High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: Multicentre randomized controlled clinical trial
et al., PLOS ONE, doi:10.1371/journal.pone.0267918, CARED, NCT04411446, May 2022
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
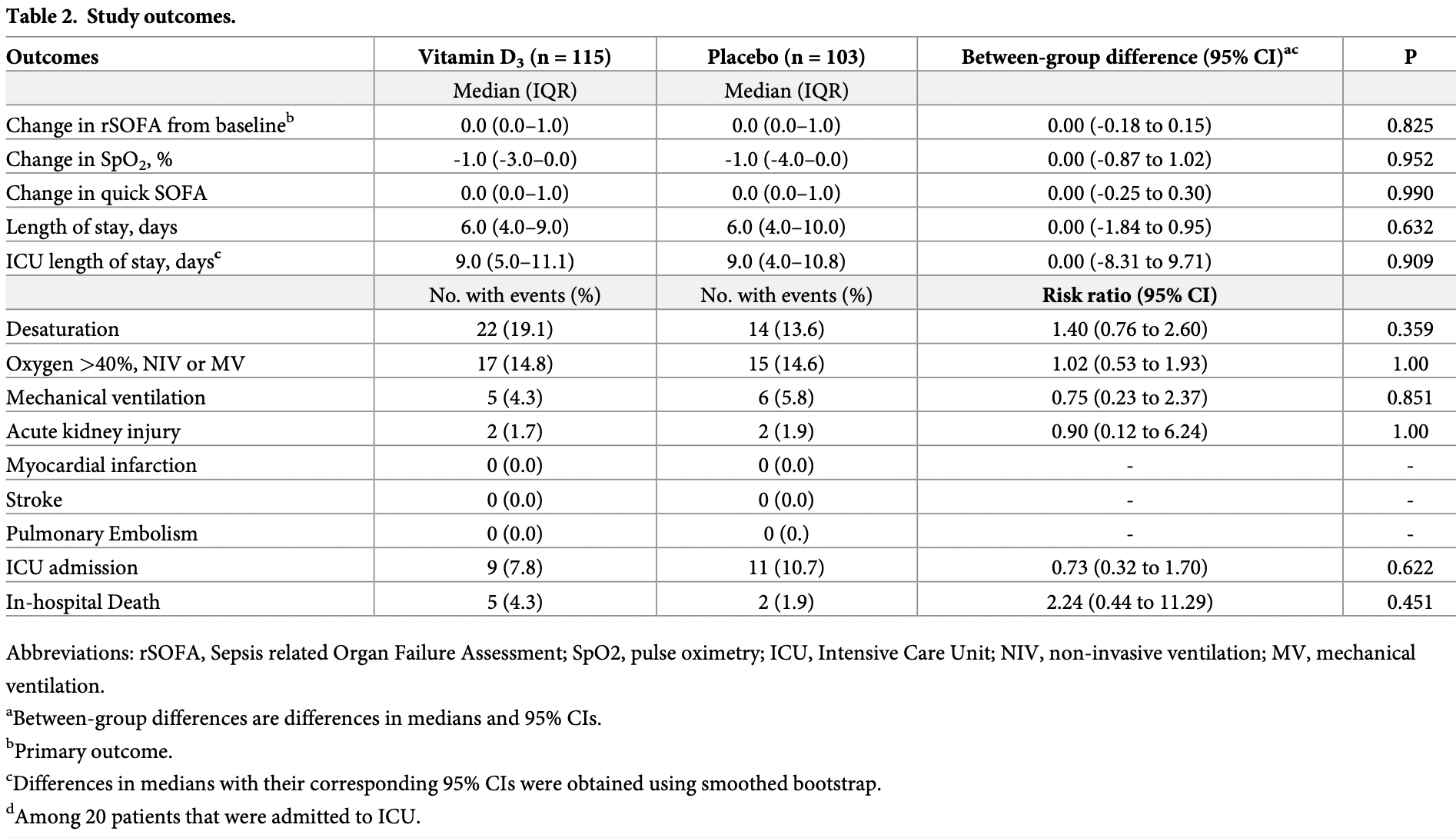
Late stage RCT with 115 patients treated with a single dose of 500,000IU cholecalciferol and 103 placebo patients, showing no significant differences. Authors do not explain why they did very late treatment with cholecalciferol instead of calcifediol or calcitriol, which would avoid several days delay in conversion. Baseline vitamin D levels were relatively high, limiting the potential benefit.
