Effectiveness of Sotrovimab and Molnupiravir in community settings in England across the Omicron BA.1 and BA.2 sublineages: emulated target trials using the OpenSAFELY platform
et al., medRxiv, doi:10.1101/2023.05.12.23289914, May 2023
OpenSAFELY retrospective 75,048 outpatients in the UK, using the clone-censor-weight approach to address immortal time bias, showing no significant difference in combined mortality/hospitalization with molnupiravir treatment.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments25.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
Study covers molnupiravir and sotrovimab.
|
risk of death/hospitalization, 9.0% higher, HR 1.09, p = 0.28, treatment 3,072, control 65,568.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
23.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Tazare et al., 16 May 2023, retrospective, United Kingdom, preprint, 31 authors, study period 16 December, 2021 - 21 May, 2022.
Effectiveness of Sotrovimab and Molnupiravir in community settings in England across the Omicron BA.1 and BA.2 sublineages: emulated target trials using the OpenSAFELY platform
doi:10.1101/2023.05.12.23289914
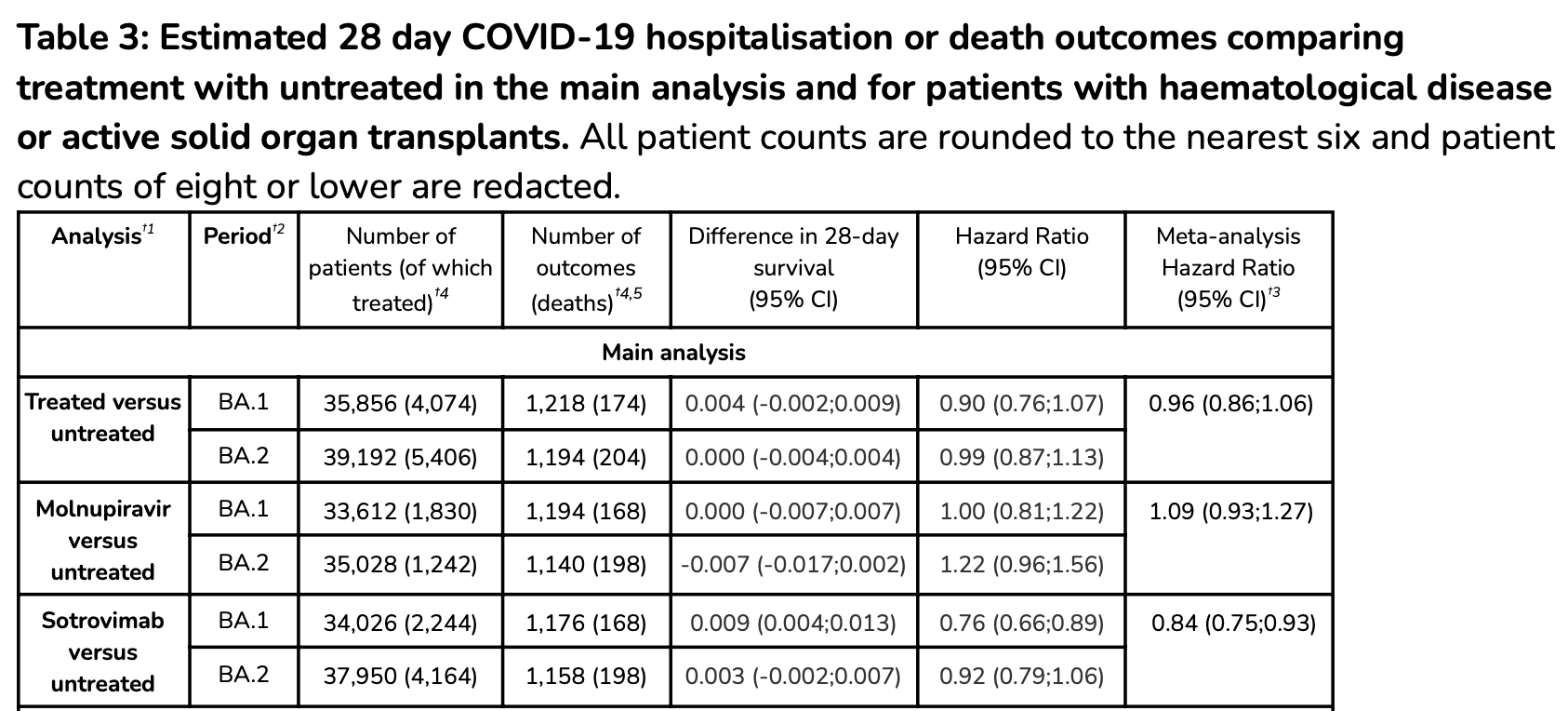
Background The effectiveness of COVID-19 monoclonal antibody and antiviral therapies against severe COVID-19 outcomes is unclear. Initial benefit was shown in unvaccinated patients and before the Omicron variant emerged. We used the OpenSAFELY platform to emulate target trials to estimate the effectiveness of sotrovimab or molnupiravir, versus no treatment.
