Calcitriol modifies tight junctions, improves barrier function, and reduces TNF‐α‐induced barrier leak in the human lung‐derived epithelial cell culture model, 16HBE 14o‐
et al., Physiological Reports, doi:10.14814/phy2.15592, Apr 2023
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study showing that calcitriol improved barrier function in human airway epithelial cells. Authors note that this mechanism could explain in part the efficacy of vitamin D seen for COVID-19 and other airway diseases.
29 preclinical studies support the efficacy of vitamin D for COVID-19:
Vitamin D has been identified by the European Food Safety Authority (EFSA) as having sufficient evidence for a causal relationship between intake and optimal immune system function27-30.
Vitamin D inhibits SARS-CoV-2 replication in vitro17,24, mitigates lung inflammation, damage, and lethality in mice with an MHV-3 model for β-CoV respiratory infections17,24, reduces SARS-CoV-2 replication in nasal epithelial cells via increased type I interferon expression20, downregulates proinflammatory cytokines IL-1β and TNF-α in SARS-CoV-2 spike protein-stimulated cells16, attenuates nucleocapsid protein-induced hyperinflammation by inactivating the NLRP3 inflammasome through the VDR-BRCC3 signaling pathway21, may be neuroprotective by protecting the blood-brain barrier, reducing neuroinflammation, and via immunomodulatory effects31, may mitigate hyperinflammation and cytokine storm by upregulating TLR10 expression which downregulates proinflammatory cytokines13, downregulates ACE2 and TMPRSS2 in human trophoblasts and minimizes spike protein-induced inflammation19, may minimize cytokine storm by dampening excessive cytokine production2, may suppress viral entry and replication via LL-37 induction11,12, and minimizes platelet aggregation mediated by SARS-CoV-2 spike protein via inhibiting integrin αIIbβ3 outside-in signaling15.
Cholecalciferol and calcifediol directly bind two allosteric pockets on the SARS-CoV-2 Spike RBD, bias the trimer toward a closed state, weaken ACE2 engagement, and reduce viral entry in cell models1.
Calcitriol may destabilize the Spike protein architecture and inhibit IL-17R dimerization, blocking viral entry and mitigating hyperinflammatory cytokine storm32.
Vitamin D improves regulatory immune cell levels and control of proinflammatory cytokines in severe COVID-1933.
Calcifediol inhibits SARS-CoV-2 papain-like protease (PLpro), a critical enzyme for viral replication14.
Symptomatic COVID-19 is associated with a lower frequency of natural killer (NK) cells and vitamin D has been shown to improve NK cell activity34,35.
1.
García-Marín et al., Exploring SARS-CoV-2 Spike RBD Pockets as Targets for Generic Drugs: A Combined Computational, Biophysical, and Biological Approach, ACS Omega, doi:10.1021/acsomega.5c05175.
2.
Alzahrani, A., A new investigation into the molecular mechanism of cholecalciferol towards reducing cytokines storm, Octahedron Drug Research, doi:10.21608/odr.2024.308273.1043.
3.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
4.
Morales-Bayuelo et al., New findings on ligand series used as SARS-CoV-2 virus inhibitors within the frameworks of molecular docking, molecular quantum similarity and chemical reactivity indices, F1000Research, doi:10.12688/f1000research.123550.3.
5.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
6.
Pandya et al., Unravelling Vitamin B12 as a potential inhibitor against SARS-CoV-2: A computational approach, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2022.100951.
7.
Mansouri et al., The impact of calcitriol and estradiol on the SARS-CoV-2 biological activity: a molecular modeling approach, Scientific Reports, doi:10.1038/s41598-022-04778-y.
8.
Song et al., Vitamin D3 and its hydroxyderivatives as promising drugs against COVID-19: a computational study, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1964601.
9.
Qayyum et al., Vitamin D and lumisterol novel metabolites can inhibit SARS-CoV-2 replication machinery enzymes, Endocrinology and Metabolism, doi:10.1152/ajpendo.00174.2021.
10.
Al-Mazaideh et al., Vitamin D is a New Promising Inhibitor to the Main Protease (Mpro) of COVID-19 by Molecular Docking, Journal of Pharmaceutical Research International, doi:10.9734/jpri/2021/v33i29B31603.
11.
Roth et al., Vitamin D-inducible antimicrobial peptide LL-37 binds SARS-CoV-2 Spike and accessory proteins ORF7a and ORF8, Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2025.1671738.
12.
Vercellino et al., Influence of Sex and 1,25α Dihydroxyvitamin D3 on SARS-CoV-2 Infection and Viral Entry, Pathogens, doi:10.3390/pathogens14080765.
13.
Knez et al., TLR10 overexpression modulates immune response in A549 lung epithelial cells challenged with SARS-CoV-2 S and N proteins, Frontiers in Immunology, doi:10.3389/fimmu.2024.1490478.
14.
Chen et al., In Vitro Characterization of Inhibition Function of Calcifediol to the Protease Activity of SARS-COV-2 PLpro, Journal of Medical Virology, doi:10.1002/jmv.70085.