Cholecalciferol was used in this study.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 44% [33‑53%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
Bolus treatment is less effective.
Pharmacokinetics and the potential side effects of high bolus doses suggest
that ongoing treatment spread over time is more appropriate.
Research has confirmed that lower dose regular treatment with vitamin D is more
effective than intermittent high-dose bolus treatment for various conditions,
including rickets and acute respiratory infections1,2. The biological mechanisms supporting these
findings involve the induction of enzymes such as 24-hydroxylase and
fibroblast growth factor 23 (FGF23) by high-dose bolus treatments. These
enzymes play roles in inactivating vitamin D, which can paradoxically reduce
levels of activated vitamin D and suppress its activation for extended periods
post-dosage. Evidence indicates that 24-hydroxylase activity may remain
elevated for several weeks following a bolus dose, leading to reduced levels
of the activated form of vitamin D. Additionally, FGF23 levels can increase
for at least three months after a large bolus dose, which also contributes to
the suppression of vitamin D activation1.
This is the 18th of 40 COVID-19 RCTs for vitamin D, which collectively show efficacy with p=0.0000001.
This is the 85th of 136 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
|
risk of death, 124.0% higher, RR 2.24, p = 0.45, treatment 5 of 115 (4.3%), control 2 of 103 (1.9%).
|
|
risk of mechanical ventilation, 25.0% lower, RR 0.75, p = 0.85, treatment 5 of 115 (4.3%), control 6 of 103 (5.8%), NNT 68.
|
|
risk of ICU admission, 27.0% lower, RR 0.73, p = 0.62, treatment 9 of 115 (7.8%), control 11 of 103 (10.7%), NNT 35.
|
|
risk of progression, 3.0% lower, OR 0.97, p = 0.82, treatment 115, control 103, Wilcoxon-Mann-Whitney, primary outcome, RR approximated with OR.
|
|
risk of progression, 32.8% lower, RR 0.67, p = 0.71, treatment 3 of 115 (2.6%), control 4 of 103 (3.9%), NNT 78, Δ rSOFA 4.
|
|
risk of progression, 79.1% higher, RR 1.79, p = 0.30, treatment 10 of 115 (8.7%), control 5 of 103 (4.9%), Δ rSOFA 3.
|
|
risk of progression, 25.4% lower, RR 0.75, p = 0.76, treatment 5 of 115 (4.3%), control 6 of 103 (5.8%), NNT 68, Δ rSOFA 2.
|
|
risk of progression, 16.0% lower, RR 0.84, p = 0.70, treatment 15 of 115 (13.0%), control 16 of 103 (15.5%), NNT 40, Δ rSOFA 1.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mariani et al., 27 May 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Argentina, peer-reviewed, mean age 59.1, 33 authors, study period 14 August, 2020 - 22 June, 2021, average treatment delay 7.0 days, dosage 500,000IU single dose, trial NCT04411446 (history) (CARED).
Contact: ja_mariani@hotmail.com.
High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: Multicentre randomized controlled clinical trial
PLOS ONE, doi:10.1371/journal.pone.0267918
Background The role of oral vitamin D 3 supplementation for hospitalized patients with COVID-19 remains to be determined. The study was aimed to evaluate whether vitamin D 3 supplementation could prevent respiratory worsening among hospitalized patients with COVID-19.
Methods and findings We designed a multicentre, randomized, double-blind, sequential, placebo-controlled clinical trial. The study was conducted in 17 second and third level hospitals, located in four
Conclusions Supplementation with a single, high dose of vitamin D 3 at admission to patients hospitalized with mild-to-moderate COVID-19 did not prevent respiratory worsening as compared with placebo.