Methods With the approval of NHS England, we derived population-based cohorts of non-hospitalised high-risk individuals in England testing positive for SARS-CoV-2 during periods of dominance of the BA.1 (16/12/2021-10/02/2022) and BA.2 (11/02/2022-21/05/2022) Omicron sublineages. We used the clone-censor-weight approach to estimate the effect of treatment with sotrovimab or molnupiravir initiated within 5 days after positive test versus no treatment. Hazard ratios (HR) for COVID-19 hospitalisation or death within 28 days were estimated using weighted Cox models.
Results
Of the 35,856 [BA.1 period] and 39,192 [BA.2 period] patients, 1,830 [BA.1] and 1,242 [BA.2] were treated with molnupiravir and 2,244 [BA.1] and 4,164 [BA.2] with sotrovimab. The estimated HRs for molnupiravir versus untreated were 1.00 (95%CI: 0.81;1.22) [BA.1] and 1.22 (0.96;1.56) [BA.2]; corresponding HRs for sotrovimab versus untreated were 0.76 (0.66;0.89) [BA.1] and 0.92 (0.79;1.06) [BA.2].
Interpretation Compared with no treatment, sotrovimab was associated with reduced risk of adverse outcomes after COVID-19 in the BA.1 period, but there was weaker evidence of benefit in the BA2 period. Molnupiravir was not associated with reduced risk in either period.
Authors' contributors The study was conceptualised by LAT, IJD, JACS, JT, LN, BZ, SJWE, BG and BMK; data was curated by LN, JT, BZ, ACAG, HJC, RH, RMS, CB, JC, JP, FH and SH; and formally analysed by LN and JT; funding was acquired by BG; the investigation was done by LN and JT; to the methodology was contributed by JT, LN, JACS, LAT, IJD, BZ, WJH, AS, CM, C; project administration was done by JT, LN, AJW, BMK, LAT and IJD; resources were provided by AM, AJW, BG, BMK and LAT; software was developed by LN, JT, ACAG, HJC, RH and RMS; the project was supervised by IJD and LAT; the study was validated by JT, LN, BZ, WJH, ACAG, HJC and RH; the results were visualised by JT and LN; the original draft was written by JT, LN and LAT; all authors were involved in draft revisions and approving the final draft for submission; all authors had full access to the OpenSAFELY platform and accept responsibility for the decision to submit for publication. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. Bennett Institute for Applied Data Science developers and principal investigators (WJH, RMS, AJW and BG) holding contracts with NHS England have access to the OpenSAFELY pseudonymised data tables as needed to develop the OpenSAFELY tools; these tools in turn enable researchers with OpenSAFELY Data Access Agreements to write and execute code for data management and data analysis (LN and JT) without direct..
References
Aggarwal, Beaty, Bennett, Change in effectiveness of sotrovimab for preventing hospitalization and mortality for at-risk COVID-19 outpatients during an Omicron BA.1 and BA.1.1-predominant phase, Int J Infect Dis IJID Off Publ Int Soc Infect Dis, doi:10.1016/j.ijid.2022.10.002
Aggarwal, Beaty, Bennett, Real-World Evidence of the Neutralizing Monoclonal Antibody Sotrovimab for Preventing Hospitalization and Mortality in COVID-19 Outpatients, J Infect Dis, doi:10.1093/infdis/jiac206
Bajema, Berry, Streja, Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. Veterans: target trial emulation studies with one-month and six-month outcomes, MedRxiv Prepr Serv Health Sci. Published online December, doi:10.1101/2022.12.05.22283134
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Blake, Leyrat, Mansfield, Propensity scores using missingness pattern information: a practical guide, Stat Med, doi:10.1002/sim.8503
Borenstein, Hedges, Higgins, Hr, A basic introduction to fixed-effect and random-effects models for meta-analysis, Res Synth Methods, doi:10.1002/jrsm.12
Brophy, Molnupiravir's authorisation was premature, BMJ, doi:10.1136/bmj.o443
Brown, Saund, Qureshi, Demographics and Outcomes of Initial Phase of COVID-19 Medicines Delivery Units Across 4 UK Centers During Peak B1.1.529 Omicron Epidemic: A Service Evaluation, Open Forum Infect Dis, doi:10.1093/ofid/ofac527
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, The Lancet, doi:10.1016/S0140-6736(22)02597-1
Cheng, Reyes, Satram, Real-World Effectiveness of Sotrovimab for the Early Treatment of COVID-19 During SARS-CoV-2 Delta and Omicron Waves in the USA, Infect Dis Ther, doi:10.1007/s40121-022-00755-0
England, Coronavirus, Interim clinical commissioning policy: Treatments for non-hospitalised patients with COVID-19
Fda, FDA updates Sotrovimab emergency use authorization
Green, Curtis, Higgins, Trends, variation, and clinical characteristics of recipients of antiviral drugs and neutralising monoclonal antibodies for covid-19 in community settings: retrospective, descriptive cohort study of 23.4 million people in OpenSAFELY, BMJ Med, doi:10.1136/bmjmed-2022-000276
Gupta, Gonzalez-Rojas, Juarez, Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, N Engl J Med, doi:10.1056/NEJMoa2107934
