Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkac256, Aug 2022
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
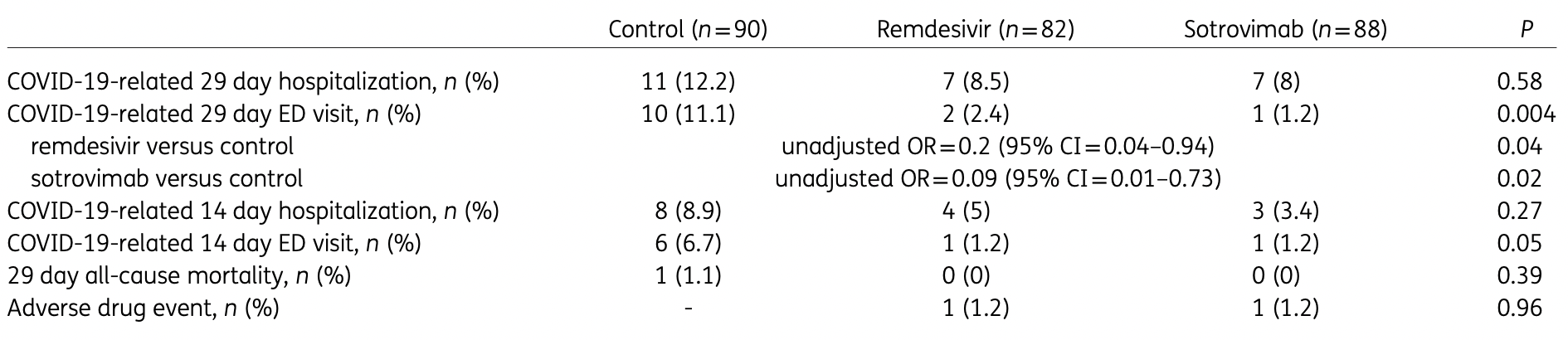
Retrospective high-risk outpatients in the USA, 82 treated with remdesivir, 88 with sotrovimab, and 90 control patients, showing significantly lower combined hospitalization/ER visits with both treatments in unadjusted results. The dominant variant was omicron B.1.1.529.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers remdesivir and sotrovimab.
|
risk of death, 66.4% lower, RR 0.34, p = 1.00, treatment 0 of 88 (0.0%), control 1 of 90 (1.1%), NNT 90, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 29.
|
|
risk of hospitalization, 34.9% lower, RR 0.65, p = 0.46, treatment 7 of 88 (8.0%), control 11 of 90 (12.2%), NNT 23, day 29.
|
|
risk of hospitalization/ER, 66.3% lower, RR 0.34, p = 0.01, treatment 7 of 88 (8.0%), control 21 of 90 (23.3%), NNT 6.5, odds ratio converted to relative risk, day 29.
|
|
risk of progression, 89.8% lower, RR 0.10, p = 0.009, treatment 1 of 88 (1.1%), control 10 of 90 (11.1%), NNT 10, ER visit, day 29.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Zhou et al., SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies, bioRxiv, doi:10.1101/2022.02.15.480166.
Piccicacco et al., 1 Aug 2022, retrospective, USA, peer-reviewed, 7 authors, study period 27 December, 2021 - 4 February, 2022, average treatment delay 4.4 days.
Contact: npiccicacco@tgh.org.
Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge
Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkac256
Background: Remdesivir and sotrovimab both have clinical trial data in the outpatient setting demonstrating reduction in the risk of hospitalizations and emergency department (ED) visits related to COVID-19. Objectives: To evaluate the effectiveness of remdesivir in comparison with sotrovimab and matched high-risk control patients in preventing COVID-19-related hospitalizations and ED visits during the Omicron B.1.1.529 surge. Patients and methods: This retrospective cohort study included outpatients positive for SARS-CoV-2, with nonsevere symptoms for ≤7 days and deemed high-risk for severe COVID-19 by an internal scoring matrix. Patients who received remdesivir or sotrovimab from 27/12/2021 to 04/02/2022 were included (n = 82 and n = 88, respectively). These were compared with a control cohort of high-risk COVID-19 outpatients who did not receive therapy (n = 90). The primary outcome was a composite of 29 day COVID-19-related hospitalizations and/or ED visits. Pre-specified secondary outcomes included components of the primary endpoint, 29 day all-cause mortality and serious adverse drug events. Results: Patients treated with remdesivir were significantly less likely to be hospitalized or visit the ED within 29 days from symptom onset (11% versus 23.3%; OR = 0.41, 95% CI = 0.17-0.95). Patients receiving sotrovimab were also less likely to be hospitalized or visit the ED (8% versus 23.3%; OR = 0.28, 95% CI = 0.11-0.71). There was no difference in the incidence of hospitalizations/ED visits between sotrovimab and remdesivir. Conclusions: Our highest-risk outpatients with Omicron-related COVID-19 who received early sotrovimab or remdesivir had significantly lower likelihoods of a hospitalization and/or ED visit.
