Real-World Evidence of the Neutralizing Monoclonal Antibody Sotrovimab for Preventing Hospitalization and Mortality in COVID-19 Outpatients
et al., The Journal of Infectious Diseases, doi:10.1093/infdis/jiac206, Apr 2022 (preprint)
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
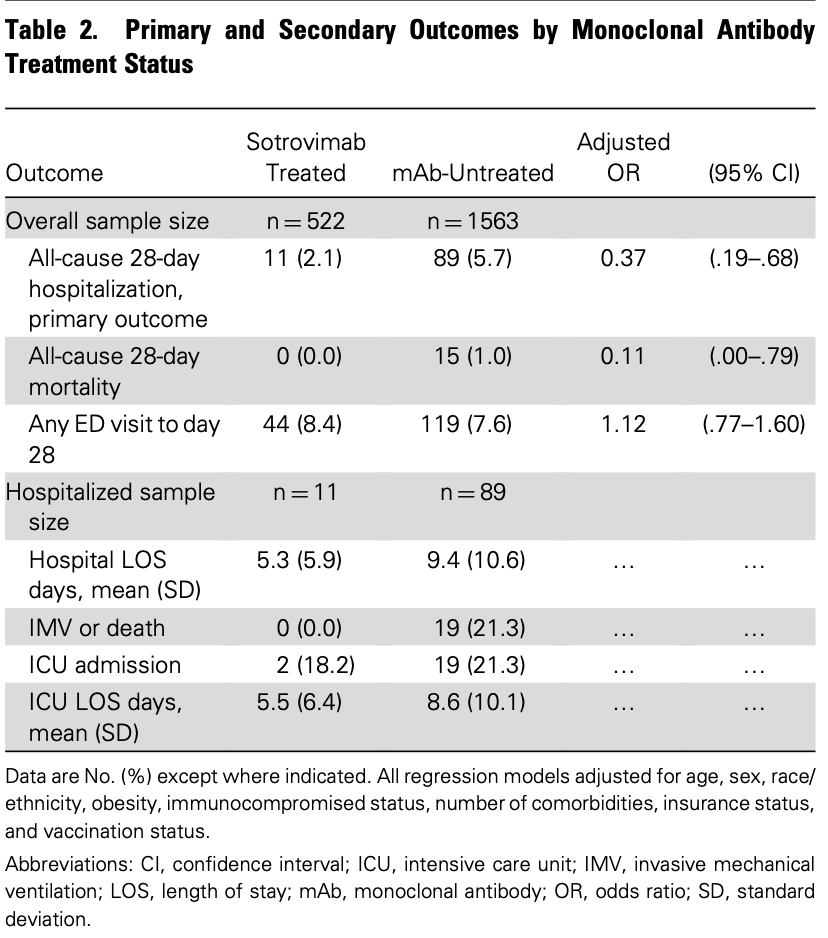
PSM retrospective 10,036 outpatients, 522 treated with sotrovimab, showing lower mortality and hospitalization with treatment.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending sotrovimab also recommended them, or
because the patient seeking out sotrovimab is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.14-6, BA.4, BA.57, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.18, and no efficacy for BA.29, XBB, XBB.1.5, ХВВ.1.9.110, XBB.1.16, BQ.1.1.45, and CL.18. US EUA has been revoked.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments11.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 88.9% lower, RR 0.11, p = 0.048, treatment 0 of 522 (0.0%), control 15 of 1,563 (1.0%), NNT 104, adjusted per study, odds ratio converted to relative risk, propensity score matching, multivariable, day 28.
|
|
risk of hospitalization, 61.6% lower, RR 0.38, p = 0.002, treatment 11 of 522 (2.1%), control 89 of 1,563 (5.7%), NNT 28, adjusted per study, odds ratio converted to relative risk, propensity score matching, multivariable, day 28, primary outcome.
|
|
ED visit, 11.0% higher, RR 1.11, p = 0.55, treatment 44 of 522 (8.4%), control 119 of 1,563 (7.6%), adjusted per study, odds ratio converted to relative risk, propensity score matching, multivariable, day 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
5.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
6.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
7.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
8.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
9.
Zhou et al., SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies, bioRxiv, doi:10.1101/2022.02.15.480166.
Aggarwal et al., 5 Apr 2022, retrospective, USA, peer-reviewed, 14 authors, study period 1 October, 2021 - 11 December, 2021.