Hernán, How to estimate the effect of treatment duration on survival outcomes using observational data, BMJ, doi:10.1136/bmj.k182
Hernán, Robins, Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available, Am J Epidemiol, doi:10.1093/aje/kwv254
Hernán, Sauer, Hernández-Díaz, Platt, Shrier, Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses, J Clin Epidemiol, doi:10.1016/j.jclinepi.2016.04.014
Hernán, The Hazards of Hazard Ratios, Epidemiol Camb Mass, doi:10.1097/EDE.0b013e3181c1ea43
Lamontagne, Agarwal, Rochwerg, A living WHO guideline on drugs for covid-19, BMJ, doi:10.1136/bmj.m3379
Lund, Richardson, Stürmer, The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application, Curr Epidemiol Rep, doi:10.1007/s40471-015-0053-5
Maringe, Benitez Majano, Exarchakou, Assessing the benefit of major surgery for elderly lung cancer patients using observational data, Int J Epidemiol, doi:10.1093/ije/dyaa057
Nab, Parker, Andrews, Changes in COVID-19-related mortality across key demographic and clinical subgroups in England from 2020 to 2022: a retrospective cohort study using the OpenSAFELY platform, Lancet Public Health, doi:10.1016/S2468-2667(23)00079-8
Nice, NICE recommends 3 treatments for COVID-19 in final draft guidance
Piccicacco, Zeitler, Ing, Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge, J Antimicrob Chemother, doi:10.1093/jac/dkac256
Schneeweiss, Avorn, A review of uses of health care utilization databases for epidemiologic research on therapeutics, J Clin Epidemiol, doi:10.1016/j.jclinepi.2004.10.012
Schneeweiss, Patrick, Stürmer, Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results, Med Care, doi:10.1097/MLR.0b013e318070c08e
Stürmer, Wang, Golightly, Keil, Lund et al., Methodological considerations when analysing and interpreting real-world data, Rheumatol Oxf Engl, doi:10.1093/rheumatology/kez320
Suissa, Immortal Time Bias in Pharmacoepidemiology, Am J Epidemiol, doi:10.1093/aje/kwm324
Vanderweele, Unmeasured confounding and hazard scales: sensitivity analysis for total, direct, and indirect effects, Eur J Epidemiol, doi:10.1007/s10654-013-9770-6
Who, Therapeutics and COVID-19: Living guideline
Wu, Carr, Harvey, WHO's Therapeutics and COVID-19 Living Guideline on mAbs needs to be reassessed, The Lancet, doi:10.1016/S0140-6736(22)01938-9
Xie, Bowe, Al-Aly, Molnupiravir and risk of hospital admission or death in adults with covid-19: emulation of a randomized target trial using electronic health records, BMJ, doi:10.1136/bmj-2022-072705
Zaqout, Almaslamani, Chemaitelly, Effectiveness of the neutralizing antibody sotrovimab among high-risk patients with mild-to-moderate SARS-CoV-2 in Qatar, Int J Infect Dis IJID Off Publ Int Soc Infect Dis, doi:10.1016/j.ijid.2022.09.023
Zheng, Green, Tazare, Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform, BMJ, doi:10.1136/bmj-2022-071932
DOI record:
{
"DOI": "10.1101/2023.05.12.23289914",
"URL": "http://dx.doi.org/10.1101/2023.05.12.23289914",
"abstract": "<jats:p>Background The effectiveness of COVID-19 monoclonal antibody and antiviral therapies against severe COVID-19 outcomes is unclear. Initial benefit was shown in unvaccinated patients and before the Omicron variant emerged. We used the OpenSAFELY platform to emulate target trials to estimate the effectiveness of sotrovimab or molnupiravir, versus no treatment. Methods With the approval of NHS England, we derived population-based cohorts of non-hospitalised high-risk individuals in England testing positive for SARS-CoV-2 during periods of dominance of the BA.1 (16/12/2021-10/02/2022) and BA.2 (11/02/2022-21/05/2022) Omicron sublineages. We used the clone-censor-weight approach to estimate the effect of treatment with sotrovimab or molnupiravir initiated within 5 days after positive test versus no treatment. Hazard ratios (HR) for COVID-19 hospitalisation or death within 28 days were estimated using weighted Cox models. Results Of the 35,856 [BA.1 period] and 39,192 [BA.2 period] patients, 1,830 [BA.1] and 1,242 [BA.2] were treated with molnupiravir and 2,244 [BA.1] and 4,164 [BA.2] with sotrovimab. The estimated HRs for molnupiravir versus untreated were 1.00 (95%CI: 0.81;1.22) [BA.1] and 1.22 (0.96;1.56) [BA.2]; corresponding HRs for sotrovimab versus untreated were 0.76 (0.66;0.89) [BA.1] and 0.92 (0.79;1.06) [BA.2]. Interpretation Compared with no treatment, sotrovimab was associated with reduced risk of adverse outcomes after COVID-19 in the BA.1 period, but there was weaker evidence of benefit in the BA2 period. Molnupiravir was not associated with reduced risk in either period.</jats:p>",
"accepted": {
"date-parts": [
[
2023,
5,
16
]
]
},
"author": [
{
"affiliation": [],
"name": "The OpenSAFELY Collaborative",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-7194-2615",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tazare",
