Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients With Mild to Moderate COVID-19
et al., JAMA, doi:10.1001/jama.2022.2832 (results published 12/4/21), COMET-ICE, NCT04545060, Dec 2021
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
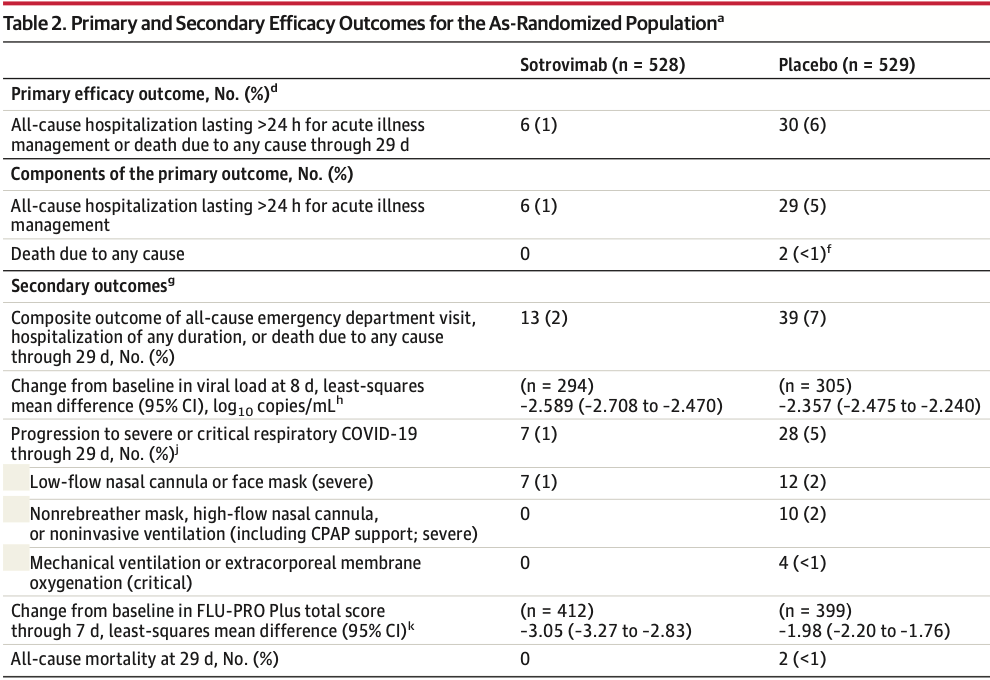
RCT 1,057 outpatients, 529 treated with sotrovimab, showing significantly lower hospitalization >24h or mortality with treatment.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
|
risk of death, 80.0% lower, RR 0.20, p = 0.50, treatment 0 of 528 (0.0%), control 2 of 529 (0.4%), NNT 264, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 29.
|
|
risk of mechanical ventilation, 88.9% lower, RR 0.11, p = 0.12, treatment 0 of 528 (0.0%), control 4 of 529 (0.8%), NNT 132, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 29.
|
|
risk of progression, 75.0% lower, RR 0.25, p < 0.001, treatment 7 of 528 (1.3%), control 28 of 529 (5.3%), NNT 25, day 29.
|
|
risk of hospitalization >24hrs or death, 79.0% lower, RR 0.21, p < 0.001, treatment 6 of 528 (1.1%), control 30 of 529 (5.7%), NNT 22, day 29, ITT, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Conflicts of interest:
research funding from the drug patent holder, employee of the drug patent holder.
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Gupta et al., 4 Dec 2021, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, 68 authors, study period 27 August, 2020 - 2 September, 2021, average treatment delay 2.6 days, trial NCT04545060 (history) (COMET-ICE).
Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients With Mild to Moderate COVID-19
JAMA, doi:10.1001/jama.2022.2832
IMPORTANCE Older patients and those with comorbidities who are infected with SARS-CoV-2 may be at increased risk of hospitalization and death. Sotrovimab is a neutralizing antibody for the treatment of high-risk patients to prevent COVID-19 progression. OBJECTIVE To evaluate the efficacy and adverse events of sotrovimab in preventing progression of mild to moderate COVID-19 to severe disease.