Contact: neil.aggarwal@cuanschutz.edu.
Real World Evidence of the Neutralizing Monoclonal Antibody Sotrovimab for Preventing Hospitalization and Mortality in COVID-19 Outpatients
doi:10.1101/2022.04.03.22273360
Background: It is not known whether sotrovimab, a neutralizing monoclonal antibody (mAb) treatment authorized for early symptomatic COVID-19 patients, is effective against the SARS-CoV-2 Delta variant to prevent progression to severe disease and mortality. Methods: Observational cohort study of non-hospitalized adult patients with SARS-CoV-2 infection from October 1 st 2021 -December 11 th 2021, using electronic health records from a statewide health system plus state-level vaccine and mortality data. We used propensity matching to select 3 patients not receiving mAbs for each patient who received outpatient sotrovimab treatment. The primary outcome was 28-day hospitalization; secondary outcomes included mortality and severity of hospitalization. Results: Of 10,036 patients with SARS-CoV-2 infection, 522 receiving sotrovimab were matched to 1,563 not receiving mAbs. Compared to mAb-untreated patients, sotrovimab treatment was associated with a 63% decrease in the odds of all-cause hospitalization (raw rate 2.1% versus 5.7%; adjusted OR 0.37, 95% CI 0.19-0.66) and an 89% decrease in the odds of allcause 28-day mortality (raw rate 0% versus 1.0%; adjusted OR 0.11, 95% CI 0.0-0.79), and may reduce respiratory disease severity among those hospitalized.
Conclusion: Real-world evidence demonstrated sotrovimab effectiveness in reducing hospitalization and all-cause 28-day mortality among COVID-19 outpatients during the Delta variant phase.
References
Angus, Optimizing the Trade-off Between Learning and Doing in a Pandemic, JAMA
Austin, An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies, Multivariate Behav Res
Austin, Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples, Stat Med
Bennett, Moffitt, Hajagos, Clinical Characterization and Prediction of Clinical Severity of SARS-CoV-2 Infection Among US Adults Using Data From the US National COVID Cohort Collaborative, JAMA Netw Open
Bernstein, Biden Administration moves to stave off shortages of monoclonal antibodies, Accessed
Cameroni, Saliba, Bowen, Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift, bioRxiv
Cavazzoni, Coronavirus (COVID-19) Update: FDA Limits Use of Certain 9. ISPOR. About real-world evidence
Dougan, Nirula, Azizad, Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19, N Engl J Med
Gupta, Gonzalez-Rojas, Juarez, Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA
Heinze, Schemper, A solution to the problem of separation in logistic regression, Stat Med
Heinze, logistf: Firth's Bias-Reduced Logistic Regression, R package version
Ho, King, Stuart, Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference, Political Analysis
Lynch, Caplan, Furlong, Bateman-House, Helpful Lessons and Cautionary Tales: How Should COVID-19 Drug Development and Access Inform Approaches to Non-Pandemic Diseases?, Am J Bioeth
Puhr, Heinze, Nold, Lusa, Geroldinger, Firth's logistic regression with rare events: accurate effect estimates and predictions?, Stat Med
Team, a language and environment for statistical computing
Vanblargan, Errico, Halfmann, An infectious SARS-CoV-2 B
Weinreich, Sivapalasingam, Norton, REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, N Engl J Med
Wynia, Beaty, Bennett, Real World Evidence of Neutralizing Monoclonal Antibodies for Preventing Hospitalization and Mortality in COVID-19 Outpatients, medRxiv
DOI record:
{
"DOI": "10.1093/infdis/jiac206",
"ISSN": [
"0022-1899",
"1537-6613"
],
"URL": "http://dx.doi.org/10.1093/infdis/jiac206",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>It is not known whether sotrovimab, a neutralizing monoclonal antibody (mAb) treatment authorized for early symptomatic coronavirus disease 2019 (COVID-19) patients, is also effective in preventing the progression of severe disease and mortality following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Delta variant infection.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>In an observational cohort study of nonhospitalized adult patients with SARS-CoV-2 infection, 1 October 2021–11 December 2021, using electronic health records from a statewide health system plus state-level vaccine and mortality data, we used propensity matching to select 3 patients not receiving mAbs for each patient who received outpatient sotrovimab treatment. The primary outcome was 28-day hospitalization; secondary outcomes included mortality and severity of hospitalization.