Author contributions Concept and design: N.P., K.Z., A.I. and J.M. Acquisition, analysis or interpretation of data: N.P., K.Z., A.I. and J.M. Drafting of manuscript: N.P., A.I., K.Z., J.F. and S.S. Critical revision of the manuscript for important intellectual content: K.K. and J.M. Statistical analysis: N.P. and K.Z.
Supplementary data Table S1 is available as Supplementary data at JAC Online.
References
Agarwal, Rochwerg, Lamontagne, A living WHO guideline on drugs for covid-19, BMJ, doi:10.1136/bmj.m3379
Andrews, Stowe, Kirsebom, Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant, N Engl J Med, doi:10.1056/NEJMoa2119451
Bhimraj, Morgan, Shumaker, IDSA Guidelines on the Treatment and Management of Patients with COVID-19
Fda, FDA Takes Actions to Expand Use of Treatment for Outpatients With Mild-to-Moderate COVID-19
Gottlieb, Vaca, Paredes, Early remdesivir to prevent progression to severe Covid-19 in outpatients, N Engl J Med, doi:10.1056/NEJMoa2116846
Gupta, Gonzalez-Rojas, Juarez, Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2022.2832
Hui, Ho, Cheung, SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo, Nature, doi:10.1038/s41586-022-04479-6
Iuliano, Brunkard, Boehmer, Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods -United States, December 2020-January 2022, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7104e4
Lee, Wong, Chai, Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis, BMJ, doi:10.1136/bmj-2021-068632
Peters, Rabinstein, Dubrock, Use of remdesivir in myasthenia gravis and COVID-19, Pharmacotherapy, doi:10.1002/phar.2524
Piccicacco, Zeitler, Montero, Effectiveness of severe acute respiratory syndrome coronavirus 2 monoclonal antibody infusions in high-risk outpatients, Open Forum Infect Dis, doi:10.1093/ofid/ofab292
Planas, Saunders, Maes, Considerable escape of SARS-CoV-2 Omicron to antibody neutralization, Nature, doi:10.1038/s41586-021-04389-z
Razonable, Ganesh, Bierle, Clinical prioritization of antispike monoclonal antibody treatment of mild to moderate Outpatient remdesivir versus sotrovimab for Omicron COVID-19, Mayo Clin Proc, doi:10.1016/j.mayocp.2021.11.017
Stolle, Nalamasu, Pergolizzi, Fact vs fallacy: the anti-vaccine discussion reloaded, Adv Ther, doi:10.1007/s12325-020-01502-y
Tseng, Ackerson, Luo, Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants, Nat Med, doi:10.1038/s41591-022-01753-y
Yek, Warner, Wiltz, Risk factors for severe COVID-19 outcomes among persons aged ≥18 years who completed a primary COVID-19 vaccination series -465 health care facilities, United States, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7101a4
Zhu, Zhang, A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.1093/jac/dkac256",
"ISSN": [
"0305-7453",
"1460-2091"
],
"URL": "http://dx.doi.org/10.1093/jac/dkac256",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Remdesivir and sotrovimab both have clinical trial data in the outpatient setting demonstrating reduction in the risk of hospitalizations and emergency department (ED) visits related to COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Objectives</jats:title>\n <jats:p>To evaluate the effectiveness of remdesivir in comparison with sotrovimab and matched high-risk control patients in preventing COVID-19-related hospitalizations and ED visits during the Omicron B.1.1.529 surge.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Patients and methods</jats:title>\n <jats:p>This retrospective cohort study included outpatients positive for SARS-CoV-2, with non-severe symptoms for ≤7 days and deemed high-risk for severe COVID-19 by an internal scoring matrix. Patients who received remdesivir or sotrovimab from 27/12/2021 to 04/02/2022 were included (n = 82 and n = 88, respectively). These were compared with a control cohort of high-risk COVID-19 outpatients who did not receive therapy (n = 90). The primary outcome was a composite of 29 day COVID-19-related hospitalizations and/or ED visits. Pre-specified secondary outcomes included components of the primary endpoint, 29 day all-cause mortality and serious adverse drug events.