15.
Wang et al., 1,25‐Dihydroxyvitamin D3 attenuates platelet aggregation potentiated by SARS‐CoV‐2 spike protein via inhibiting integrin αIIbβ3 outside‐in signaling, Cell Biochemistry and Function, doi:10.1002/cbf.4039.
16.
Alcalá-Santiago et al., Disentangling the Immunomodulatory Effects of Vitamin D on the SARS-CoV-2 Virus by In Vitro Approaches, The 14th European Nutrition Conference FENS 2023, doi:10.3390/proceedings2023091415.
17.
Campolina-Silva et al., Dietary Vitamin D Mitigates Coronavirus-Induced Lung Inflammation and Damage in Mice, Viruses, doi:10.3390/v15122434.
18.
Moatasim et al., Potent Antiviral Activity of Vitamin B12 against Severe Acute Respiratory Syndrome Coronavirus 2, Middle East Respiratory Syndrome Coronavirus, and Human Coronavirus 229E, Microorganisms, doi:10.3390/microorganisms11112777.
19.
Vargas-Castro et al., Calcitriol prevents SARS-CoV spike-induced inflammation in human trophoblasts through downregulating ACE2 and TMPRSS2 expression, The Journal of Steroid Biochemistry and Molecular Biology, doi:10.1016/j.jsbmb.2024.106625.
20.
Sposito et al., Age differential CD13 and interferon expression in airway epithelia affect SARS-CoV-2 infection - effects of vitamin D, Mucosal Immunology, doi:10.1016/j.mucimm.2023.08.002.
21.
Chen (B) et al., Vitamin D3 attenuates SARS‐CoV‐2 nucleocapsid protein‐caused hyperinflammation by inactivating the NLRP3 inflammasome through the VDR‐BRCC3 signaling pathway in vitro and in vivo, MedComm, doi:10.1002/mco2.318.
22.
Rybakovsky et al., Calcitriol modifies tight junctions, improves barrier function, and reduces TNF‐α‐induced barrier leak in the human lung‐derived epithelial cell culture model, 16HBE 14o‐, Physiological Reports, doi:10.14814/phy2.15592.
23.
DiGuilio et al., The multiphasic TNF-α-induced compromise of Calu-3 airway epithelial barrier function, Experimental Lung Research, doi:10.1080/01902148.2023.2193637.
24.
Pickard et al., Discovery of re-purposed drugs that slow SARS-CoV-2 replication in human cells, PLOS Pathogens, doi:10.1371/journal.ppat.1009840.
25.
Mok et al., Calcitriol, the active form of vitamin D, is a promising candidate for COVID-19 prophylaxis, bioRxiv, doi:10.1101/2020.06.21.162396.
26.
Fernandes de Souza et al., Lung Inflammation Induced by Inactivated SARS-CoV-2 in C57BL/6 Female Mice Is Controlled by Intranasal Instillation of Vitamin D, Cells, doi:10.3390/cells12071092.
27.
Galmés et al., Suboptimal Consumption of Relevant Immune System Micronutrients Is Associated with a Worse Impact of COVID-19 in Spanish Populations, Nutrients, doi:10.3390/nu14112254.
28.
Galmés (B) et al., Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework, Nutrients, doi:10.3390/nu12092738.
29.
EFSA, Scientific Opinion on the substantiation of a health claim related to vitamin D and contribution to the normal function of the immune system pursuant to Article 14 of Regulation (EC) No 1924/2006, EFSA Journal, doi:10.2903/j.efsa.2015.4096.
30.
EFSA (B), Scientific Opinion on the substantiation of health claims related to vitamin D and normal function of the immune system and inflammatory response (ID 154, 159), maintenance of normal muscle function (ID 155) and maintenance of normal cardiovascular function (ID 159) pursuant to Article 13(1) of Regulation (E, EFSA Journal, doi:10.2903/j.efsa.2010.1468.
31.
Gotelli et al., Understanding the immune-endocrine effects of vitamin D in SARS-CoV-2 infection: a role in protecting against neurodamage?, Neuroimmunomodulation, doi:10.1159/000533286.
32.
Fadel et al., Targeting asparagine and cysteine in SARS-CoV-2 variants and human pro-inflammatory mediators to alleviate COVID-19 severity; a cross-section and in-silico study, Scientific Reports, doi:10.1038/s41598-025-19359-y.
33.
Saheb Sharif-Askari et al., Increased blood immune regulatory cells in severe COVID-19 with autoantibodies to type I interferons, Scientific Reports, doi:10.1038/s41598-023-43675-w.
Rybakovsky et al., 11 Apr 2023, peer-reviewed, 7 authors.