Conclusions Among nonhospitalized patients with mild to moderate COVID-19 and at risk of disease progression, a single intravenous dose of sotrovimab, compared with placebo, signifi-cantly reduced the risk of a composite end point of all-cause hospitalization or death through day 29. The findings support sotrovimab as a treatment option for nonhospitalized, high-risk patients with mild to moderate COVID-19, although efficacy against SARS-CoV-2 variants that have emerged since the study was completed is unknown. Role of the Funder/Sponsor: The sponsors designed the trial and were involved in the conduct of the trial, data collection, management, analysis, and interpretation; participated in the preparation and review of the manuscript; but did not have the right to veto publication. The authors selected the journal and made the decision to submit the manuscript for publication. Group Information: A list of the COMET-ICE Investigators appears in Supplement 3.
Data Sharing Statement: See Supplement 4. Additional Contributions: We thank Courtney St Amour, PhD (Cello Health Communications/ SciFluent), for medical writing support, which was funded by Vir Biotechnology Inc and GlaxoSmithKline. We also thank Krystyna Grycz, BA, Jordan Clark, BS, and Minnie Kuo, MS (all 3 employed by Vir Biotechnology Inc), for clinical operations support. These individuals were compensated as part of their normal salaries.
References
Cameroni, Bowen, Rosen, Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift, Nature. Published online, doi:10.1038/s41586-021-04386-2
Cervantes, Martin, Frank, Experiences of Latinx individuals hospitalized for COVID-19: a qualitative study, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.0684?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2022.2832
Chen, Nirula, Heller, None
Craft, Travassos, Palacios, Openshaw, Inadequate minority representation within SARS-CoV-2 vaccine trials, Am J Trop Med Hyg, doi:10.4269/ajtmh.20-1294
Fajnzylber, Regan, Coxen, Massachusetts Consortium for Pathogen Readiness. SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat Commun, doi:10.1038/s41467-020-19057-5
Flores, Frontera, Andrasik, Assessment of the inclusion of racial/ethnic minority, female, and older individuals in vaccine clinical trials, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.37640?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2022.2832
Focosi, Maggi, Neutralising antibody escape of SARS-CoV-2 spike protein: risk assessment for antibody-based Covid-19 therapeutics and vaccines, Rev Med Virol, doi:10.1002/rmv.2231
Gao, Ding, Dong, Risk factors for severe and critically ill COVID-19 patients: a review, Allergy, doi:10.1111/all.14657
Gaudinski, Coates, Houser, Study Team. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults, PLoS Med, doi:10.1371/journal.pmed.1002493
Gupta, Gonzalez-Rojas, Juarez, Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med, doi:10.1056/NEJMoa2107934
Han, Poon, Powers, Iii, Leidy et al., Using the Influenza Patient-Reported Outcome (FLU-PRO) diary to evaluate symptoms of influenza viral infection in a healthy human challenge model, BMC Infect Dis, doi:10.1186/s12879-018-3220-8
Hwang, Shih, Cani, Arvin, Fink et al., Group sequential designs using a family of type I error probability spending functions, Stat Med, doi:10.1038/s41586-020-2538-8
Investigators, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Lan, Demets, Discrete sequential boundaries for clinical trials, Biometrika, doi:10.2307/2336502
Lempp, Soriaga, Montiel-Ruiz, Lectins enhance SARS-CoV-2 infection and influence neutralizing antibodies, Nature, doi:10.1038/s41586-021-03925-1
Levey, Bosch, Lewis, Greene, Rogers et al., Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients With Mild to Moderate COVID-19 Original Investigation Research jama.com, Ann Intern Med, doi:10.7326/0003-4819-130-6-199903160-00002
Liu, Wei, Zhang, V2 and 501Y.V3 variants of SARS-CoV-2 lose binding to bamlanivimab in vitro, MAbs, doi:10.1080/19420862.2021.1919285
Lustig, Zuckerman, Nemet, Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel, Euro Surveill, doi:10.2807/1560-7917.ES.2021.26.26.2100557
Pinto, Park, Beltramello, Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody, Nature, doi:10.1038/s41586-020-2349-y
Riley, Chen, Matthay, Excess mortality among Latino people in California during the COVID-19 pandemic, SSM Popul Health, doi:10.1016/j.ssmph.2021.100860
Seyedalinaghi, Afsahi, Mohssenipour, Late complications of COVID-19; a systematic review of current evidence, Arch Acad Emerg Med
Starr, Greaney, Dingens, Bloom, Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016, Cell Rep Med, doi:10.1016/j.xcrm.2021.100255
Stokes, Zambrano, Anderson, Coronavirus disease 2019 case surveillance-United States, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm6924e2
Vanblargan, Errico, Halfmann, An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies, Nat Med. Published online, doi:10.1038/s41591-021-01678-y
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review, JAMA, doi:10.1001/jama.2020.12839?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2022.2832
Zheng, Yu, Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study, BMJ, doi:10.1136/bmj.m1443
DOI record:
{
"DOI": "10.1001/jama.2022.2832",
"ISSN": [
"0098-7484"
],
"URL": "http://dx.doi.org/10.1001/jama.2022.2832",
"author": [
{
"affiliation": [
