Prevention of covid-19 and other acute respiratory infections with cod liver oil supplementation, a low dose vitamin D supplement: quadruple blinded, randomised placebo controlled trial
et al., BMJ, doi:10.1136/bmj-2022-071245, NCT04609423, Sep 2022
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
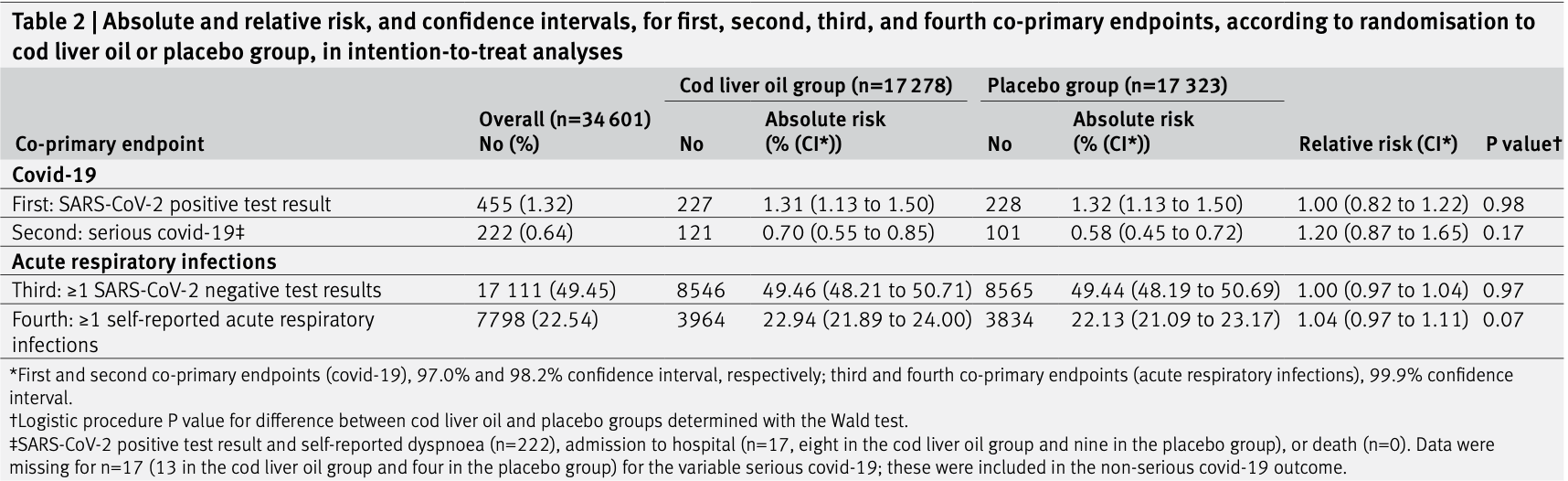
RCT 17,278 low-risk patients (zero mortality) treated with 5mL/day cod liver oil (~400IU vitamin D) and 17,323 placebo patients in Norway, showing no significant differences with treatment. The placebo group had higher vitamin D at baseline, and both groups had comparable vitamin D during treatment (74 vs. 63 nmol/L). 23% of control patients took vitamin D supplements and 62% consumed fatty fish (typically a good source of vitamin D). Adherence was low (<70% for "strict" compliance, which only required >0.5L consumed, or treatment for > "2-3" months).
This is the 27th of 40 COVID-19 RCTs for vitamin D, which collectively show efficacy with p=0.0000001.
