Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkac256, Aug 2022
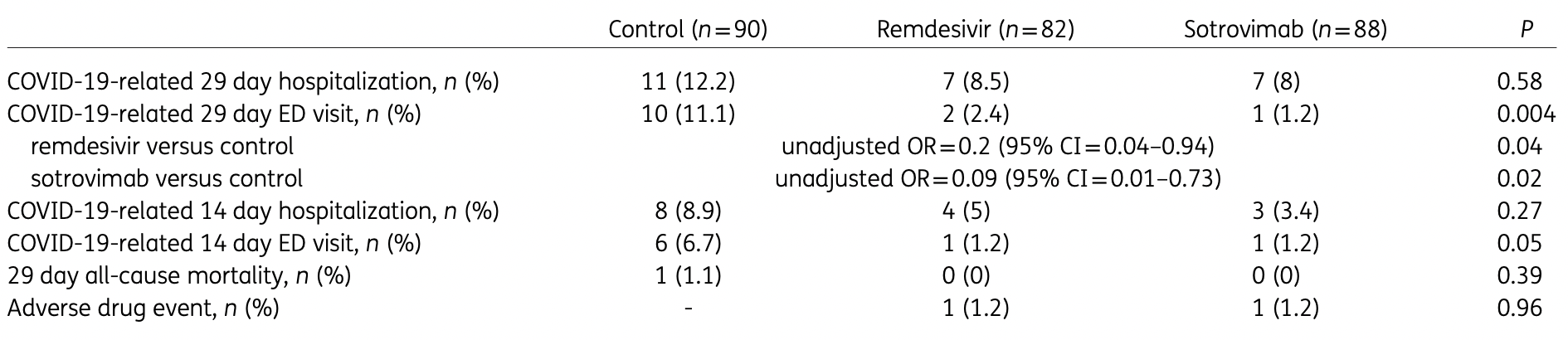
Retrospective high-risk outpatients in the USA, 82 treated with remdesivir, 88 with sotrovimab, and 90 control patients, showing significantly lower combined hospitalization/ER visits with both treatments in unadjusted results. The dominant variant was omicron B.1.1.529.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Remdesivir efficacy disappears with longer
followup. Mixed-effects meta-regression of efficacy as a function of
followup duration across all remdesivir studies shows decreasing efficacy with
longer followup15. This may reflect
antiviral efficacy being offset by serious adverse effects of treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments16.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers remdesivir and sotrovimab.
|
risk of death, 65.6% lower, RR 0.34, p = 1.00, treatment 0 of 82 (0.0%), control 1 of 90 (1.1%), NNT 90, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 29.
|
|
risk of hospitalization, 30.2% lower, RR 0.70, p = 0.47, treatment 7 of 82 (8.5%), control 11 of 90 (12.2%), NNT 27, day 29.
|
|
risk of hospitalization/ER, 52.5% lower, RR 0.48, p = 0.05, treatment 9 of 82 (11.0%), control 21 of 90 (23.3%), NNT 8.1, odds ratio converted to relative risk, day 29.
|
|
risk of progression, 78.0% lower, RR 0.22, p = 0.03, treatment 2 of 82 (2.4%), control 10 of 90 (11.1%), NNT 12, day 29.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
13.
Mohammed et al., Bradycardia associated with remdesivir treatment in coronavirus disease 2019 patients: A propensity score-matched analysis, Medicine, doi:10.1097/MD.0000000000044501.
Piccicacco et al., 1 Aug 2022, retrospective, USA, peer-reviewed, 7 authors, study period 27 December, 2021 - 4 February, 2022, average treatment delay 4.0 days, ER visit.
Contact: npiccicacco@tgh.org.
Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge
Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkac256
Background: Remdesivir and sotrovimab both have clinical trial data in the outpatient setting demonstrating reduction in the risk of hospitalizations and emergency department (ED) visits related to COVID-19. Objectives: To evaluate the effectiveness of remdesivir in comparison with sotrovimab and matched high-risk control patients in preventing COVID-19-related hospitalizations and ED visits during the Omicron B.1.1.529 surge. Patients and methods: This retrospective cohort study included outpatients positive for SARS-CoV-2, with nonsevere symptoms for ≤7 days and deemed high-risk for severe COVID-19 by an internal scoring matrix. Patients who received remdesivir or sotrovimab from 27/12/2021 to 04/02/2022 were included (n = 82 and n = 88, respectively). These were compared with a control cohort of high-risk COVID-19 outpatients who did not receive therapy (n = 90). The primary outcome was a composite of 29 day COVID-19-related hospitalizations and/or ED visits. Pre-specified secondary outcomes included components of the primary endpoint, 29 day all-cause mortality and serious adverse drug events. Results: Patients treated with remdesivir were significantly less likely to be hospitalized or visit the ED within 29 days from symptom onset (11% versus 23.3%; OR = 0.41, 95% CI = 0.17-0.95). Patients receiving sotrovimab were also less likely to be hospitalized or visit the ED (8% versus 23.3%; OR = 0.28, 95% CI = 0.11-0.71). There was no difference in the incidence of hospitalizations/ED visits between sotrovimab and remdesivir. Conclusions: Our highest-risk outpatients with Omicron-related COVID-19 who received early sotrovimab or remdesivir had significantly lower likelihoods of a hospitalization and/or ED visit.
Author contributions Concept and design: N.P., K.Z., A.I. and J.M. Acquisition, analysis or interpretation of data: N.P., K.Z., A.I. and J.M. Drafting of manuscript: N.P., A.I., K.Z., J.F. and S.S. Critical revision of the manuscript for important intellectual content: K.K. and J.M. Statistical analysis: N.P. and K.Z.
Supplementary data Table S1 is available as Supplementary data at JAC Online.
References
Agarwal, Rochwerg, Lamontagne, A living WHO guideline on drugs for covid-19, BMJ, doi:10.1136/bmj.m3379
Andrews, Stowe, Kirsebom, Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant, N Engl J Med, doi:10.1056/NEJMoa2119451
Bhimraj, Morgan, Shumaker, IDSA Guidelines on the Treatment and Management of Patients with COVID-19
Fda, FDA Takes Actions to Expand Use of Treatment for Outpatients With Mild-to-Moderate COVID-19
Gottlieb, Vaca, Paredes, Early remdesivir to prevent progression to severe Covid-19 in outpatients, N Engl J Med, doi:10.1056/NEJMoa2116846
Gupta, Gonzalez-Rojas, Juarez, Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2022.2832
Hui, Ho, Cheung, SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo, Nature, doi:10.1038/s41586-022-04479-6
Iuliano, Brunkard, Boehmer, Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods -United States, December 2020-January 2022, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7104e4
Lee, Wong, Chai, Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis, BMJ, doi:10.1136/bmj-2021-068632
Peters, Rabinstein, Dubrock, Use of remdesivir in myasthenia gravis and COVID-19, Pharmacotherapy, doi:10.1002/phar.2524
Piccicacco, Zeitler, Montero, Effectiveness of severe acute respiratory syndrome coronavirus 2 monoclonal antibody infusions in high-risk outpatients, Open Forum Infect Dis, doi:10.1093/ofid/ofab292
Planas, Saunders, Maes, Considerable escape of SARS-CoV-2 Omicron to antibody neutralization, Nature, doi:10.1038/s41586-021-04389-z
Razonable, Ganesh, Bierle, Clinical prioritization of antispike monoclonal antibody treatment of mild to moderate Outpatient remdesivir versus sotrovimab for Omicron COVID-19, Mayo Clin Proc, doi:10.1016/j.mayocp.2021.11.017
Stolle, Nalamasu, Pergolizzi, Fact vs fallacy: the anti-vaccine discussion reloaded, Adv Ther, doi:10.1007/s12325-020-01502-y
Tseng, Ackerson, Luo, Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants, Nat Med, doi:10.1038/s41591-022-01753-y
Yek, Warner, Wiltz, Risk factors for severe COVID-19 outcomes among persons aged ≥18 years who completed a primary COVID-19 vaccination series -465 health care facilities, United States, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7101a4
Zhu, Zhang, A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.1093/jac/dkac256",
"ISSN": [
"0305-7453",
"1460-2091"
],