Contact: mullinj@mlhs.org.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Calcitriol modifies tight junctions, improves barrier function, and reduces TNF‐α‐induced barrier leak in the human lung‐derived epithelial cell culture model, 16HBE 14o‐
Physiological Reports, doi:10.14814/phy2.15592
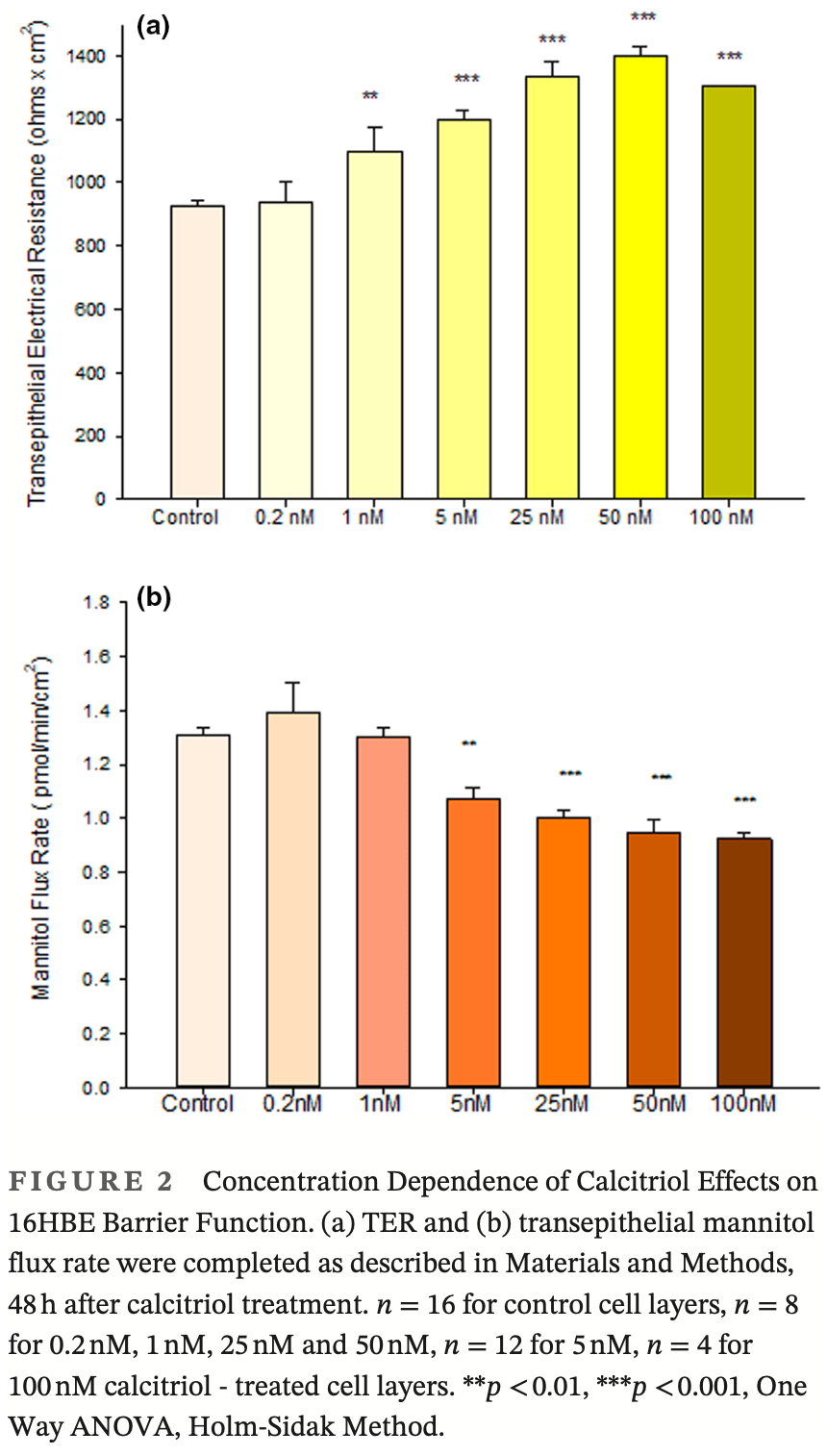
Using the 16HBE 14o-human airway epithelial cell culture model, calcitriol (Vitamin D) was shown to improve barrier function by two independent metrics -increased transepithelial electrical resistance (TER) and reduced transepithelial diffusion of 14 C-D-mannitol (J m ). Both effects were concentration dependent and active out to 168 h post-treatment. Barrier improvement associated with changes in the abundance of specific tight junctional (TJ) proteins in detergent-soluble fractions, most notably decreased claudin-2. TNF-αinduced compromise of barrier function could be attenuated by calcitriol with a concentration dependence similar to that observed for improvement of control barrier function. TNFαinduced increases in claudin-2 were partially reversed by calcitriol. The ERK 1,2 inhibitor, U0126, itself improved 16HBE barrier function indicating MAPK pathway regulation of 16HBE barrier function. Calcitriol's action was additive to the effect of U0126 in reducing TNF-α -induced barrier compromise, suggesting that calcitriol may be acting through a non-ERK pathway in its blunting of TNF-α -induced barrier compromise. This was supported by calcitriol being without effect on pERK levels elevated by the action of TNF-α. Lack of effect of TNF-α on the death marker, caspase-3, and the inability of calcitriol to decrease the elevated LC3B II level caused by TNF-α, suggest that calcitriol's barrier improvement does not involve a cell death pathway. Calcitriol's improvement of control barrier function was not additive to barrier improvement induced by retinoic acid (Vitamin A). Calcitriol improvement and protection of airway barrier function could in part explain Vitamin D's reported clinical efficacy in COVID-19 and other airway diseases.