This is the 98th of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
|
risk of ICU admission, 0.3% higher, RR 1.00, p = 1.00, treatment 4 of 17,278 (0.0%), control 4 of 17,323 (0.0%).
|
|
risk of hospitalization, 10.9% lower, RR 0.89, p = 1.00, treatment 8 of 17,278 (0.0%), control 9 of 17,323 (0.1%), NNT 17692.
|
|
risk of severe case, 20.0% higher, RR 1.20, p = 0.17, treatment 121 of 17,278 (0.7%), control 101 of 17,323 (0.6%).
|
|
risk of case, no change, RR 1.00, p = 0.98, treatment 227 of 17,278 (1.3%), control 228 of 17,323 (1.3%), NNT 42377.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Brunvoll et al., 7 Sep 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Norway, peer-reviewed, mean age 44.9, 15 authors, study period 10 November, 2020 - 2 June, 2021, dosage 400IU daily, this trial uses multiple treatments in the treatment arm (combined with cod liver oil) - results of individual treatments may vary, trial NCT04609423 (history).
Contact: arne@meg.no.
Prevention of covid-19 and other acute respiratory infections with cod liver oil supplementation, a low dose vitamin D supplement: quadruple blinded, randomised placebo controlled trial
BMJ, doi:10.1136/bmj-2022-071245
Objective To determine if daily supplementation with cod liver oil, a low dose vitamin D supplement, in winter, prevents SARS-CoV-2 infection, serious covid-19, or other acute respiratory infections in adults in Norway.
References
Asher, Tintle, Myers, Lockshon, Bacareza et al., Blood omega-3 fatty acids and death from COVID-19: A pilot study, Prostaglandins Leukot Essent Fatty Acids, doi:10.1016/j.plefa.2021.102250
Bassatne, Basbous, Chakhtoura, El Zein, Rahme et al., The link between COVID-19 and VItamin D (VIVID): A systematic review and metaanalysis, Metabolism, doi:10.1016/j.metabol.2021.154753
Bolland, Avenell, Grey, Vitamin D and acute respiratory infection: secondary analysis of a previous randomised controlled trial and updated metaanalyses, medRxiv, doi:10.1101/2022.02.03.22270409
Butler-Laporte, Nakanishi, Mooser, Vitamin D and COVID-19 susceptibility and severity in the COVID-19 Host Genetics Initiative: A Mendelian randomization study, PLoS Med, doi:10.1371/journal.pmed.1003605
Chen, Mei, Xie, Low vitamin D levels do not aggravate COVID-19 risk or death, and vitamin D supplementation does not improve outcomes in hospitalized patients with COVID-19: a metaanalysis and GRADE assessment of cohort studies and RCTs, Nutr J, doi:10.1186/s12937-021-00744-y
Deschasaux-Tanguy, Srour, Bourhis, Nutritional risk factors for SARS-CoV-2 infection: a prospective study within the NutriNet-Santé cohort, BMC Med, doi:10.1186/s12916-021-02168-1
Greiller, Martineau, Modulation of the immune response to respiratory viruses by vitamin D, Nutrients, doi:10.3390/nu7064240
Holt, Talaei, Greenig, Risk factors for developing COVID-19: a population-based longitudinal study (COVIDENCE UK), Thorax, doi:10.1136/thoraxjnl-2021-217487
Holter, Pischke, De Boer, Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2010540117
Jiang, Li, Wang, Effect of marine-derived n-3 polyunsaturated fatty acids on major eicosanoids: a systematic review and meta-analysis from 18 randomized controlled trials, PLoS One, doi:10.1371/journal.pone.0147351
Jolliffe, Cajr, Sluyter, Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587(21)00051-6on15
Jolliffe, Holt, Greenig, Vitamin D supplements for prevention of Covid-19 or other acute respiratory infections: a phase 3 randomized controlled trial (CORONAVIT), medRxiv, doi:10.1101/2022.03.22.22271707
Kaya, Pamukçu, Yakar, The role of vitamin D deficiency on COVID-19: a systematic review and meta-analysis of observational studies, Epidemiol Health, doi:10.4178/epih.e2021074
Kiecolt-Glaser, Belury, Andridge, Malarkey, Hwang et al., Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial, Brain Behav Immun, doi:10.1016/j.bbi.2012.05.011
Ma, Zhou, Heianza, Qi, Habitual use of vitamin D supplements and risk of coronavirus disease 2019 (COVID-19) infection: a prospective study in UK Biobank, Am J Clin Nutr, doi:10.1093/ajcn/nqaa381
Mai, Langhammer, Chen, Cajr, Cod liver oil intake and incidence of asthma in Norwegian adults--the HUNT study, Thorax, doi:10.1136/thoraxjnl-2012-202061