{
"name": "Albion Finch Medical, William Osler Health Centre, Toronto, Ontario, Canada"
}
],
"family": "Gupta",
"given": "Anil",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Optimus U Corp, Miami, Florida"
}
],
"family": "Gonzalez-Rojas",
"given": "Yaneicy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Florida International Medical Research, Miami"
}
],
"family": "Juarez",
"given": "Erick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Pontevedra, Spain"
}
],
"family": "Crespo Casal",
"given": "Manuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pines Care Research Center, Pembroke Pines, Florida"
}
],
"family": "Moya",
"given": "Jaynier",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil"
}
],
"family": "Rodrigues Falci",
"given": "Diego",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sarkis Clinical Trials, Gainesville, Florida"
}
],
"family": "Sarkis",
"given": "Elias",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centex Studies, McAllen, Texas"
}
],
"family": "Solis",
"given": "Joel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vir Biotechnology Inc, San Francisco, California"
}
],
"family": "Zheng",
"given": "Hanzhe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "GlaxoSmithKline, Brentford, England"
}
],
"family": "Scott",
"given": "Nicola",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vir Biotechnology Inc, San Francisco, California"
}
],
"family": "Cathcart",
"given": "Andrea L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vir Biotechnology Inc, San Francisco, California"
}
],
"family": "Parra",
"given": "Sergio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vir Biotechnology Inc, San Francisco, California"
}
],
"family": "Sager",
"given": "Jennifer E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "GlaxoSmithKline, Brentford, England"
}
],
"family": "Austin",
"given": "Daren",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "GlaxoSmithKline, Cambridge, Massachusetts"
}
],
"family": "Peppercorn",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vir Biotechnology Inc, San Francisco, California"
}
],
"family": "Alexander",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vir Biotechnology Inc, San Francisco, California"
}
],
"family": "Yeh",
"given": "Wendy W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Central Texas Clinical Research, Austin"
}
],
"family": "Brinson",
"given": "Cynthia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vir Biotechnology Inc, San Francisco, California"
}
],
"family": "Aldinger",
"given": "Melissa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Departments of Global Health and Medicine, University of Washington, Seattle"
},
{
"name": "Fred Hutchinson Cancer Research Center, Seattle, Washington"
}
],
"family": "Shapiro",
"given": "Adrienne E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Free",
"given": "Almena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Johnson",
"given": "Kimball",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Cordasco",
"given": "Edward",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Little",
"given": "Raymond",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Bajwa",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Doshi",
"given": "Ankur",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Focil",
"given": "Augusto",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Hussain",
"given": "Rubaba (Rubie)",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Bostick",
"given": "Greg",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Somodevilla",
"given": "Guillermo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Ali",
"given": "Hasan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Kowalczyk",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Mittal",
"given": "Shilpi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Caso",
"given": "Jorge",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Goisse",
"given": "Marcy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Espinal",
"given": "Ladynez",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Zepeda",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Nguyen",
"given": "Thinh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Martinez",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Alvarez",
"given": "German",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Pucillo",
"given": "Ronald",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Seep",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Parikh",
"given": "Naval",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Escobar",
"given": "Victor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Curra",
"given": "Armando",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Dal Maso",
"given": "Vinicius",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "O'Mahony",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Ramacciotti",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Diaz",
"given": "Jorge",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Luz",
"given": "Kleber",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Ruane",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Mochlera",
"given": "Bharat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Roldan Sanchez",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Hernandez",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Fernandez",
