplan, Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system, J Pain Symptom Manage, doi:10.1016/S0885-3924(96)00274-6
Zhang, Ghosh, Basavarajappa, Molecular dynamics simulations and functional studies reveal that hBD-2 binds SARS-CoV-2 spike RBD and blocks viral entry into ACE2 expressing cells, bioRxiv, doi:10.1101/2021.01.07.425621
DOI record:
{
"DOI": "10.1136/bmj-2022-071230",
"ISSN": [
"1756-1833"
],
"URL": "http://dx.doi.org/10.1136/bmj-2022-071230",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Objective</jats:title>\n <jats:p>To determine the effect of population level implementation of a test-and-treat approach to correction of suboptimal vitamin D status (25-hydroxyvitamin D (25(OH)D) <75 nmol/L) on risk of all cause acute respiratory tract infection and covid 19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Design</jats:title>\n <jats:p>Phase 3 open label randomised controlled trial.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Setting</jats:title>\n <jats:p>United Kingdom.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Participants</jats:title>\n <jats:p>6200 people aged ≥16 years who were not taking vitamin D supplements at baseline.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Interventions</jats:title>\n <jats:p>Offer of a postal finger prick test of blood 25(OH)D concentration with provision of a six month supply of lower dose vitamin D (800 IU/day, n=1550) or higher dose vitamin D (3200 IU/day, n=1550) to those with blood 25(OH)D concentration <75 nmol/L, compared with no offer of testing or supplementation (n=3100). Follow-up was for six months.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Main outcome measures</jats:title>\n <jats:p>The primary outcome was the proportion of participants with at least one swab test or doctor confirmed acute respiratory tract infection of any cause. A secondary outcome was the proportion of participants with swab test confirmed covid-19. Logistic regression was used to calculate odds ratios and associated 95% confidence intervals. The primary analysis was conducted by intention to treat.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Of 3100 participants offered a vitamin D test, 2958 (95.4%) accepted and 2674 (86.3%) had 25(OH)D concentrations <75 nmol/L and received vitamin D supplements (n=1328 lower dose, n=1346 higher dose). Compared with 136/2949 (4.6%) participants in the no offer group, at least one acute respiratory tract infection of any cause occurred in 87/1515 (5.7%) in the lower dose group (odds ratio 1.26, 95% confidence interval 0.96 to 1.66) and 76/1515 (5.0%) in the higher dose group (1.09, 0.82 to 1.46). Compared with 78/2949 (2.6%) participants in the no offer group, 55/1515 (3.6%) developed covid-19 in the lower dose group (1.39, 0.98 to 1.97) and 45/1515 (3.0%) in the higher dose group (1.13, 0.78 to 1.63).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Among people aged 16 years and older with a high baseline prevalence of suboptimal vitamin D status, implementation of a population level test-and-treat approach to vitamin D supplementation was not associated with a reduction in risk of all cause acute respiratory tract infection or covid-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Trial registration</jats:title>\n <jats:p>\n ClinicalTrials.gov\n <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" xlink:href=\"NCT04579640\" ext-link-type=\"clintrialgov\">NCT04579640</jats:ext-link>\n .\n </jats:p>\n </jats:sec>",
"alternative-id": [
"10.1136/bmj-2022-071230"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-3592-1945",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jolliffe",
"given": "David A",
"sequence": "first"
},
{
"affiliation": [],
"family": "Holt",
"given": "Hayley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Greenig",
"given": "Matthew",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6901-3665",
"affiliation": [],
"authenticated-orcid": false,
"family": "Talaei",
"given": "Mohammad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perdek",
"given": "Natalia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0369-2885",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pfeffer",
"given": "Paul",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0816-9276",
"affiliation": [],
"authenticated-orcid": false,
"family": "Vivaldi",
"given": "Giulia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maltby",
"given": "Sheena",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2535-4545",
"affiliation": [],
"authenticated-orcid": false,
"family": "Symons",
"given": "Jane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barlow",
"given": "Nicola L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Normandale",
"given": "Alexa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcha",
"given": "Rajvinder",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2885-1299",
"affiliation": [],
"authenticated-orcid": false,
"family": "Richter",
"given": "Alex G",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9300-5569",
"affiliation": [],
"authenticated-orcid": false,
"family": "Faustini",
"given": "Sian E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Orton",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ford",
"given": "David",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5225-000X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lyons",
"given": "Ronan A",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1218-1008",
"affiliation": [],
"authenticated-orcid": false,
"family": "Davies",
"given": "Gwyneth A",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0606-8167",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kee",
"given": "Frank",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Griffiths",
"given": "Christopher J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Norrie",
"given": "John",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7022-3056",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sheikh",
"given": "Aziz",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7273-8691",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shaheen",
"given": "Seif O",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Relton",
"given": "Clare",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5387-1721",
"affiliation": [],
"authenticated-orcid": false,
"family": "Martineau",
"given": "Adrian R",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04579640",