Manson, Brannon, Rosen, Taylor, Vitamin D deficiency -is there really a pandemic?, N Engl J Med, doi:10.1056/NEJMp1608005
Martineau, Cates, Urashima, Vitamin D for the management of asthma, Cochrane Database Syst Rev, doi:10.1002/14651858.CD011511.pub2
Oscanoa, Amado, Vidal, Laird, Ghashut et al., The relationship between the severity and mortality of SARS-CoV-2 infection and 25-hydroxyvitamin D concentration -a metaanalysis, Adv Respir Med, doi:10.5603/ARM.a2021.0037
Pham, Rahman, Majidi, Waterhouse, Neale, Acute respiratory tract infection and 25-hydroxyvitamin D concentration: a systematic review and meta-analysis, Int J Environ Res Public Health, doi:10.3390/ijerph16173020
Raposo, Fondell, Ström, Intake of vitamin C, vitamin E, selenium, zinc and polyunsaturated fatty acids and upper respiratory tract infection-a prospective cohort study, Eur J Clin Nutr, doi:10.1038/ejcn.2016.261
Story, Essential sufficiency of zinc, ω-3 polyunsaturated fatty acids, vitamin D and magnesium for prevention and treatment of COVID-19, diabetes, cardiovascular diseases, lung diseases and cancer, Biochimie, doi:10.1016/j.biochi.2021.05.013
Talaei, Faustini, Holt, Determinants of pre-vaccination antibody responses to SARS-CoV-2: a population-based longitudinal study (COVIDENCE UK), BMC Med, doi:10.1186/s12916-022-02286-4
Villasis-Keever, López-Alarcón, Miranda-Novales, Efficacy and safety of vitamin D supplementation to prevent COVID-19 in frontline healthcare workers. a randomized clinical trial, Arch Med Res, doi:10.1016/j.arcmed.2022.04.003
Vlieg-Boerstra, De, Meyer, Nutrient supplementation for prevention of viral respiratory tract infections in healthy subjects: A systematic review and meta-analysis, Allergy, doi:10.1111/all.15136
Wei, Meng, Li, Wang, Chen, The effects of low-ratio n-6/n-3 PUFA on biomarkers of inflammation: a systematic review and metaanalysis, Food Funct, doi:10.1039/D0FO01976C
Wise, Covid-19: Evidence is lacking to support vitamin D's role in treatment and prevention, BMJ, doi:10.1136/bmj.m4912
Zárate, Jaber-Vazdekis, Tejera, Pérez, Rodríguez, Significance of long chain polyunsaturated fatty acids in human health, Clin Transl Med, doi:10.1186/s40169-017-0153-6
DOI record:
{
"DOI": "10.1136/bmj-2022-071245",
"ISSN": [
"1756-1833"
],
"URL": "http://dx.doi.org/10.1136/bmj-2022-071245",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Objective</jats:title>\n <jats:p>To determine if daily supplementation with cod liver oil, a low dose vitamin D supplement, in winter, prevents SARS-CoV-2 infection, serious covid-19, or other acute respiratory infections in adults in Norway.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Design</jats:title>\n <jats:p>Quadruple blinded, randomised placebo controlled trial.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Setting</jats:title>\n <jats:p>Norway, 10 November 2020 to 2 June 2021.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Participants</jats:title>\n <jats:p>34 601 adults (aged 18-75 years), not taking daily vitamin D supplements.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Intervention</jats:title>\n <jats:p>5 mL/day of cod liver oil (10 µg of vitamin D, n=17 278) or placebo (n=17 323) for up to six months.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Main outcome measures</jats:title>\n <jats:p>Four co-primary endpoints were predefined: the first was a positive SARS-CoV-2 test result determined by reverse transcriptase-quantitative polymerase chain reaction and the second was serious covid-19, defined as self-reported dyspnoea, admission to hospital, or death. Other acute respiratory infections were indicated by the third and fourth co-primary endpoints: a negative SARS-CoV-2 test result and self-reported symptoms. Side effects related to the supplementation were self-reported. The fallback method was used to handle multiple comparisons.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Supplementation with cod liver oil was not associated with a reduced risk of any of the co-primary endpoints. Participants took the supplement (cod liver oil or placebo) for a median of 164 days, and 227 (1.31%) participants in the cod liver oil group and 228 (1.32%) participants in the placebo group had a positive SARS-CoV-2 test result (relative risk 1.00, multiple comparison adjusted confidence interval 0.82 to 1.22). Serious covid-19 was identified in 121 (0.70%) participants in the cod liver oil group and in 101 (0.58%) participants in the placebo group (1.20, 0.87 to 1.65). 