"given": "Alfredo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Leavitt",
"given": "Glenn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Azizad",
"given": "Masoud",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Afzal",
"given": "Haider",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Fatakia",
"given": "Adil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Narejos Perez",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Stadnik",
"given": "Claudio Marcel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Gorgos",
"given": "Linda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Sachdeva",
"given": "Yessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Segura",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Quandros",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Perry",
"given": "Russell",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the COMET-ICE Investigators"
}
],
"family": "Sher",
"given": "Lawrence",
"sequence": "additional"
},
{
"affiliation": [],
"name": "COMET-ICE Investigators",
"sequence": "additional"
}
],
"container-title": [
"JAMA"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
14
]
],
"date-time": "2022-03-14T15:03:45Z",
"timestamp": 1647270225000
},
"deposited": {
"date-parts": [
[
2022,
4,
5
]
],
"date-time": "2022-04-05T15:02:48Z",
"timestamp": 1649170968000
},
"indexed": {
"date-parts": [
[
2022,
4,
21
]
],
"date-time": "2022-04-21T06:26:03Z",
"timestamp": 1650522363106
},
"is-referenced-by-count": 1,
"issn-type": [
{
"type": "print",
"value": "0098-7484"
}
],
"issue": "13",
"issued": {
"date-parts": [
[
2022,
4,
5
]
]
},
"journal-issue": {
"issue": "13",
"published-print": {
"date-parts": [
[
2022,
4,
5
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jama/articlepdf/2790246/jama_gupta_2022_oi_220023_1648650464.44846.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "1236",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2022,
4,
5
]
]
},
"published-print": {
"date-parts": [
[
2022,
4,
5
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"article-title": "Late complications of COVID-19; a systematic review of current evidence.",
"author": "SeyedAlinaghi",
"issue": "1",
"journal-title": "Arch Acad Emerg Med",
"key": "joi220023r2",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1111/all.v76.2",
"article-title": "Risk factors for severe and critically ill COVID-19 patients: a review.",
"author": "Gao",
"doi-asserted-by": "publisher",
"first-page": "428",
"issue": "2",
"journal-title": "Allergy",
"key": "joi220023r3",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.2807/1560-7917.ES.2021.26.26.2100557",
"article-title": "Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel.",
"author": "Lustig",
"doi-asserted-by": "crossref",
"issue": "26",
"journal-title": "Euro Surveill",
"key": "joi220023r5",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2349-y",
"article-title": "Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody.",
"author": "Pinto",
"doi-asserted-by": "publisher",
"first-page": "290",
"issue": "7815",
"journal-title": "Nature",
"key": "joi220023r7",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1371/journal.pmed.1002493",
"article-title": "Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults.",
"author": "Gaudinski",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "PLoS Med",
"key": "joi220023r8",
"volume": "15",
"year": "2018"
},
{
"DOI": "10.1002/rmv.2231",
"article-title": "Neutralising antibody escape of SARS-CoV-2 spike protein: risk assessment for antibody-based Covid-19 therapeutics and vaccines.",
"author": "Focosi",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "Rev Med Virol",
"key": "joi220023r9",
"volume": "31",
"year": "2021"
},
{
"article-title": "Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016.",
"author": "Starr",
"issue": "4",
"journal-title": "Cell Rep Med",
"key": "joi220023r10",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1080/19420862.2021.1919285",
"article-title": "501Y.V2 and 501Y.V3 variants of SARS-CoV-2 lose binding to bamlanivimab in vitro.",
"author": "Liu",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "MAbs",
"key": "joi220023r11",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03925-1",
"article-title": "Lectins enhance SARS-CoV-2 infection and influence neutralizing antibodies.",
"author": "Lempp",
"doi-asserted-by": "publisher",
"first-page": "342",
"issue": "7880",
"journal-title": "Nature",
"key": "joi220023r13",
"volume": "598",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab.",
"author": "Gupta",
"doi-asserted-by": "publisher",
"first-page": "1941",
"issue": "21",
"journal-title": "N Engl J Med",
"key": "joi220023r14",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.7326/0003-4819-130-6-199903160-00002",
"article-title": "A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation.",
"author": "Levey",
"doi-asserted-by": "publisher",
"first-page": "461",
"issue": "6",
"journal-title": "Ann Intern Med",
"key": "joi220023r15",
"volume": "130",
"year": "1999"
},
{
"DOI": "10.1186/s12879-018-3220-8",
"article-title": "Using the Influenza Patient-Reported Outcome (FLU-PRO) diary to evaluate symptoms of influenza viral infection in a healthy human challenge model.",
"author": "Han",
"doi-asserted-by": "publisher",
"first-page": "353",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "joi220023r17",