AUTHOR CONTRIBUTIONS
ACKNOWLEDGMENTS The authors are very grateful to Ms. Terri Olshefski and Ms. Elene Mironidis of the Editorial Office of the Lankenau Institute for Medical Research for their work in formatting and editing our manuscript for publication. The assistance of Ms. Elizabeth Newberry in proofreading is also gratefully acknowledged. We are very thankful to Dr. Mazen Hassan and Ms. Kari Heller for their help in obtaining needed reference material.
FUNDING INFORMATION Financial support for this research came in part from a research grant from the Sharpe-Strumia Research Foundation (JMM) and NIH grant AI139392 (RNH).
ETHICAL STATEMENT This Study used neither animal nor human subjects. All science was conducted in an ethical manner.
References
Aggarwal, Suzuki, Taylor, Bhargava, Rao, Contrasting effects of ERK on tight junction integrity in differentiated and under-differentiated Caco-2 cell monolayers, The Biochemical Journal, doi:10.1042/BJ20100249
Alenmyr, Uller, Greiff, Högestätt, Zygmunt, TRPV4-mediated calcium influx and ciliary activity in human native airway epithelial cells, Basic & Clinical Pharmacology & Toxicology, doi:10.1111/bcpt.12135
Amoozadeh, Dan, Xiao, Waheed, Szászi, Tumor necrosis factor-α induces a biphasic change in claudin-2 expression in tubular epithelial cells: Role in barrier functions, American Journal of Physiology. Cell Physiology, doi:10.1152/ajpcell.00388.2014
Anand, Kaul, Vitamin D3-dependent pathway regulates TACO gene transcription, Biochemical and Biophysical Research Communications, doi:10.1016/j.bbrc.2003.09.087
Barbin, Roisin, Zalc, Tumor necrosis factor alpha activates the phosphorylation of ERK, SAPK/JNK, and P38 kinase in primary cultures of neurons, Neurochemical Research, doi:10.1023/a:1011086426652
Brenner, Schöttker, Vitamin D insufficiency may account for almost nine of ten COVID-19 deaths: Time to act. Comment on: "vitamin D deficiency and outcome of COVID-19 patients, Nutrients, doi:10.3390/nu12123642
Bücker, Zakrzewski, Wiegand, Pieper, Fromm et al., Zinc prevents intestinal epithelial barrier dysfunction induced by alphahemolysin-producing Escherichia coli 536 infection in porcine colon, Veterinary Microbiology, doi:10.1016/j.vetmic.2020.108632
Callaghan, Rybakovsky, Ferrick, Thomas, Mullin, Retinoic acid improves baseline barrier function and attenuates TNF-α-induced barrier leak in human bronchial epithelial cell culture model, 16HBE 14o, PLoS One, doi:10.1371/journal.pone.0242536
Cantorna, Snyder, Arora, Vitamin a and vitamin D regulate the microbial complexity, barrier function and the mucosal immune responses to insure intestinal homeostasis, Critical Reviews in Biochemistry and Molecular Biology
Castillo, Entrenas Costa, Vaquero Barrios, Alcalá Díaz, López Miranda et al., Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, The Journal of Steroid Biochemistry and Molecular Biology, doi:10.1016/j.jsbmb.2020.105751
Chen, Lu, Zhang, Sun, Vitamin D receptor deletion leads to the destruction of tight and Adherens junctions in lungs, Tissue Barriers, doi:10.1080/21688370.2018.1540904
Chowdhury, Howat, Phillips, Lackie, Interactions between endothelial cells and epithelial cells in a combined cell model of airway mucosa: Effects on tight junction permeability, Experimental Lung Research, doi:10.3109/01902140903026582
Colpitts, Baumert, Claudins in viral infection: From entry to spread, Pflügers Archiv, doi:10.1007/s00424-016-1908-4
Cozens, Yezzi, Kunzelmann, Ohrui, Chin et al., CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells, American Journal of Respiratory Cell and Molecular Biology, doi:10.1165/ajrcmb.10.1.7507342
Daneshkhah, Agrawal, Eshein, Subramanian, Roy et al., Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients, Aging Clinical and Experimental Research, doi:10.1007/s40520-020-01677-y
Diguilio, Rybakovsky, Abdavies, Chamoun, Flounders et al., Micronutrient improvement of epithelial barrier function in various disease states: A case for adjuvant therapy, International Journal of Molecular Sciences, doi:10.3390/ijms23062995
Droessler, Cornelius, Boehm, Stein, Brunner et al., Barrier perturbation in porcine Peyer's patches by tumor necrosis factor is associated with a dysregulation of Claudins, Frontiers in Physiology, doi:10.3389/fphys.2022.889552
Durgan, Tao, Walters, Florey, Schmidt et al., SOS1 and Ras regulate epithelial tight junction formation in the human airway through EMP1, EMBO Reports, doi:10.15252/embr.201439218
Fernandez-Robredo, González-Zamora, Recalde, Bilbao-Malavé, Bezunartea et al., Vitamin D protects against oxidative stress and inflammation in human retinal cells, Antioxidants, doi:10.3390/antiox9090838
Furuse, Sasaki, Tsukita, Manner of interaction of heterogeneous claudin species within and between tight junction strands, The Journal of Cell Biology, doi:10.1083/jcb.147.4.891