"URL": "http://dx.doi.org/10.1093/jac/dkac256",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Remdesivir and sotrovimab both have clinical trial data in the outpatient setting demonstrating reduction in the risk of hospitalizations and emergency department (ED) visits related to COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Objectives</jats:title>\n <jats:p>To evaluate the effectiveness of remdesivir in comparison with sotrovimab and matched high-risk control patients in preventing COVID-19-related hospitalizations and ED visits during the Omicron B.1.1.529 surge.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Patients and methods</jats:title>\n <jats:p>This retrospective cohort study included outpatients positive for SARS-CoV-2, with non-severe symptoms for ≤7 days and deemed high-risk for severe COVID-19 by an internal scoring matrix. Patients who received remdesivir or sotrovimab from 27/12/2021 to 04/02/2022 were included (n = 82 and n = 88, respectively). These were compared with a control cohort of high-risk COVID-19 outpatients who did not receive therapy (n = 90). The primary outcome was a composite of 29 day COVID-19-related hospitalizations and/or ED visits. Pre-specified secondary outcomes included components of the primary endpoint, 29 day all-cause mortality and serious adverse drug events.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Patients treated with remdesivir were significantly less likely to be hospitalized or visit the ED within 29 days from symptom onset (11% versus 23.3%; OR = 0.41, 95% CI = 0.17–0.95). Patients receiving sotrovimab were also less likely to be hospitalized or visit the ED (8% versus 23.3%; OR = 0.28, 95% CI = 0.11–0.71). There was no difference in the incidence of hospitalizations/ED visits between sotrovimab and remdesivir.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Our highest-risk outpatients with Omicron-related COVID-19 who received early sotrovimab or remdesivir had significantly lower likelihoods of a hospitalization and/or ED visit.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6246-7533",
"affiliation": [
{
"name": "Department of Pharmacy, Tampa General Hospital , Tampa, FL , USA"
}
],
"authenticated-orcid": false,
"family": "Piccicacco",
"given": "Nicholas",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Pharmacy, Tampa General Hospital , Tampa, FL , USA"
}
],
"family": "Zeitler",
"given": "Kristen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy, Vanderbilt University Medical Center , Nashville, TN , USA"
}
],
"family": "Ing",
"given": "Austin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Internal Medicine, University of South Florida Morsani College of Medicine , Tampa, FL , USA"
}
],
"family": "Montero",
"given": "Jose",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Esoteric Testing/R&D and Microbiology Laboratories, Tampa General Hospital , Tampa, FL , USA"
}
],
"family": "Faughn",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Esoteric Testing/R&D and Microbiology Laboratories, Tampa General Hospital , Tampa, FL , USA"
}
],
"family": "Silbert",
"given": "Suzane",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3384-152X",
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Internal Medicine, University of South Florida Morsani College of Medicine , Tampa, FL , USA"
},
{
"name": "Global Emerging Diseases Institute, Tampa General Hospital , Tampa, FL , USA"
}
],
"authenticated-orcid": false,
"family": "Kim",
"given": "Kami",
"sequence": "additional"
}
],
"container-title": "Journal of Antimicrobial Chemotherapy",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
1
]
],
"date-time": "2022-08-01T16:50:21Z",
"timestamp": 1659372621000
},
"deposited": {
"date-parts": [
[
2022,
8,
1
]
],
"date-time": "2022-08-01T16:50:37Z",
"timestamp": 1659372637000
},
"indexed": {
"date-parts": [
[
2022,
8,
2
]
],
"date-time": "2022-08-02T04:28:47Z",
"timestamp": 1659414527716
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/journals/pages/open_access/funder_policies/chorus/standard_publication_model",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
1
]
],
"date-time": "2022-08-01T00:00:00Z",
"timestamp": 1659312000000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/jac/advance-article-pdf/doi/10.1093/jac/dkac256/45210080/dkac256.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jac/advance-article-pdf/doi/10.1093/jac/dkac256/45210080/dkac256.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
8,
1
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
1
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"author": "Johns Hopkins University",
"key": "2022080116401611900_dkac256-B1"
},
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A novel coronavirus from patients with pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "N Engl J Med",
"key": "2022080116401611900_dkac256-B2",
"volume": "382",
"year": "2020"
},
{
"author": "CDC",
"key": "2022080116401611900_dkac256-B3"
},
{
"DOI": "10.15585/mmwr.mm7101a4",