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Of 10 036 patients with SARS-CoV-2 infection, 522 receiving sotrovimab were matched to 1563 not receiving mAbs. Compared to mAb-untreated patients, sotrovimab treatment was associated with a 63% decrease in the odds of all-cause hospitalization (raw rate 2.1% vs 5.7%; adjusted odds ratio [aOR], 0.37; 95% confidence interval [CI], .19–.66) and an 89% decrease in the odds of all-cause 28-day mortality (raw rate 0% vs 1.0%; aOR, 0.11; 95% CI, .0–.79), and may reduce respiratory disease severity among those hospitalized.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Real-world evidence demonstrated sotrovimab effectiveness in reducing hospitalization and all-cause 28-day mortality among COVID-19 outpatients during the Delta variant phase.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0922-8824",
"affiliation": [
{
"name": "Department of Medicine, University of Colorado School of Medicine , Aurora, Colorado , USA"
}
],
"authenticated-orcid": false,
"family": "Aggarwal",
"given": "Neil R",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Biostatistics and Informatics, Colorado School of Public Health , Aurora, Colorado , USA"
}
],
"family": "Beaty",
"given": "Laurel E",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Section of Informatics and Data Science, Department of Pediatrics, University of Colorado School of Medicine , Aurora, Colorado , USA"
},
{
"name": "Colorado Clinical and Translational Sciences Institute, University of Colorado Anschutz Medical Campus , Aurora, Colorado , USA"
}
],
"family": "Bennett",
"given": "Tellen D",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics and Informatics, Colorado School of Public Health , Aurora, Colorado , USA"
},
{
"name": "Colorado Clinical and Translational Sciences Institute, University of Colorado Anschutz Medical Campus , Aurora, Colorado , USA"
}
],
"family": "Carlson",
"given": "Nichole E",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Colorado School of Medicine , Aurora, Colorado , USA"
}
],
"family": "Davis",
"given": "Christopher B",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Colorado School of Medicine , Aurora, Colorado , USA"
},
{
"name": "Colorado Clinical and Translational Sciences Institute, University of Colorado Anschutz Medical Campus , Aurora, Colorado , USA"
},
{
"name": "Department of Family Medicine, University of Colorado School of Medicine , Aurora, Colorado , USA"
}
],
"family": "Kwan",
"given": "Bethany M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics and Informatics, Colorado School of Public Health , Aurora, Colorado , USA"
}
],
"family": "Mayer",
"given": "David A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Section of Informatics and Data Science, Department of Pediatrics, University of Colorado School of Medicine , Aurora, Colorado , USA"
}
],
"family": "Ong",
"given": "Toan C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Section of Informatics and Data Science, Department of Pediatrics, University of Colorado School of Medicine , Aurora, Colorado , USA"
}
],
"family": "Russell",
"given": "Seth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Research Informatics, Children’s Hospital Colorado , Aurora, Colorado , USA"
}
],
"family": "Steele",
"given": "Jeffrey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics and Informatics, Colorado School of Public Health , Aurora, Colorado , USA"
}
],
"family": "Wogu",
"given": "Adane F",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of Colorado School of Medicine , Aurora, Colorado , USA"
},
{
"name": "Center for Bioethics and Humanities, University of Colorado, Anschutz Medical Campus , Aurora, Colorado , USA"
},
{
"name": "Department of Health Systems Management and Policy, Colorado School of Public Health , Aurora, Colorado , USA"
}
],
"family": "Wynia",
"given": "Matthew K",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Colorado School of Medicine , Aurora, Colorado , USA"
}
],
"family": "Zane",
"given": "Richard D",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Colorado School of Medicine , Aurora, Colorado , USA"
},
{
"name": "Colorado Clinical and Translational Sciences Institute, University of Colorado Anschutz Medical Campus , Aurora, Colorado , USA"
}
],
"family": "Ginde",
"given": "Adit A",
"sequence": "additional"