Supporting information S1
References
Amrein, Schnedl, Holl, Riedl, Christopher et al., Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial, JAMA, doi:10.1001/jama.2014.13204
Anka, Tahir, Abubakar, Alsabbagh, Zian et al., Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management, Scand J Immunol, doi:10.1111/sji.12998
Annweiler, Beaudenon, Simon, Guenet, Otekpo, Vitamin D supplementation prior to or during COVID-19 associated with better 3-month survival in geriatric patients: Extension phase of the GERIA-COVID study, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2021.105958
Atef, Vitamin D assays in clinical laboratory: Past, present and future challenges, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2017.02.011
Bergman, Lindh, Bjo ¨rkhem-Bergman, Lindh, Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, PLoS One, doi:10.1371/journal.pone.0065835
Briggs, Gormally, Li, Browning, Treggiari et al., Early but not late convalescent plasma is associated with better survival in moderate-to-severe COVID-19, PLoS One, doi:10.1371/journal.pone.0254453
Castillo, Costa, Barrios, Alcala ´dı ´az, Lo ´pez Miranda et al., Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2020.105751
Costagliola, Nuzzi, Spada, Comberiati, Verduci et al., Nutraceuticals in Viral Infections: An Overview of the Immunomodulating Properties, Nutrients, doi:10.3390/nu13072410
Divine, Norton, Baro ´n, Juarez-Colunga, The Wilcoxon-Mann-Whitney Procedure Fails as a Test of Medians, Am Stat, doi:10.1080/00031305.2017.1305291
Ferder, Martı ´n Gime ´nez, Vm, Inserra, Tajer et al., Vitamin D supplementation as a rational pharmacological approach in the COVID-19 pandemic, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00186.2020
Festic, Bansal, Kor, Gajic, US Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS). SpO2/FiO2 ratio on hospital admission is an indicator of early acute respiratory distress syndrome development among patients at risk, J Intensive Care Med, doi:10.1177/0885066613516411
Getachew, Tizabi, Vitamin D and COVID-19: Role of ACE2, age, gender, and ethnicity, J Med Virol, doi:10.1002/jmv.27075
Grove, Osokogu, Al-Khudairy, Mehrabian, Zanganeh et al., Association between vitamin D supplementation or serum vitamin D level and susceptibility to SARS-CoV-2 infection or COVID-19 including clinical course, morbidity and mortality outcomes? A systematic review, BMJ Open, doi:10.1136/bmjopen-2020-043737
Heart, Lung ; Ginde, Brower, Caterino, Finck et al., Early High-Dose Vitamin D3 for Critically Ill, Vitamin D-Deficient Patients, N Engl J Med, doi:10.1056/NEJMoa1911124
Jovic, Ali, Ibrahim, Jessop, Tarassoli et al., Could Vitamins Help in the Fight Against COVID-19?, Nutrients, doi:10.3390/nu12092550
Kearns, Alvarez, Tangpricha, Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review, Endocr Pract, doi:10.4158/EP13265.RA
Kraus, Medenwald, Ludwig-Kraus, Do extreme summers increase blood vitamin D (25-hydroxyvitamin D) levels?, PLoS One, doi:10.1371/journal.pone.0242230
Leaf, Ginde, Vitamin D3 to Treat COVID-19: Different Disease, Same Answer, JAMA, doi:10.1001/jama.2020.26850
Mariani, Gime ´nez, Vmm, Bergam, Tajer et al., Association Between Vitamin D Deficiency and COVID-19 Incidence, Complications, and Mortality in 46 Countries: An Ecological Study, Health Secur, doi:10.1089/hs.2020.0137
Mariani, Tajer, Antonietti, Inserra, Ferder et al., High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: A structured summary of a study protocol for a randomised controlled trial (CARED-TRIAL), Trials, doi:10.1186/s13063-021-05073-3
Martineau, Forouhi, Vitamin D for COVID-19: a case to answer?, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587%2820%2930268-0
Martineau, Jolliffe, Hooper, Greenberg, Aloia et al., Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, BMJ, doi:10.1136/bmj.i6583
Meltzer, Best, Zhang, Vokes, Arora et al., Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.19722
Mora, Iwata, Von Andrian, Vitamin effects on the immune system: vitamins A and D take centre stage, Nat Rev Immunol, doi:10.1038/nri2378
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.26848
Osterholm, Preparing for the next pandemic, N Engl J Med, doi:10.1056/NEJMp058068
Pandharipande, Shintani, Hagerman, Jacques, Rice et al., Derivation and validation of Spo2/Fio2 ratio to impute for Pao2/Fio2 ratio in the respiratory component of the Sequential Organ Failure Assessment score, Crit Care Med, doi:10.1097/CCM.0b013e31819cefa9