"volume": "18",
"year": "2018"
},
{
"DOI": "10.15585/mmwr.mm6924e2",
"article-title": "Coronavirus disease 2019 case surveillance—United States, January 22-May 30, 2020.",
"author": "Stokes",
"doi-asserted-by": "publisher",
"first-page": "759",
"issue": "24",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "joi220023r18",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.2307/2336502",
"article-title": "Discrete sequential boundaries for clinical trials.",
"author": "Lan",
"doi-asserted-by": "publisher",
"first-page": "659",
"issue": "3",
"journal-title": "Biometrika",
"key": "joi220023r19",
"volume": "70",
"year": "1983"
},
{
"DOI": "10.1002/(ISSN)1097-0258",
"article-title": "Group sequential designs using a family of type I error probability spending functions.",
"author": "Hwang",
"doi-asserted-by": "publisher",
"first-page": "1439",
"issue": "12",
"journal-title": "Stat Med",
"key": "joi220023r20",
"volume": "9",
"year": "1990"
},
{
"DOI": "10.1038/s41586-020-2538-8",
"article-title": "A perspective on potential antibody-dependent enhancement of SARS-CoV-2.",
"author": "Arvin",
"doi-asserted-by": "publisher",
"first-page": "353",
"issue": "7821",
"journal-title": "Nature",
"key": "joi220023r21",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.12839",
"article-title": "Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review.",
"author": "Wiersinga",
"doi-asserted-by": "publisher",
"first-page": "782",
"issue": "8",
"journal-title": "JAMA",
"key": "joi220023r22",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1443",
"article-title": "Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study.",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "m1443",
"journal-title": "BMJ",
"key": "joi220023r23",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"article-title": "SARS-CoV-2 viral load is associated with increased disease severity and mortality.",
"author": "Fajnzylber",
"doi-asserted-by": "publisher",
"first-page": "5493",
"issue": "1",
"journal-title": "Nat Commun",
"key": "joi220023r24",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19.",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "229",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "joi220023r25",
"volume": "384",
"year": "2021"
},
{
"article-title": "Excess mortality among Latino people in California during the COVID-19 pandemic.",
"author": "Riley",
"journal-title": "SSM Popul Health",
"key": "joi220023r26",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2020.37640",
"article-title": "Assessment of the inclusion of racial/ethnic minority, female, and older individuals in vaccine clinical trials.",
"author": "Flores",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "JAMA Netw Open",
"key": "joi220023r27",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.4269/ajtmh.20-1294",
"article-title": "Inadequate minority representation within SARS-CoV-2 vaccine trials.",
"author": "Craft",
"doi-asserted-by": "publisher",
"first-page": "32",
"issue": "1",
"journal-title": "Am J Trop Med Hyg",
"key": "joi220023r28",
"volume": "104",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.0684",
"article-title": "Experiences of Latinx individuals hospitalized for COVID-19: a qualitative study.",
"author": "Cervantes",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "JAMA Netw Open",
"key": "joi220023r29",
"volume": "4",
"year": "2021"
},
{
"article-title": "Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift.",
"author": "Cameroni",
"journal-title": "Nature",
"key": "joi220023r31"
},
{
"article-title": "An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies.",
"author": "VanBlargan",
"journal-title": "Nat Med",
"key": "joi220023r32"
},
{
"key": "joi220023r1",
"unstructured": "World Health Organization. WHO coronavirus (COVID-19) dashboard. Accessed January 3, 2022. https://covid19.who.int"
},
{
"key": "joi220023r4",
"unstructured": "US Centers for Disease Control and Prevention. SARS-CoV-2 variant classifications and definitions. Accessed January 13, 2022. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html"
},
{
"key": "joi220023r6",
"unstructured": "National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. Accessed January 28, 2022. https://www.covid19treatmentguidelines.nih.gov"
},
{
"key": "joi220023r12",
"unstructured": "US Food and Drug Administration. Fact sheet for healthcare providers emergency use authorization (EUA) of sotrovimab. Accessed February 10, 2022. https://www.fda.gov/media/149534/download"
},
{
"key": "joi220023r16",
"unstructured": "US Food and Drug Administration. COVID-19: developing drugs and biological products for treatment or prevention. Published February 2021. Accessed September 17, 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/covid-19-developing-drugs-and-biological-products-treatment-or-prevention"
},
{
"key": "joi220023r30",
"unstructured": "National Institutes of Health. OpenData Portal: SARS-CoV-2 variants and therapeutics. Updated February 3, 2022. Accessed February 10, 2022. https://opendata.ncats.nih.gov/variant/activity"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jama/fullarticle/2790246"
}
},
"score": 1,
"short-container-title": [
"JAMA"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": [
"Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients With Mild to Moderate COVID-19"
],
"type": "journal-article",
"volume": "327"
}