}
],
"container-title": "The Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
5,
16
]
],
"date-time": "2022-05-16T20:56:06Z",
"timestamp": 1652734566000
},
"deposited": {
"date-parts": [
[
2023,
5,
24
]
],
"date-time": "2023-05-24T02:44:26Z",
"timestamp": 1684896266000
},
"funder": [
{
"DOI": "10.13039/100006108",
"doi-asserted-by": "publisher",
"name": "National Center for Advancing Translational Sciences"
},
{
"DOI": "10.13039/100000002",
"award": [
"UL1TR002525",
"UL1TR002535-03S3",
"UL1TR002535-04S2"
],
"doi-asserted-by": "publisher",
"name": "National Institutes of Health"
}
],
"indexed": {
"date-parts": [
[
2023,
5,
25
]
],
"date-time": "2023-05-25T02:50:04Z",
"timestamp": 1684983004669
},
"is-referenced-by-count": 18,
"issue": "12",
"issued": {
"date-parts": [
[
2022,
5,
16
]
]
},
"journal-issue": {
"issue": "12",
"published-online": {
"date-parts": [
[
2022,
5,
16
]
]
},
"published-print": {
"date-parts": [
[
2022,
12,
13
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/pages/standard-publication-reuse-rights",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
5,
16
]
],
"date-time": "2022-05-16T00:00:00Z",
"timestamp": 1652659200000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/jid/advance-article-pdf/doi/10.1093/infdis/jiac206/43914521/jiac206.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jid/article-pdf/226/12/2129/50430385/jiac206.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jid/article-pdf/226/12/2129/50430385/jiac206.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "2129-2136",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
5,
16
]
]
},
"published-online": {
"date-parts": [
[
2022,
5,
16
]
]
},
"published-other": {
"date-parts": [
[
2022,
12,
15
]
]
},
"published-print": {
"date-parts": [
[
2022,
12,
13
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"author": "Centers for Disease Control and Prevention",
"key": "2023052402435640300_jiac206-B1"
},
{
"author": "COVID-19 Treatment Guidelines Panel",
"key": "2023052402435640300_jiac206-B2"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab plus etesevimab in mild or moderate Covid-19",
"author": "Dougan",
"doi-asserted-by": "crossref",
"first-page": "1382",
"journal-title": "N Engl J Med",
"key": "2023052402435640300_jiac206-B3",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1001/jama.2022.2832",
"article-title": "Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1236",
"journal-title": "JAMA",
"key": "2023052402435640300_jiac206-B4",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "2023052402435640300_jiac206-B5",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1080/15265161.2021.1974975",
"article-title": "Helpful lessons and cautionary tales: how should COVID-19 drug development and access inform approaches to non-pandemic diseases?",
"author": "Lynch",
"doi-asserted-by": "crossref",
"first-page": "4",
"journal-title": "Am J Bioeth",
"key": "2023052402435640300_jiac206-B6",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1101/2021.12.23.21268244",
"article-title": "Effectiveness of casirivimab and imdevimab, and sotrovimab during Delta variant surge: a prospective cohort study and comparative effectiveness randomized trial",
"author": "Huang",
"doi-asserted-by": "publisher",
"key": "2023052402435640300_jiac206-B7",
"volume-title": "medRxiv",
"year": "2021"
},
{
"DOI": "10.1016/j.mayocp.2021.12.002",
"article-title": "Effectiveness of monoclonal antibodies in preventing severe COVID-19 with emergence of the Delta variant",
"author": "O’Horo",
"doi-asserted-by": "crossref",
"first-page": "327",
"journal-title": "Mayo Clin Proc",
"key": "2023052402435640300_jiac206-B8",
"volume": "97",
"year": "2022"
},
{
"DOI": "10.1172/JCI151697",
"article-title": "Intravenous bamlanivimab use associates with reduced hospitalization in high-risk patients with mild to moderate COVID-19",
"author": "Ganesh",
"doi-asserted-by": "crossref",
"first-page": "e151697",
"journal-title": "J Clin Invest",
"key": "2023052402435640300_jiac206-B9",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.101102",
"article-title": "Casirivimab-imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19",
"author": "Razonable",
"doi-asserted-by": "crossref",
"first-page": "101102",
"journal-title": "EClinicalMedicine",
"key": "2023052402435640300_jiac206-B10",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1101/2021.04.09.21255219",
"article-title": "Early experience with neutralizing monoclonal antibody therapy for COVID-19",