Peng, Liu, Zheng, Lu, Hou et al., Immunological Aspects of SARS-CoV-2 Infection and the Putative Beneficial Role of Vitamin-D, Int J Mol Sci, doi:10.3390/ijms22105251
Petrelli, Luciani, Perego, Dognini, Colombelli et al., Therapeutic and prognostic role of vitamin D for COVID-19 infection: A systematic review and meta-analysis of 43 observational studies, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2021.105883
Pham, Waterhouse, Baxter, Romero, Mcleod et al., The effect of vitamin D supplementation on acute respiratory tract infection in older Australian adults: an analysis of data from the D-Health Trial, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587%2820%2930380-6
Rice, Wheeler, Bernard, Hayden, Schoenfeld et al., Institute ARDS Network. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS, Chest, doi:10.1378/chest.07-0617
Schulz, Altman, Moher, Group, CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials, BMJ, doi:10.1136/bmj.c332
Seymour, Liu, Iwashyna, Brunkhorst, Rea et al., Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), JAMA, doi:10.1001/jama.2016.0288
Silberstein, Vitamin, A simpler alternative to tocilizumab for trial in COVID-19?, Med Hypotheses, doi:10.1016/j.mehy.2020.109767
Stroehlein, Wallqvist, Iannizzi, Mikolajewska, Metzendorf et al., Vitamin D supplementation for the treatment of COVID-19: a living systematic review, Cochrane Database Syst Rev, doi:10.1002/14651858.CD015043
Teymoori-Rad, Shokri, Salimi, Marashi, The interplay between vitamin D and viral infections, Rev Med Virol, doi:10.1002/rmv.2032
Torjesen, Covid-19: Public health agencies review whether vitamin D supplements could reduce risk, BMJ, doi:10.1136/bmj.m2475
Vincent, Moreno, Takala, Willatts, Mendonc ¸a et al., The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine, Intensive Care Med, doi:10.1007/BF01709751
Yisak, Ewunetei, Kefale, Mamuye, Teshome et al., Effects of Vitamin D on COVID-19 Infection and Prognosis: A Systematic Review, Risk Manag Healthc Policy, doi:10.2147/RMHP.S291584
DOI record:
{
"DOI": "10.1371/journal.pone.0267918",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0267918",
"abstract": "<jats:sec id=\"sec001\">\n<jats:title>Background</jats:title>\n<jats:p>The role of oral vitamin D<jats:sub>3</jats:sub> supplementation for hospitalized patients with COVID-19 remains to be determined. The study was aimed to evaluate whether vitamin D<jats:sub>3</jats:sub> supplementation could prevent respiratory worsening among hospitalized patients with COVID-19.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec002\">\n<jats:title>Methods and findings</jats:title>\n<jats:p>We designed a multicentre, randomized, double-blind, sequential, placebo-controlled clinical trial. The study was conducted in 17 second and third level hospitals, located in four provinces of Argentina, from 14 August 2020 to 22 June 2021. We enrolled 218 adult patients, hospitalized in general wards with SARS-CoV-2 confirmed infection, mild-to-moderate COVID-19 and risk factors for disease progression. Participants were randomized to a single oral dose of 500 000 IU of vitamin D<jats:sub>3</jats:sub> or matching placebo. Randomization ratio was 1:1, with permuted blocks and stratified for study site, diabetes and age (≤60 vs >60 years). The primary outcome was the change in the respiratory Sepsis related Organ Failure Assessment score between baseline and the highest value recorded up to day 7. Secondary outcomes included the length of hospital stay; intensive care unit admission; and in-hospital mortality. Overall, 115 participants were assigned to vitamin D<jats:sub>3</jats:sub> and 105 to placebo (mean [SD] age, 59.1 [10.7] years; 103 [47.2%] women). There were no significant differences in the primary outcome between groups (median [IQR] 0.0 [0.0–1.0] vs 0.0 [0.0–1.0], for vitamin D<jats:sub>3</jats:sub> and placebo, respectively; <jats:italic>p</jats:italic> = 0.925). Median [IQR] length of hospital stay was not significantly different between vitamin D<jats:sub>3</jats:sub> group (6.0 [4.0–9.0] days) and placebo group (6.0 [4.0–10.0] days; <jats:italic>p</jats:italic> = 0.632). There were no significant differences for intensive care unit admissions (7.8% vs 10.7%; RR 0.73; 95% CI 0.32 to 1.70; <jats:italic>p</jats:italic> = 0.622), or in-hospital mortality (4.3% vs 1.9%; RR 2.24; 95% CI 0.44 to 11.29; <jats:italic>p</jats:italic> = 0.451). There were no significant differences in serious adverse events (vitamin D<jats:sub>3</jats:sub> = 14.8%, placebo = 11.7%).