Gitter, Bendfeldt, Schulzke, Fromm, Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis, The FASEB Journal, doi:10.1096/fj.99-0898com
Groeger, Meyle, Epithelial barrier and oral bacterial infection, Periodontology, doi:10.1111/prd.12094
Guttman, Finlay, Tight junctions as targets of infectious agents, Biochimica et Biophysica Acta, doi:10.1016/j.bbamem.2008.10.028
Haws, Krouse, Xia, Gruenert, Wine, CFTR channels in immortalized human airway cells, The American Journal of Physiology, doi:10.1152/ajplung.1992.263.6.L692
Hayashi, Okamatsu, Ogasawara, Tsugawa, Isoda et al., Oral supplementation of the vitamin D metabolite 25(OH)D 3 against influenza virus infection in mice, Nutrients
Infante, Buoso, Pieri, Lupisella, Nuccetelli et al., Low vitamin D status at admission as a risk factor for poor survival in hospitalized patients with COVID-10: An Italian retrospective study, Journal of the American College of Nutrition, doi:10.1080/07315724.2021.1877580
Inoue, Akimoto, Homma, Tanaka, Sagara, Airway epithelial dysfunction in asthma: Relevant to epidermal growth factor receptors and airway epithelial cells, Journal of Clinical Medicine, doi:10.3390/jcm9113698
Katz, Yue, Xue, Increased risk for COVID-19 in patients with vitamin D deficiency, Nutrition, doi:10.1016/j.nut.2020.111106
Khare, Godbole, Pawar, Mohan, Pandey et al., Calcitriol [1, 25[OH]2 D3] pre-and post-treatment suppresses inflammatory response to influenza a (H1N1) infection in human lung A549 epithelial cells, European Journal of Nutrition, doi:10.1007/s00394-012-0449-7
Krug, Schulzke, Fromm, Tight junction, selective permeability, and related diseases, Seminars in Cell & Developmental Biology, doi:10.1016/j.semcdb.2014.09.002
Langberg, Rotem, Fenig, Koren, Ravid, Vitamin D protects keratinocytes from deleterious effects of ionizing radiation, The British Journal of Dermatology, doi:10.1111/j.1365-2133.2008.08797.x
Lee, Lau, Chusilp, Filler, Li et al., Protective effects of vitamin D against injury in intestinal epithelium, Pediatric Surgery International, doi:10.1007/s00383-019-04586-y
Li, Dong, Zhao, Song, Tang et al., 1,25-Dihydroxyvitamin D3 prevents toluene diisocyanate-induced airway epithelial barrier disruption, International Journal of Molecular Medicine, doi:10.3892/ijmm.2015.2214
Liu, Sun, Wang, Zhang, Zhao et al., Low vitamin D status is associated with coronavirus disease 2019 outcomes: A systematic review and meta-analysis, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.12.077
Lyu, Zhang, Dai, Xiang, Li et al., Calcitriol inhibits apoptosis via activation of autophagy in hyperosmotic stress stimulated corneal epithelial cells in vivo and in vitro, Experimental Eye Research, doi:10.1016/j.exer.2020.108210
Ma, Ende, Alvarado, Christensen, Kalish et al., Topical vitamin D may modulate human Sinonasal mucosal responses to house dust mite antigen, American Journal of Rhinology & Allergy, doi:10.1177/1945892420905432
Meckel, Li, Lim, Kocherginsky, Weber et al., Serum 25-hydroxyvitamin D concentration is inversely associated with mucosal inflammation in patients with ulcerative colitis, The American Journal of Clinical Nutrition, doi:10.3945/ajcn.115.123786
Merzon, Tworowski, Gorohovski, Vinker, Golan Cohen et al., Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study, The FEBS Journal, doi:10.1111/febs.15495
Mohanty, Kamolvit, Hertting, Brauner, Vitamin D strengthens the bladder epithelial barrier by inducing tight junction proteins during E. coli urinary tract infection, Cell and Tissue Research, doi:10.1007/s00441-019-03162-z
Mullin, Agostino, Rendon-Huerta, Thornton, Keynote review: Epithelial and endothelial barriers in human disease, Drug Discovery Today, doi:10.1016/S1359-6446(05)03379-9
Mullin, Leatherman, Valenzano, Huerta, Verrechio et al., Ras mutation impairs epithelial barrier function to a wide range of nonelectrolytes, Molecular Biology of the Cell, doi:10.1091/mbc.e05-04-0294
Munshi, Hussein, Toraih, Elshazli, Jardak et al., Vitamin D insufficiency as a potential culprit in critical COVID-19 patients, Journal of Medical Virology, doi:10.1002/jmv.26360
Murdaca, Pioggia, Negrini, Vitamin D and Covid-19: An update on evidence and potential therapeutic implications, Clinical and Molecular Allergy, doi:10.1186/s12948-020-00139-0
Nelson, Powell, Price, Hersom, Yelich et al., Assessment of serum 25-hydroxyvitamin D concentrations of beef cows and calves across seasons and geographical locations, Journal of Animal Science, doi:10.2527/jas.2016-0611
Ohaegbulam, Swalih, Patel, Smith, Perrin, Vitamin D supplementation in COVID-19 patients: A clinical case series, American Journal of Therapeutics, doi:10.1097/MJT.0000000000001222