"registry": "10.18810/clinical-trials-gov"
},
{
"clinical-trial-number": "nct04330599",
"registry": "10.18810/clinical-trials-gov"
},
{
"clinical-trial-number": "nct04641195",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "BMJ",
"container-title-short": "BMJ",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2022,
9,
8
]
],
"date-time": "2022-09-08T00:00:47Z",
"timestamp": 1662595247000
},
"deposited": {
"date-parts": [
[
2022,
9,
8
]
],
"date-time": "2022-09-08T04:00:59Z",
"timestamp": 1662609659000
},
"funder": [
{
"DOI": "10.13039/100015652",
"doi-asserted-by": "publisher",
"name": "Barts Charity"
}
],
"indexed": {
"date-parts": [
[
2022,
9,
12
]
],
"date-time": "2022-09-12T20:27:26Z",
"timestamp": 1663014446868
},
"is-referenced-by-count": 2,
"issued": {
"date-parts": [
[
2022,
9,
7
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
7
]
],
"date-time": "2022-09-07T00:00:00Z",
"timestamp": 1662508800000
}
}
],
"link": [
{
"URL": "http://data.bmj.org/tdm/10.1136/bmj-2022-071230",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmj-2022-071230",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e071230",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2022,
9,
7
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
7
]
]
},
"publisher": "BMJ",
"reference": [
{
"DOI": "10.1016/S0140-6736(21)00306-8",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.1"
},
{
"DOI": "10.1007/s10654-020-00671-y",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.2"
},
{
"DOI": "10.1016/S2213-2600(21)00075-8",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.3"
},
{
"DOI": "10.3390/nu7064240",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.4"
},
{
"DOI": "10.1002/jbm4.10405",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.5"
},
{
"DOI": "10.1038/s41590-021-01080-3",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.6"
},
{
"DOI": "10.1101/2020.12.02.408153",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.7"
},
{
"DOI": "10.1101/2021.01.07.425621",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.8"
},
{
"DOI": "10.1111/febs.15495",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.9"
},
{
"DOI": "10.1093/pubmed/fdaa095",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.10"
},
{
"DOI": "10.1093/ajcn/nqaa381",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.11"
},
{
"DOI": "10.1136/thoraxjnl-2021-217487",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.12"
},
{
"DOI": "10.1186/s12916-022-02286-4",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.13"
},
{
"DOI": "10.1136/bmjnph-2021-000250",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.14"
},
{
"DOI": "10.3389/fpubh.2021.736665",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.15"
},
{
"DOI": "10.1210/clinem/dgab892",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.16"
},
{
"DOI": "10.1542/peds.2011-3029",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.17"
},
{
"DOI": "10.1136/bmjopen-2012-001663",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.18"
},
{
"DOI": "10.1136/thoraxjnl-2015-206996",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.19"
},
{
"DOI": "10.1056/NEJMoa1915176",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.20"
},
{
"DOI": "10.1016/S2213-8587(20)30380-6",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.21"
},
{
"DOI": "10.1016/S2213-8587(21)00051-6",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.22"
},
{
"DOI": "10.1016/j.arcmed.2022.04.003",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.23"
},
{
"DOI": "10.1136/bmj.c1066",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.24"
},
{
"DOI": "10.1101/2022.03.03.22271835",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.25"
},
{
"key": "2022090721000803000_378.sep07_6.e071230.26"
},
{
"DOI": "10.1177/0004563216662073",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.27"
},
{
"DOI": "10.1007/s00198-005-1867-7",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.28"
},
{
"DOI": "10.1016/j.pbiomolbio.2006.02.003",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.29"
},
{
"DOI": "10.1016/j.jsbmb.2006.12.016",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.30"
},
{
"DOI": "10.1136/bmj.2.5213.1665",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.31"
},
{
"DOI": "10.1016/S0885-3924(96)00274-6",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.32"
},
{
"DOI": "10.7861/clinmed.2021-0037",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.33"
},
{
"DOI": "10.1186/s12885-020-6525-0",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.34"
},
{
"DOI": "10.1186/s12874-019-0754-4",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.35"
},
{
"DOI": "10.1080/01621459.1955.10501294",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.36"
},
{
"DOI": "10.3945/ajcn.2009.28441",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.37"
},
{
"DOI": "10.1023/A:1010933404324",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.38"
},
{
"DOI": "10.1093/cid/ciz801",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.39"
},
{
"DOI": "10.1371/journal.pmed.1003605",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.40"
},
{
"DOI": "10.1093/ajcn/87.3.688",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.41"
},
{
"DOI": "10.1136/bmj.i6583",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.42"
},
{
"DOI": "10.1016/j.jcv.2021.104795",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.43"
},
{
"DOI": "10.1136/bmjopen-2020-048151",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.44"
},
{
"DOI": "10.1136/bmjopen-2020-045343",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.45"
},
{
"DOI": "10.1016/S2213-8587(21)00263-1",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.46"
},
{
"key": "2022090721000803000_378.sep07_6.e071230.47",
"unstructured": "Office for National Statistics. Overview of the UK population: August 2019. 2019"
},
{
"DOI": "10.1136/heartjnl-2016-309573",
"doi-asserted-by": "publisher",
"key": "2022090721000803000_378.sep07_6.e071230.48"
}
],
"reference-count": 48,
"references-count": 48,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.bmj.com/lookup/doi/10.1136/bmj-2022-071230"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Engineering"
],
"subtitle": [],
"title": "Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and covid-19: phase 3 randomised controlled trial (CORONAVIT)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy"
}