</jats:p>\n</jats:sec>\n<jats:sec id=\"sec003\">\n<jats:title>Conclusions</jats:title>\n<jats:p>Among hospitalized patients with mild-to-moderate COVID-19 and risk factors, a single high oral dose of vitamin D<jats:sub>3</jats:sub> as compared with placebo, did not prevent the respiratory worsening.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec004\">\n<jats:title>Trial registration</jats:title>\n<jats:p>ClincicalTrials.gov Identifier: <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/show/NCT04411446\" xlink:type=\"simple\">NCT04411446</jats:ext-link>.</jats:p>\n</jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9372-6817",
"affiliation": [],
"authenticated-orcid": true,
"family": "Mariani",
"given": "Javier",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-7676-4264",
"affiliation": [],
"authenticated-orcid": true,
"family": "Antonietti",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tajer",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferder",
"given": "León",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6671-874X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Inserra",
"given": "Felipe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sanchez Cunto",
"given": "Milagro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brosio",
"given": "Diego",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ross",
"given": "Fernando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zylberman",
"given": "Marcelo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "López",
"given": "Daniel Emilio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luna Hisano",
"given": "Cecilia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maristany Batisda",
"given": "Sebastián",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pace",
"given": "Gabriela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salvatore",
"given": "Adrián",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hogrefe",
"given": "Jimena Fernanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turela",
"given": "Marcela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gaido",
"given": "Andrés",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodera",
"given": "Beatriz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Banega",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iglesias",
"given": "María Eugenia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rzepeski",
"given": "Mariela",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9615-3746",
"affiliation": [],
"authenticated-orcid": true,
"family": "Gomez Portillo",
"given": "Juan Manuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bertelli",
"given": "Magalí",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vilela",
"given": "Andrés",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heffner",
"given": "Leandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Annetta",
"given": "Verónica Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moracho",
"given": "Lucila",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carmona",
"given": "Maximiliano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Melito",
"given": "Graciela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martínez",
"given": "María José",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luna",
"given": "Gloria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vensentini",
"given": "Natalia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Manucha",
"given": "Walter",
"sequence": "additional"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2022,
5,
27
]
],
"date-time": "2022-05-27T17:35:37Z",
"timestamp": 1653672937000
},
"deposited": {
"date-parts": [
[
2022,
5,
27
]
],
"date-time": "2022-05-27T17:36:25Z",
"timestamp": 1653672985000
},
"editor": [
{
"affiliation": [],
"family": "Putzu",
"given": "Alessandro",
"sequence": "first"
}
],
"funder": [
{
"award": [
"FONCyT IP COVID-19-931"
],
"name": "Agencia Nacional de Promoción Científica, Desarrollo Tecnológico e Innovación"
}
],
"indexed": {
"date-parts": [
[
2022,
5,
27
]
],
"date-time": "2022-05-27T18:11:16Z",
"timestamp": 1653675076136
},
"is-referenced-by-count": 0,
"issue": "5",
"issued": {
"date-parts": [
[
2022,
5,
27
]
]
},
"journal-issue": {
"issue": "5",
"published-online": {
"date-parts": [
[
2022,
5,
27
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