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Patients treated with remdesivir were significantly less likely to be hospitalized or visit the ED within 29 days from symptom onset (11% versus 23.3%; OR = 0.41, 95% CI = 0.17–0.95). Patients receiving sotrovimab were also less likely to be hospitalized or visit the ED (8% versus 23.3%; OR = 0.28, 95% CI = 0.11–0.71). There was no difference in the incidence of hospitalizations/ED visits between sotrovimab and remdesivir.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Our highest-risk outpatients with Omicron-related COVID-19 who received early sotrovimab or remdesivir had significantly lower likelihoods of a hospitalization and/or ED visit.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6246-7533",
"affiliation": [
{
"name": "Department of Pharmacy, Tampa General Hospital , Tampa, FL , USA"
}
],
"authenticated-orcid": false,
"family": "Piccicacco",
"given": "Nicholas",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Pharmacy, Tampa General Hospital , Tampa, FL , USA"
}
],
"family": "Zeitler",
"given": "Kristen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy, Vanderbilt University Medical Center , Nashville, TN , USA"
}
],
"family": "Ing",
"given": "Austin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Internal Medicine, University of South Florida Morsani College of Medicine , Tampa, FL , USA"
}
],
"family": "Montero",
"given": "Jose",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Esoteric Testing/R&D and Microbiology Laboratories, Tampa General Hospital , Tampa, FL , USA"
}
],
"family": "Faughn",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Esoteric Testing/R&D and Microbiology Laboratories, Tampa General Hospital , Tampa, FL , USA"
}
],
"family": "Silbert",
"given": "Suzane",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3384-152X",
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Internal Medicine, University of South Florida Morsani College of Medicine , Tampa, FL , USA"
},
{
"name": "Global Emerging Diseases Institute, Tampa General Hospital , Tampa, FL , USA"
}
],
"authenticated-orcid": false,
"family": "Kim",
"given": "Kami",
"sequence": "additional"
}
],
"container-title": "Journal of Antimicrobial Chemotherapy",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
1
]
],
"date-time": "2022-08-01T16:50:21Z",
"timestamp": 1659372621000
},
"deposited": {
"date-parts": [
[
2022,
8,
1
]
],
"date-time": "2022-08-01T16:50:37Z",
"timestamp": 1659372637000
},
"indexed": {
"date-parts": [
[
2022,
8,
2
]
],
"date-time": "2022-08-02T04:28:47Z",
"timestamp": 1659414527716
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/journals/pages/open_access/funder_policies/chorus/standard_publication_model",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
1
]
],
"date-time": "2022-08-01T00:00:00Z",
"timestamp": 1659312000000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/jac/advance-article-pdf/doi/10.1093/jac/dkac256/45210080/dkac256.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jac/advance-article-pdf/doi/10.1093/jac/dkac256/45210080/dkac256.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
8,
1
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
1
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"author": "Johns Hopkins University",
"key": "2022080116401611900_dkac256-B1"
},
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A novel coronavirus from patients with pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "N Engl J Med",
"key": "2022080116401611900_dkac256-B2",
"volume": "382",
"year": "2020"
},
{
"author": "CDC",
"key": "2022080116401611900_dkac256-B3"
},
{
"DOI": "10.15585/mmwr.mm7101a4",
"article-title": "Risk factors for severe COVID-19 outcomes among persons aged ≥18 years who completed a primary COVID-19 vaccination series - 465 health care facilities, United States, December 2020-October 2021",
"author": "Yek",
"doi-asserted-by": "crossref",
"first-page": "19",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2022080116401611900_dkac256-B4",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-04389-z",
"article-title": "Considerable escape of SARS-CoV-2 Omicron to antibody neutralization",
"author": "Planas",
"doi-asserted-by": "crossref",
"first-page": "671",