"article-title": "Risk factors for severe COVID-19 outcomes among persons aged ≥18 years who completed a primary COVID-19 vaccination series - 465 health care facilities, United States, December 2020-October 2021",
"author": "Yek",
"doi-asserted-by": "crossref",
"first-page": "19",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2022080116401611900_dkac256-B4",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-04389-z",
"article-title": "Considerable escape of SARS-CoV-2 Omicron to antibody neutralization",
"author": "Planas",
"doi-asserted-by": "crossref",
"first-page": "671",
"journal-title": "Nature",
"key": "2022080116401611900_dkac256-B5",
"volume": "602",
"year": "2022"
},
{
"author": "FDA",
"key": "2022080116401611900_dkac256-B6"
},
{
"author": "FDA",
"key": "2022080116401611900_dkac256-B7"
},
{
"DOI": "10.1001/jama.2022.2832",
"article-title": "Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1236",
"journal-title": "JAMA",
"key": "2022080116401611900_dkac256-B8",
"volume": "327",
"year": "2022"
},
{
"author": "Gilead Sciences, Inc",
"key": "2022080116401611900_dkac256-B9"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early remdesivir to prevent progression to severe Covid-19 in outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N Engl J Med",
"key": "2022080116401611900_dkac256-B10",
"volume": "386",
"year": "2022"
},
{
"author": "NIH",
"key": "2022080116401611900_dkac256-B11"
},
{
"author": "Bhimraj",
"key": "2022080116401611900_dkac256-B12",
"year": "2022"
},
{
"DOI": "10.1136/bmj.m3379",
"article-title": "A living WHO guideline on drugs for covid-19",
"author": "Agarwal",
"doi-asserted-by": "crossref",
"first-page": "m3379",
"journal-title": "BMJ",
"key": "2022080116401611900_dkac256-B13",
"volume": "370",
"year": "2020"
},
{
"author": "FDA",
"key": "2022080116401611900_dkac256-B14"
},
{
"DOI": "10.1016/j.mayocp.2021.11.017",
"article-title": "Clinical prioritization of antispike monoclonal antibody treatment of mild to moderate COVID-19",
"author": "Razonable",
"doi-asserted-by": "crossref",
"first-page": "26",
"journal-title": "Mayo Clin Proc",
"key": "2022080116401611900_dkac256-B15",
"volume": "97",
"year": "2022"
},
{
"DOI": "10.1093/ofid/ofab292",
"article-title": "Effectiveness of severe acute respiratory syndrome coronavirus 2 monoclonal antibody infusions in high-risk outpatients",
"author": "Piccicacco",
"doi-asserted-by": "crossref",
"first-page": "ofab292",
"journal-title": "Open Forum Infect Dis",
"key": "2022080116401611900_dkac256-B16",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.15585/mmwr.mm7104e4",
"article-title": "Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022",
"author": "Iuliano",
"doi-asserted-by": "crossref",
"first-page": "146",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2022080116401611900_dkac256-B17",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-01753-y",
"article-title": "Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants",
"author": "Tseng",
"doi-asserted-by": "crossref",
"first-page": "1063",
"journal-title": "Nat Med",
"key": "2022080116401611900_dkac256-B18",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2119451",
"article-title": "Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant",
"author": "Andrews",
"doi-asserted-by": "crossref",
"first-page": "1532",
"journal-title": "N Engl J Med",
"key": "2022080116401611900_dkac256-B19",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04479-6",
"article-title": "SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo",
"author": "Hui",
"doi-asserted-by": "crossref",
"first-page": "715",
"journal-title": "Nature",
"key": "2022080116401611900_dkac256-B20",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1136/bmj-2021-068632",
"article-title": "Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "e068632",
"journal-title": "BMJ",
"key": "2022080116401611900_dkac256-B21",
"volume": "376",
"year": "2022"
},
{
"DOI": "10.1002/phar.2524",
"article-title": "Use of remdesivir in myasthenia gravis and COVID-19",
"author": "Peters",
"doi-asserted-by": "crossref",
"first-page": "546",
"journal-title": "Pharmacotherapy",
"key": "2022080116401611900_dkac256-B22",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1007/s12325-020-01502-y",
"article-title": "Fact vs fallacy: the anti-vaccine discussion reloaded",
"author": "Stolle",
"doi-asserted-by": "crossref",
"first-page": "4481",
"journal-title": "Adv Ther",
"key": "2022080116401611900_dkac256-B23",
"volume": "37",
"year": "2020"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jac/advance-article/doi/10.1093/jac/dkac256/6652940"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Pharmacology (medical)",
"Pharmacology",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge",
"type": "journal-article"
}
piccicacco