5,
27
]
],
"date-time": "2022-05-27T00:00:00Z",
"timestamp": 1653609600000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0267918",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0267918",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2022,
5,
27
]
]
},
"published-online": {
"date-parts": [
[
2022,
5,
27
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"DOI": "10.1152/ajplung.00186.2020",
"article-title": "Vitamin D supplementation as a rational pharmacological approach in the COVID-19 pandemic",
"author": "L Ferder",
"doi-asserted-by": "crossref",
"first-page": "L941",
"issue": "6",
"journal-title": "Am J Physiol Lung Cell Mol Physiol",
"key": "pone.0267918.ref001",
"volume": "319",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m2475",
"article-title": "Covid-19: Public health agencies review whether vitamin D supplements could reduce risk",
"author": "I. Torjesen",
"doi-asserted-by": "crossref",
"first-page": "m2475",
"journal-title": "BMJ",
"key": "pone.0267918.ref002",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2020.109767",
"article-title": "Vitamin D: A simpler alternative to tocilizumab for trial in COVID-19?",
"author": "M. Silberstein",
"doi-asserted-by": "crossref",
"first-page": "109767",
"journal-title": "Med Hypotheses",
"key": "pone.0267918.ref003",
"volume": "140",
"year": "2020"
},
{
"DOI": "10.3390/nu13072410",
"article-title": "Nutraceuticals in Viral Infections: An Overview of the Immunomodulating Properties",
"author": "G Costagliola",
"doi-asserted-by": "crossref",
"first-page": "2410",
"issue": "7",
"journal-title": "Nutrients",
"key": "pone.0267918.ref004",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1002/rmv.2032",
"article-title": "The interplay between vitamin D and viral infections.",
"author": "M Teymoori-Rad",
"doi-asserted-by": "crossref",
"first-page": "e2032",
"issue": "2",
"journal-title": "Rev Med Virol",
"key": "pone.0267918.ref005",
"volume": "29",
"year": "2019"
},
{
"DOI": "10.3390/ijms22105251",
"article-title": "Immunological Aspects of SARS-CoV-2 Infection and the Putative Beneficial Role of Vitamin-D.",
"author": "MY Peng",
"doi-asserted-by": "crossref",
"first-page": "5251",
"issue": "10",
"journal-title": "Int J Mol Sci.",
"key": "pone.0267918.ref006",
"volume": "22",
"year": "2021"
},
{
"article-title": "Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management",
"author": "AU Anka",
"first-page": "e12998",
"issue": "4",
"journal-title": "Scand J Immunol",
"key": "pone.0267918.ref007",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1056/NEJMp058068",
"article-title": "Preparing for the next pandemic",
"author": "MT Osterholm",
"doi-asserted-by": "crossref",
"first-page": "1839",
"issue": "18",
"journal-title": "N Engl J Med",
"key": "pone.0267918.ref008",
"volume": "352",
"year": "2005"
},
{
"DOI": "10.1038/nri2378",
"article-title": "Vitamin effects on the immune system: vitamins A and D take centre stage",
"author": "JR Mora",
"doi-asserted-by": "crossref",
"first-page": "685",
"issue": "9",
"journal-title": "Nat Rev Immunol",
"key": "pone.0267918.ref009",
"volume": "8",
"year": "2008"
},
{
"DOI": "10.1002/jmv.27075",
"article-title": "Vitamin D and COVID-19: Role of ACE2, age, gender, and ethnicity",
"author": "B Getachew",
"doi-asserted-by": "crossref",
"first-page": "5285",
"issue": "9",
"journal-title": "J Med Virol",
"key": "pone.0267918.ref010",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.jsbmb.2021.105883",
"article-title": "Therapeutic and prognostic role of vitamin D for COVID-19 infection: A systematic review and meta-analysis of 43 observational studies",
"author": "F Petrelli",
"doi-asserted-by": "crossref",
"first-page": "105883",
"journal-title": "J Steroid Biochem Mol Biol",
"key": "pone.0267918.ref011",
"volume": "211",
"year": "2021"
},
{
"DOI": "10.2147/RMHP.S291584",
"article-title": "Effects of Vitamin D on COVID-19 Infection and Prognosis: A Systematic Review.",
"author": "H Yisak",
"doi-asserted-by": "crossref",
"first-page": "31",
"journal-title": "Risk Manag Healthc Policy",
"key": "pone.0267918.ref012",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1089/hs.2020.0137",
"article-title": "Association Between Vitamin D Deficiency and COVID-19 Incidence, Complications, and Mortality in 46 Countries: An Ecological Study",
"author": "J Mariani",
"doi-asserted-by": "crossref",
"first-page": "302",
"issue": "3",
"journal-title": "Health Secur",