Pan, Borovac, Spicer, Hoenderop, Bindels et al., The epithelial sodium/proton exchanger, NHE3, is necessary for renal and intestinal calcium (re)absorption, American Journal of Physiology. Renal Physiology
Peralta Soler, Mullin, Knudsen, Marano, Tissue remodeling during tumor necrosis factorinduced apoptosis in LLC-PK1 renal epithelial cells, The American Journal of Physiology, doi:10.1152/ajprenal.1996.270.5.F869
Petecchia, Sabatini, Usai, Caci, Varesio et al., Cytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ERK1/2-pathway, Laboratory Investigation, doi:10.1038/labinvest.2012.67
Randolph, Simon, Characterization of retinol metabolism in cultured human epidermal keratinocytes, The Journal of Biological Chemistry
Rosenthal, Günzel, Theune, Czichos, Schulzke et al., Water channels and barriers formed by claudins, Annals of the New York Academy of Sciences, doi:10.1111/nyas.13383
Rybakovsky, Diguilio, Geagan, Pham, Harty et al., Calcitriol modifies tight junctions, improves barrier function, and reduces TNF-α-induced barrier leak in the human lungderived epithelial cell culture model, 16HBE 14o, Physiological Reports
Sabetta, Depetrillo, Cipriani, Smardin, Burns et al., Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults, PLoS One, doi:10.1371/journal.pone.0011088
Sawada, Tight junction-related human diseases, Pathology International, doi:10.1111/pin.12021
Schlingmann, Overgaard, Molina, Lynn, Mitchell et al., Regulation of claudin/ zonula occludens-1 complexes by hetero-claudin interactions, Nature Communications, doi:10.1038/ncomms12276
Sekiyama, Gon, Terakado, Takeshita, Kozu et al., Glucocorticoids enhance airway epithelial barrier integrity, International Immunopharmacology, doi:10.1016/j.intimp.2011.12.006
Shashikanth, France, Xiao, Haest, Rizzo et al., Tight junction channel regulation by interclaudin interference, Nature Communications, doi:10.1038/s41467-022-31587-8
Shintani, Maruoka, Gon, Koyama, Yoshida et al., Nuclear factor erythroid 2-related factor 2 (Nrf2) regulates airway epithelial barrier integrity, Allergology International, doi:10.1016/j.alit.2015.06.004
Sonoda, Furuse, Sasaki, Yonemura, Katahira et al., Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier, The Journal of Cell Biology, doi:10.1083/jcb.147.1.195
Surman, Penkert, Jones, Sealy, Hurwitz, Vitamin supplementation at the time of immunization with a cold-adapted influenza virus vaccine corrects poor mucosal antibody responses in mice deficient for vitamins a and D, Clinical and Vaccine Immunology, doi:10.1128/CVI.00739-15
Sweerus, Lachowicz-Scroggins, Gordon, Lafemina, Huang et al., Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma, The Journal of Allergy and Clinical Immunology, doi:10.1016/j.jaci.2016.02.035
Torres-Flores, Arias, Tight junctions go viral!, Viruses, doi:10.3390/v7092865
Uddin, Seumois, Lau, Rytila, Davies et al., Enhancement of neutrophil function by the bronchial epithelium stimulated by epidermal growth factor, The European Respiratory Journal, doi:10.1183/09031936.00144307
Valenzano, Diguilio, Mercado, Teter, To et al., Remodeling of tight junctions and enhancement of barrier integrity of the CACO-2 intestinal epithelial cell layer by micronutrients, PLoS One
Vargas-Robles, Castro-Ochoa, Citalán-Madrid, Schnoor, Beneficial effects of nutritional supplements on intestinal epithelial barrier functions in experimental colitis models, World Journal of Gastroenterology, doi:10.3748/wjg.v25.i30.4181
Wine, Finkbeiner, Haws, Krouse, Moon et al., CFTR and other Clchannels in human airway cells, The Japanese Journal of Physiology
Xatzipsalti, Papadopoulos, Cellular and animals models for rhinovirus infection in asthma, Contributions to Microbiology, doi:10.1159/000107053
Xiong, Zhou, Liu, Yi, Wang et al., 1α,25-Dihydroxyvitamin D3 promotes angiogenesis by alleviating AGEs-induced autophagy, Archives of Biochemistry and Biophysics, doi:10.1016/j.abb.2021.109041
Yamada, Kanda, Retinoic acid promotes barrier functions in human iPSC-derived intestinal epithelial monolayers, Journal of Pharmacological Sciences, doi:10.1016/j.jphs.2019.06.012
Zhu, Rogers, Burke-Gaffney, Hellewell, Jeffery, Cytokine-induced airway epithelial ICAM-1 upregulation: Quantification by high-resolution scanning and transmission electron microscopy, The European Respiratory Journal, doi:10.1183/09031936.99.13613299
DOI record:
{
"DOI": "10.14814/phy2.15592",
"ISSN": [
"2051-817X",
"2051-817X"
],
"URL": "http://dx.doi.org/10.14814/phy2.15592",
"alternative-id": [
"10.14814/phy2.15592"
],
"author": [
{
"affiliation": [
{
"name": "The Lankenau Institute for Medical Research Wynnewood Pennsylvania USA"
}
],
"family": "Rybakovsky",
"given": "Elizabeth",
"sequence": "first"
},
{
"affiliation": [
{
"name": "The Lankenau Institute for Medical Research Wynnewood Pennsylvania USA"
}
],
"family": "DiGuilio",