8546 (49.46%) and 8565 (49.44%) participants in the cod liver oil and placebo groups, respectively, had ≥1 negative SARS-CoV-2 test results (1.00, 0.97 to 1.04). 3964 (22.94%) and 3834 (22.13%) participants in the cod liver oil and placebo groups, respectively, reported ≥1 acute respiratory infections (1.04, 0.97 to 1.11). Only low grade side effects were reported in the cod liver oil and placebo groups.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>Supplementation with cod liver oil in the winter did not reduce the incidence of SARS-CoV-2 infection, serious covid-19, or other acute respiratory infections compared with placebo.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Trial registration</jats:title>\n <jats:p>\n ClinicalTrials.gov\n <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" xlink:href=\"NCT04609423\" ext-link-type=\"clintrialgov\">NCT04609423</jats:ext-link>\n .\n </jats:p>\n </jats:sec>",
"alternative-id": [
"10.1136/bmj-2022-071245"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-0917-9898",
"affiliation": [],
"authenticated-orcid": false,
"family": "Brunvoll",
"given": "Sonja H",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-1922-0751",
"affiliation": [],
"authenticated-orcid": false,
"family": "Nygaard",
"given": "Anders B",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9294-2510",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ellingjord-Dale",
"given": "Merete",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5717-6985",
"affiliation": [],
"authenticated-orcid": false,
"family": "Holland",
"given": "Petter",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5674-3522",
"affiliation": [],
"authenticated-orcid": false,
"family": "Istre",
"given": "Mette Stausland",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4968-2295",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kalleberg",
"given": "Karl Trygve",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3964-4351",
"affiliation": [],
"authenticated-orcid": false,
"family": "Søraas",
"given": "Camilla L",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8674-9703",
"affiliation": [],
"authenticated-orcid": false,
"family": "Holven",
"given": "Kirsten B",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7986-4204",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ulven",
"given": "Stine M",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3002-4030",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hjartåker",
"given": "Anette",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haider",
"given": "Trond",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2445-1258",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lund-Johansen",
"given": "Fridtjof",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4375-2697",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dahl",
"given": "John Arne",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3262-8260",
"affiliation": [],
"authenticated-orcid": false,
"family": "Meyer",
"given": "Haakon E",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1622-591X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Søraas",
"given": "Arne",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04609423",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "BMJ",
"container-title-short": "BMJ",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2022,
9,
8
]
],
"date-time": "2022-09-08T00:00:18Z",
"timestamp": 1662595218000
},
"deposited": {
"date-parts": [
[
2022,
9,
8
]
],
"date-time": "2022-09-08T04:01:26Z",
"timestamp": 1662609686000
},
"indexed": {
"date-parts": [
[
2022,
9,
8
]
],
"date-time": "2022-09-08T04:46:17Z",
"timestamp": 1662612377360
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2022,
9,
7
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
7
]
],
"date-time": "2022-09-07T00:00:00Z",
"timestamp": 1662508800000
}
}
],
"link": [
{