"key": "pone.0267918.ref013",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"article-title": "Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study",
"author": "M Entrenas Castillo",
"doi-asserted-by": "crossref",
"first-page": "105751",
"journal-title": "J Steroid Biochem Mol Biol",
"key": "pone.0267918.ref014",
"volume": "203",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.19722",
"article-title": "Association of Vitamin D Status and Other Clinical Characteristics With COVID-19",
"author": "DO Meltzer",
"doi-asserted-by": "crossref",
"first-page": "e2019722",
"issue": "9",
"journal-title": "Test Results. JAMA Netw Open",
"key": "pone.0267918.ref015",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.26848",
"article-title": "Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial",
"author": "IH Murai",
"doi-asserted-by": "crossref",
"first-page": "1053",
"issue": "11",
"journal-title": "JAMA",
"key": "pone.0267918.ref016",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2020-043737",
"article-title": "Association between vitamin D supplementation or serum vitamin D level and susceptibility to SARS-CoV-2 infection or COVID-19 including clinical course, morbidity and mortality outcomes? A systematic review",
"author": "A Grove",
"doi-asserted-by": "crossref",
"first-page": "e043737",
"issue": "5",
"journal-title": "BMJ Open",
"key": "pone.0267918.ref017",
"volume": "11",
"year": "2021"
},
{
"article-title": "Vitamin D supplementation for the treatment of COVID-19: a living systematic review.",
"author": "JK Stroehlein",
"first-page": "CD015043",
"issue": "5",
"journal-title": "Cochrane Database Syst Rev",
"key": "pone.0267918.ref018",
"volume": "5",
"year": "2021"
},
{
"article-title": "Vitamin D3 to Treat COVID-19: Different Disease",
"author": "DE Leaf",
"first-page": "1047",
"issue": "11",
"journal-title": "Same Answer. JAMA",
"key": "pone.0267918.ref019",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1016/S2213-8587(20)30268-0",
"article-title": "Vitamin D for COVID-19: a case to answer",
"author": "AR Martineau",
"doi-asserted-by": "crossref",
"first-page": "735",
"issue": "9",
"journal-title": "Lancet Diabetes Endocrinol",
"key": "pone.0267918.ref020",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1186/s13063-021-05073-3",
"article-title": "High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: A structured summary of a study protocol for a randomised controlled trial (CARED-TRIAL).",
"author": "J Mariani",
"doi-asserted-by": "crossref",
"first-page": "111",
"issue": "1",
"journal-title": "Trials",
"key": "pone.0267918.ref021",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1136/bmj.c332",
"article-title": "CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials",
"author": "KF Schulz",
"doi-asserted-by": "crossref",
"first-page": "c332",
"journal-title": "BMJ",
"key": "pone.0267918.ref022",
"volume": "340",
"year": "2010"
},
{
"DOI": "10.1007/BF01709751",
"article-title": "The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine",
"author": "JL Vincent",
"doi-asserted-by": "crossref",
"first-page": "707",
"issue": "7",
"journal-title": "Intensive Care Med",
"key": "pone.0267918.ref023",
"volume": "22",
"year": "1996"
},
{
"DOI": "10.1097/CCM.0b013e31819cefa9",
"article-title": "Derivation and validation of Spo2/Fio2 ratio to impute for Pao2/Fio2 ratio in the respiratory component of the Sequential Organ Failure Assessment score",
"author": "PP Pandharipande",
"doi-asserted-by": "crossref",
"first-page": "1317",
"issue": "4",
"journal-title": "Crit Care Med",
"key": "pone.0267918.ref024",
"volume": "37",
"year": "2009"
},
{
"DOI": "10.1378/chest.07-0617",
"article-title": "Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS",
"author": "National Institutes of Health, National Heart, Lung, and Blood Institute ARDS Network.",
"doi-asserted-by": "crossref",
"first-page": "410",
"issue": "2",
"journal-title": "Chest",
"key": "pone.0267918.ref025",
"volume": "132",
"year": "2007"
},
{
"DOI": "10.1001/jama.2016.0288",
"article-title": "Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3).",
"author": "CW Seymour",
"doi-asserted-by": "crossref",
"first-page": "762",
"issue": "8",
"journal-title": "JAMA",
"key": "pone.0267918.ref026",
"volume": "315",
"year": "2016"
},
{
"DOI": "10.1016/j.jsbmb.2017.02.011",
"article-title": "Vitamin D assays in clinical laboratory: Past, present and future challenges",
"author": "SH Atef",