"given": "Katherine M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Lankenau Institute for Medical Research Wynnewood Pennsylvania USA"
}
],
"family": "Valenzano",
"given": "Mary Carmen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biology Drexel University Philadelphia Pennsylvania USA"
}
],
"family": "Geagan",
"given": "Sophie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biology Drexel University Philadelphia Pennsylvania USA"
}
],
"family": "Pham",
"given": "Kaithlyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathobiology and Microbiology University of Pennsylvania, School of Veterinary Medicine Philadelphia Pennsylvania USA"
}
],
"family": "Harty",
"given": "Ronald N.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5285-7530",
"affiliation": [
{
"name": "The Lankenau Institute for Medical Research Wynnewood Pennsylvania USA"
}
],
"authenticated-orcid": false,
"family": "Mullin",
"given": "James M.",
"sequence": "additional"
}
],
"container-title": "Physiological Reports",
"container-title-short": "Physiological Reports",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
4,
11
]
],
"date-time": "2023-04-11T08:05:35Z",
"timestamp": 1681200335000
},
"deposited": {
"date-parts": [
[
2023,
4,
11
]
],
"date-time": "2023-04-11T08:05:40Z",
"timestamp": 1681200340000
},
"funder": [
{
"DOI": "10.13039/100000002",
"award": [
"AI139392"
],
"doi-asserted-by": "publisher",
"name": "National Institutes of Health"
}
],
"indexed": {
"date-parts": [
[
2023,
4,
12
]
],
"date-time": "2023-04-12T04:33:35Z",
"timestamp": 1681274015254
},
"is-referenced-by-count": 0,
"issue": "7",
"issued": {
"date-parts": [
[
2023,
4
]
]
},
"journal-issue": {
"issue": "7",
"published-print": {
"date-parts": [
[
2023,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 10,
"start": {
"date-parts": [
[
2023,
4,
11
]
],
"date-time": "2023-04-11T00:00:00Z",
"timestamp": 1681171200000
}
}
],
"link": [
{
"URL": "https://physoc.onlinelibrary.wiley.com/doi/pdf/10.14814/phy2.15592",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.14814",
"published": {
"date-parts": [
[
2023,
4
]
]
},
"published-online": {
"date-parts": [
[
2023,
4,
11
]
]
},
"published-print": {
"date-parts": [
[
2023,
4
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1042/BJ20100249",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_2_1"
},
{
"DOI": "10.1111/bcpt.12135",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_3_1"
},
{
"DOI": "10.1152/ajpcell.00388.2014",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_4_1"
},
{
"DOI": "10.1016/j.bbrc.2003.09.087",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_5_1"
},
{
"DOI": "10.1023/a:1011086426652",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_6_1"
},
{
"DOI": "10.3390/nu12123642",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_7_1"
},
{
"DOI": "10.1016/j.vetmic.2020.108632",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_8_1"
},
{
"DOI": "10.1371/journal.pone.0242536",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_9_1"
},
{
"DOI": "10.1080/10409238.2019.1611734",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_10_1"
},
{
"DOI": "10.1080/21688370.2018.1540904",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_11_1"
},
{
"DOI": "10.3109/01902140903026582",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_12_1"
},
{
"DOI": "10.1007/s00424‐016‐1908‐4",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_13_1"
},
{
"DOI": "10.1165/ajrcmb.10.1.7507342",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_14_1"
},
{
"DOI": "10.1007/s40520‐020‐01677‐y",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_15_1"
},
{
"DOI": "10.3390/ijms23062995",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_16_1"
},
{
"DOI": "10.3389/fphys.2022.889552",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_17_1"
},
{
"DOI": "10.15252/embr.201439218",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_18_1"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_19_1"
},
{
"DOI": "10.3390/antiox9090838",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_20_1"
},
{
"DOI": "10.1083/jcb.147.4.891",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_21_1"
},
{
"DOI": "10.1096/fj.99‐0898com",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_22_1"
},
{
"DOI": "10.1111/prd.12094",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_23_1"
},
{
"DOI": "10.1016/j.bbamem.2008.10.028",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_24_1"
},
{
"DOI": "10.1152/ajplung.1992.263.6.L692",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_25_1"
},
{
"DOI": "10.3390/nu12072000",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_26_1"
},
{
"DOI": "10.1080/07315724.2021.1877580",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_27_1"
},
{