"journal-title": "Nature",
"key": "2022080116401611900_dkac256-B5",
"volume": "602",
"year": "2022"
},
{
"author": "FDA",
"key": "2022080116401611900_dkac256-B6"
},
{
"author": "FDA",
"key": "2022080116401611900_dkac256-B7"
},
{
"DOI": "10.1001/jama.2022.2832",
"article-title": "Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1236",
"journal-title": "JAMA",
"key": "2022080116401611900_dkac256-B8",
"volume": "327",
"year": "2022"
},
{
"author": "Gilead Sciences, Inc",
"key": "2022080116401611900_dkac256-B9"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early remdesivir to prevent progression to severe Covid-19 in outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N Engl J Med",
"key": "2022080116401611900_dkac256-B10",
"volume": "386",
"year": "2022"
},
{
"author": "NIH",
"key": "2022080116401611900_dkac256-B11"
},
{
"author": "Bhimraj",
"key": "2022080116401611900_dkac256-B12",
"year": "2022"
},
{
"DOI": "10.1136/bmj.m3379",
"article-title": "A living WHO guideline on drugs for covid-19",
"author": "Agarwal",
"doi-asserted-by": "crossref",
"first-page": "m3379",
"journal-title": "BMJ",
"key": "2022080116401611900_dkac256-B13",
"volume": "370",
"year": "2020"
},
{
"author": "FDA",
"key": "2022080116401611900_dkac256-B14"
},
{
"DOI": "10.1016/j.mayocp.2021.11.017",
"article-title": "Clinical prioritization of antispike monoclonal antibody treatment of mild to moderate COVID-19",
"author": "Razonable",
"doi-asserted-by": "crossref",
"first-page": "26",
"journal-title": "Mayo Clin Proc",
"key": "2022080116401611900_dkac256-B15",
"volume": "97",
"year": "2022"
},
{
"DOI": "10.1093/ofid/ofab292",
"article-title": "Effectiveness of severe acute respiratory syndrome coronavirus 2 monoclonal antibody infusions in high-risk outpatients",
"author": "Piccicacco",
"doi-asserted-by": "crossref",
"first-page": "ofab292",
"journal-title": "Open Forum Infect Dis",
"key": "2022080116401611900_dkac256-B16",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.15585/mmwr.mm7104e4",
"article-title": "Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022",
"author": "Iuliano",
"doi-asserted-by": "crossref",
"first-page": "146",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2022080116401611900_dkac256-B17",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-01753-y",
"article-title": "Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants",
"author": "Tseng",
"doi-asserted-by": "crossref",
"first-page": "1063",
"journal-title": "Nat Med",
"key": "2022080116401611900_dkac256-B18",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2119451",
"article-title": "Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant",
"author": "Andrews",
"doi-asserted-by": "crossref",
"first-page": "1532",
"journal-title": "N Engl J Med",
"key": "2022080116401611900_dkac256-B19",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04479-6",
"article-title": "SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo",
"author": "Hui",
"doi-asserted-by": "crossref",
"first-page": "715",
"journal-title": "Nature",
"key": "2022080116401611900_dkac256-B20",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1136/bmj-2021-068632",
"article-title": "Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "e068632",
"journal-title": "BMJ",
"key": "2022080116401611900_dkac256-B21",
"volume": "376",
"year": "2022"
},
{
"DOI": "10.1002/phar.2524",
"article-title": "Use of remdesivir in myasthenia gravis and COVID-19",
"author": "Peters",
"doi-asserted-by": "crossref",
"first-page": "546",
"journal-title": "Pharmacotherapy",
"key": "2022080116401611900_dkac256-B22",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1007/s12325-020-01502-y",
"article-title": "Fact vs fallacy: the anti-vaccine discussion reloaded",
"author": "Stolle",
"doi-asserted-by": "crossref",
"first-page": "4481",
"journal-title": "Adv Ther",
"key": "2022080116401611900_dkac256-B23",
"volume": "37",
"year": "2020"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jac/advance-article/doi/10.1093/jac/dkac256/6652940"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Pharmacology (medical)",
"Pharmacology",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge",
"type": "journal-article"
}