"doi-asserted-by": "crossref",
"first-page": "136",
"journal-title": "J Steroid Biochem Mol Biol",
"key": "pone.0267918.ref027",
"volume": "175",
"year": "2018"
},
{
"DOI": "10.1080/00031305.2017.1305291",
"article-title": "The Wilcoxon–Mann–Whitney Procedure Fails as a Test of Medians.",
"author": "GW Divine",
"doi-asserted-by": "crossref",
"first-page": "278",
"issue": "3",
"journal-title": "Am Stat",
"key": "pone.0267918.ref028",
"volume": "72",
"year": "2018"
},
{
"DOI": "10.1371/journal.pone.0065835",
"article-title": "Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials",
"author": "P Bergman",
"doi-asserted-by": "crossref",
"first-page": "e65835",
"issue": "6",
"journal-title": "PLoS One",
"key": "pone.0267918.ref029",
"volume": "8",
"year": "2013"
},
{
"DOI": "10.1136/bmj.i6583",
"article-title": "Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data",
"author": "AR Martineau",
"doi-asserted-by": "crossref",
"first-page": "i6583",
"journal-title": "BMJ",
"key": "pone.0267918.ref030",
"volume": "356",
"year": "2017"
},
{
"DOI": "10.1016/S2213-8587(20)30380-6",
"article-title": "The effect of vitamin D supplementation on acute respiratory tract infection in older Australian adults: an analysis of data from the D-Health Trial",
"author": "H Pham",
"doi-asserted-by": "crossref",
"first-page": "69",
"issue": "2",
"journal-title": "Lancet Diabetes Endocrinol",
"key": "pone.0267918.ref031",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.3390/nu12092550",
"article-title": "Could Vitamins Help in the Fight Against COVID-19?",
"author": "TH Jovic",
"doi-asserted-by": "crossref",
"first-page": "2550",
"issue": "9",
"journal-title": "Nutrients",
"key": "pone.0267918.ref032",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.4158/EP13265.RA",
"article-title": "Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review",
"author": "MD Kearns",
"doi-asserted-by": "crossref",
"first-page": "341",
"issue": "4",
"journal-title": "Endocr Pract",
"key": "pone.0267918.ref033",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1016/j.jsbmb.2021.105958",
"article-title": "Vitamin D supplementation prior to or during COVID-19 associated with better 3-month survival in geriatric patients: Extension phase of the GERIA-COVID study",
"author": "C Annweiler",
"doi-asserted-by": "crossref",
"first-page": "105958",
"journal-title": "J Steroid Biochem Mol Biol",
"key": "pone.0267918.ref034",
"volume": "213",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0242230",
"article-title": "Do extreme summers increase blood vitamin D (25-hydroxyvitamin D) levels?",
"author": "FB Kraus",
"doi-asserted-by": "crossref",
"first-page": "e0242230",
"issue": "11",
"journal-title": "PLoS One",
"key": "pone.0267918.ref035",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1001/jama.2014.13204",
"article-title": "Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial",
"author": "K Amrein",
"doi-asserted-by": "crossref",
"first-page": "1520",
"issue": "15",
"journal-title": "JAMA",
"key": "pone.0267918.ref036",
"volume": "312",
"year": "2014"
},
{
"DOI": "10.1056/NEJMoa1911124",
"article-title": "Early High-Dose Vitamin D3 for Critically Ill, Vitamin D-Deficient Patients",
"author": "National Heart, Lung, and Blood Institute PETAL Clinical Trials Network",
"doi-asserted-by": "crossref",
"first-page": "2529",
"issue": "26",
"journal-title": "N Engl J Med",
"key": "pone.0267918.ref037",
"volume": "381",
"year": "2019"
},
{
"DOI": "10.1371/journal.pone.0254453",
"article-title": "Early but not late convalescent plasma is associated with better survival in moderate-to-severe COVID-19.",
"author": "N Briggs",
"doi-asserted-by": "crossref",
"first-page": "e0254453",
"issue": "7",
"journal-title": "PLoS One",
"key": "pone.0267918.ref038",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1177/0885066613516411",
"article-title": "SpO2/FiO2 ratio on hospital admission is an indicator of early acute respiratory distress syndrome development among patients at risk.",
"author": "US Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG–LIPS)",
"doi-asserted-by": "crossref",
"first-page": "209",
"issue": "4",
"journal-title": "J Intensive Care Med",
"key": "pone.0267918.ref039",
"volume": "30",
"year": "2015"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0267918"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: Multicentre randomized controlled clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "17"
}