"URL": "http://data.bmj.org/tdm/10.1136/bmj-2022-071245",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmj-2022-071245",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e071245",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2022,
9,
7
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
7
]
]
},
"publisher": "BMJ",
"reference": [
{
"DOI": "10.1136/bmjnph-2021-000250",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.1"
},
{
"DOI": "10.5603/ARM.a2021.0037",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.2"
},
{
"DOI": "10.4178/epih.e2021074",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.3"
},
{
"DOI": "10.1016/j.metabol.2021.154753",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.4"
},
{
"DOI": "10.1136/bmj.m4912",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.5"
},
{
"DOI": "10.1186/s12937-021-00744-y",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.6"
},
{
"DOI": "10.1371/journal.pmed.1003605",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.7"
},
{
"DOI": "10.1186/s12916-022-02286-4",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.8"
},
{
"DOI": "10.1136/thoraxjnl-2021-217487",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.9"
},
{
"DOI": "10.3390/nu7064240",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.10"
},
{
"DOI": "10.3390/ijerph16173020",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.11"
},
{
"DOI": "10.1016/S2213-8587(21)00051-6",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.12"
},
{
"DOI": "10.1016/j.biochi.2021.05.013",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.13"
},
{
"DOI": "10.1039/D0FO01976C",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.14"
},
{
"DOI": "10.1186/s40169-017-0153-6",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.15"
},
{
"DOI": "10.1371/journal.pone.0147351",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.16"
},
{
"DOI": "10.1016/j.bbi.2012.05.011",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.17"
},
{
"DOI": "10.1073/pnas.2010540117",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.18"
},
{
"key": "2022090721001328000_378.sep07_9.e071245.19",
"unstructured": "US Department of Health and Human Services. Food and Drug Administration, Center for Drug Evaluation and Research (CDER), and Center for Biologics Evaluation and Research (CBER). Multiple Endpoints in Clinical Trials, Guidance for Industry . https://www.fda.gov/media/102657/."
},
{
"DOI": "10.1101/2022.03.22.22271707",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.20"
},
{
"DOI": "10.1016/j.arcmed.2022.04.003",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.21"
},
{
"DOI": "10.1093/ajcn/nqaa381",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.22"
},
{
"DOI": "10.1101/2022.02.03.22270409",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.23"
},
{
"DOI": "10.1056/NEJMp1608005",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.24"
},
{
"key": "2022090721001328000_378.sep07_9.e071245.25",
"unstructured": "Nordic Council of Ministers, Nordic Council of Ministers Secretariat. Nordic Nutrition Recommendations, 2012: Integrating nutrition and physical activity. https://www.norden.org/en/publication/nordic-nutrition-recommendations-2012."
},
{
"DOI": "10.1186/s12916-021-02168-1",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.26"
},
{
"DOI": "10.1038/ejcn.2016.261",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.27"
},
{
"DOI": "10.1016/j.plefa.2021.102250",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.28"
},
{
"DOI": "10.1002/14651858.CD011511.pub2",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.29"
},
{
"DOI": "10.1136/thoraxjnl-2012-202061",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.30"
},
{
"DOI": "10.1111/all.15136",
"doi-asserted-by": "publisher",
"key": "2022090721001328000_378.sep07_9.e071245.31"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.bmj.com/lookup/doi/10.1136/bmj-2022-071245"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Engineering"
],
"subtitle": [],
"title": "Prevention of covid-19 and other acute respiratory infections with cod liver oil supplementation, a low dose vitamin D supplement: quadruple blinded, randomised placebo controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy"
}