"DOI": "10.3390/jcm9113698",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_28_1"
},
{
"DOI": "10.1016/j.nut.2020.111106",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_29_1"
},
{
"DOI": "10.1007/s00394‐012‐0449‐7",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_30_1"
},
{
"DOI": "10.1016/j.semcdb.2014.09.002",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_31_1"
},
{
"DOI": "10.1111/j.1365‐2133.2008.08797.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_32_1"
},
{
"DOI": "10.1007/s00383‐019‐04586‐y",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_33_1"
},
{
"DOI": "10.3892/ijmm.2015.2214",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_34_1"
},
{
"DOI": "10.1016/j.ijid.2020.12.077",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_35_1"
},
{
"DOI": "10.1016/j.exer.2020.108210",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_36_1"
},
{
"DOI": "10.1177/1945892420905432",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_37_1"
},
{
"DOI": "10.3945/ajcn.115.123786",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_38_1"
},
{
"DOI": "10.1111/febs.15495",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_39_1"
},
{
"DOI": "10.1007/s00441‐019‐03162‐z",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_40_1"
},
{
"DOI": "10.1016/S1359‐6446(05)03379‐9",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_41_1"
},
{
"DOI": "10.1091/mbc.e05‐04‐0294",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_42_1"
},
{
"DOI": "10.1002/jmv.26360",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_43_1"
},
{
"DOI": "10.1186/s12948‐020‐00139‐0",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_44_1"
},
{
"DOI": "10.2527/jas.2016‐0611",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_45_1"
},
{
"DOI": "10.1097/MJT.0000000000001222",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_46_1"
},
{
"DOI": "10.1152/ajprenal.00504.2010",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_47_1"
},
{
"DOI": "10.1152/ajprenal.1996.270.5.F869",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_48_1"
},
{
"DOI": "10.1038/labinvest.2012.67",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_49_1"
},
{
"DOI": "10.1016/S0021-9258(18)98336-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_50_1"
},
{
"DOI": "10.1111/nyas.13383",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_51_1"
},
{
"DOI": "10.1371/journal.pone.0011088",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_52_1"
},
{
"DOI": "10.1111/pin.12021",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_53_1"
},
{
"DOI": "10.1038/ncomms12276",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_54_1"
},
{
"DOI": "10.1016/j.intimp.2011.12.006",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_55_1"
},
{
"DOI": "10.1038/s41467‐022‐31587‐8",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_56_1"
},
{
"DOI": "10.1016/j.alit.2015.06.004",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_57_1"
},
{
"DOI": "10.1083/jcb.147.1.195",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_58_1"
},
{
"DOI": "10.1128/CVI.00739‐15",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_59_1"
},
{
"DOI": "10.1016/j.jaci.2016.02.035",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_60_1"
},
{
"DOI": "10.3390/v7092865",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_61_1"
},
{
"DOI": "10.1183/09031936.00144307",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_62_1"
},
{
"DOI": "10.1371/journal.pone.0133926",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_63_1"
},
{
"DOI": "10.3748/wjg.v25.i30.4181",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_64_1"
},
{
"article-title": "CFTR and other Cl‐channels in human airway cells",
"author": "Wine J. J.",
"first-page": "S199",
"issue": "2",
"journal-title": "The Japanese Journal of Physiology",
"key": "e_1_2_9_65_1",
"volume": "44",
"year": "1994"
},
{
"DOI": "10.1159/000107053",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_66_1"
},
{
"DOI": "10.1016/j.abb.2021.109041",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_67_1"
},
{
"DOI": "10.1016/j.jphs.2019.06.012",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_68_1"
},
{
"DOI": "10.1183/09031936.99.13613299",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_69_1"
}
],
"reference-count": 68,
"references-count": 68,
"relation": {},
"resource": {
"primary": {
"URL": "https://physoc.onlinelibrary.wiley.com/doi/10.14814/phy2.15592"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Physiology (medical)",
"Physiology"
],
"subtitle": [],
"title": "Calcitriol modifies tight junctions, improves barrier function, and reduces\n <scp>TNF‐α‐induced</scp>\n barrier leak in the human\n <scp>lung‐derived</scp>\n epithelial cell culture model,\n <scp>16HBE</scp>\n 14o‐",
"type": "journal-article",
"volume": "11"
}