"given": "John",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1821-7246",
"affiliation": [],
"authenticated-orcid": false,
"family": "Nab",
"given": "Linda",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1814-6692",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zheng",
"given": "Bang",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9162-4999",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hulme",
"given": "William J",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7246-2074",
"affiliation": [],
"authenticated-orcid": false,
"family": "Green",
"given": "Amelia CA",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3429-9576",
"affiliation": [],
"authenticated-orcid": false,
"family": "Curtis",
"given": "Helen J",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3157-1127",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mahalingasivam",
"given": "Viyaasan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5295-4370",
"affiliation": [],
"authenticated-orcid": false,
"family": "Higgins",
"given": "Rose",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1637-837X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Schultze",
"given": "Anna",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5364-8757",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bhaskaran",
"given": "Krishnan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2098-1278",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mehrkar",
"given": "Amir",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3701-4997",
"affiliation": [],
"authenticated-orcid": false,
"family": "Schaffer",
"given": "Andrea L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Rebecca M",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0113-2593",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bates",
"given": "Christopher",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4681-4873",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cockburn",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parry",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hester",
"given": "Frank",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harper",
"given": "Sam",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0362-6717",
"affiliation": [],
"authenticated-orcid": false,
"family": "Eggo",
"given": "Rosalind M",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4932-6135",
"affiliation": [],
"authenticated-orcid": false,
"family": "Walker",
"given": "Alex J",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7585-4743",
"affiliation": [],
"authenticated-orcid": false,
"family": "Marks",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brown",
"given": "Michael",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8739-9565",
"affiliation": [],
"authenticated-orcid": false,
"family": "Maringe",
"given": "Camille",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4097-4577",
"affiliation": [],
"authenticated-orcid": false,
"family": "Leyrat",
"given": "Clemence",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1474-2596",
"affiliation": [],
"authenticated-orcid": false,
"family": "Evans",
"given": "Stephen JW",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5127-4728",
"affiliation": [],
"authenticated-orcid": false,
"family": "Goldacre",
"given": "Ben",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3786-9063",
"affiliation": [],
"authenticated-orcid": false,
"family": "MacKenna",
"given": "Brian",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8496-6053",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sterne",
"given": "Jonathan AC",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8848-9493",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tomlinson",
"given": "Laurie A",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8970-1406",
"affiliation": [],
"authenticated-orcid": false,
"family": "Douglas",
"given": "Ian J",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
5,
16
]
],
"date-time": "2023-05-16T21:15:21Z",
"timestamp": 1684271721000
},
"deposited": {
"date-parts": [
[
2023,
5,
16
]
],
"date-time": "2023-05-16T21:15:21Z",
"timestamp": 1684271721000
},
"group-title": "Epidemiology",
"indexed": {
"date-parts": [
[
2023,
5,
17
]
],
"date-time": "2023-05-17T04:55:30Z",
"timestamp": 1684299330035
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
5,
16
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2023.05.12.23289914",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2023,
5,
16
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2023,
5,
16
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2023.05.12.23289914"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Effectiveness of Sotrovimab and Molnupiravir in community settings in England across the Omicron BA.1 and BA.2 sublineages: emulated target trials using the OpenSAFELY platform",
"type": "posted-content"
}