"author": "Jarrett",
"doi-asserted-by": "crossref",
"key": "2023052402435640300_jiac206-B11",
"volume-title": "JMIRx Med",
"year": "2021"
},
{
"DOI": "10.1101/2022.01.09.22268963",
"article-title": "Real world evidence of neutralizing monoclonal antibodies for preventing hospitalization and mortality in COVID-19 outpatients",
"author": "Wynia",
"doi-asserted-by": "publisher",
"key": "2023052402435640300_jiac206-B12",
"volume-title": "medRxiv",
"year": "2022"
},
{
"author": "International Society for Pharmacoeconomics and Outcomes Research",
"key": "2023052402435640300_jiac206-B13"
},
{
"DOI": "10.1001/jama.2020.4984",
"article-title": "Optimizing the trade-off between learning and doing in a pandemic",
"author": "Angus",
"doi-asserted-by": "crossref",
"first-page": "1895",
"journal-title": "JAMA",
"key": "2023052402435640300_jiac206-B14",
"volume": "323",
"year": "2020"
},
{
"author": "Colorado Department of Public Health and Environment",
"key": "2023052402435640300_jiac206-B15"
},
{
"DOI": "10.1080/00273171.2011.568786",
"article-title": "An introduction to propensity score methods for reducing the effects of confounding in observational studies",
"author": "Austin",
"doi-asserted-by": "crossref",
"first-page": "399",
"journal-title": "Multivariate Behav Res",
"key": "2023052402435640300_jiac206-B16",
"volume": "46",
"year": "2011"
},
{
"DOI": "10.1093/pan/mpl013",
"article-title": "Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference",
"author": "Ho",
"doi-asserted-by": "crossref",
"first-page": "199",
"journal-title": "Political Anal",
"key": "2023052402435640300_jiac206-B17",
"volume": "15",
"year": "2007"
},
{
"DOI": "10.1002/sim.3697",
"article-title": "Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples",
"author": "Austin",
"doi-asserted-by": "crossref",
"first-page": "3083",
"journal-title": "Stat Med",
"key": "2023052402435640300_jiac206-B18",
"volume": "28",
"year": "2009"
},
{
"author": "National Institute of Health.",
"key": "2023052402435640300_jiac206-B19"
},
{
"author": "Heinz",
"key": "2023052402435640300_jiac206-B20",
"year": "2020"
},
{
"DOI": "10.1002/sim.1047",
"article-title": "A solution to the problem of separation in logistic regression",
"author": "Heinze",
"doi-asserted-by": "crossref",
"first-page": "2409",
"journal-title": "Stat Med",
"key": "2023052402435640300_jiac206-B21",
"volume": "21",
"year": "2002"
},
{
"article-title": "Firth's logistic regression with rare events: accurate effect estimates and predictions?",
"author": "Puhr",
"first-page": "2302",
"journal-title": "Stat Med",
"key": "2023052402435640300_jiac206-B22",
"volume": "36",
"year": "2017"
},
{
"author": "R Core Team",
"key": "2023052402435640300_jiac206-B23",
"volume-title": "R: a language and environment for statistical computing",
"year": "2020"
},
{
"article-title": "Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift",
"author": "Cameroni",
"key": "2023052402435640300_jiac206-B24",
"volume-title": "Nature",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04594-4",
"article-title": "Antibody evasion properties of SARS-CoV-2 Omicron sublineages",
"author": "Iketani",
"doi-asserted-by": "crossref",
"first-page": "553",
"journal-title": "Nature",
"key": "2023052402435640300_jiac206-B25",
"volume": "604",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2201933",
"article-title": "Efficacy of antiviral agents against the SARS-CoV-2 Omicron Subvariant BA.2",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "1475",
"journal-title": "N Engl J Med",
"key": "2023052402435640300_jiac206-B26",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1101/2022.04.21.22274060",
"article-title": "Effectiveness of the neutralizing antibody sotrovimab among high-risk patients with mild to moderate SARS-CoV-2 in Qatar",
"author": "Zaqout",
"doi-asserted-by": "publisher",
"key": "2023052402435640300_jiac206-B27",
"volume-title": "medRxiv",
"year": "2022"
},
{
"author": "National Academies",
"key": "2023052402435640300_jiac206-B28",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.16901",
"article-title": "Clinical characterization and prediction of clinical severity of SARS-CoV-2 infection among US adults using data from the US National COVID Cohort Collaborative",
"author": "Bennett",
"doi-asserted-by": "crossref",
"first-page": "e2116901",
"journal-title": "JAMA Netw Open",
"key": "2023052402435640300_jiac206-B29",
"volume": "4",
"year": "2021"
},
{
"author": "Food and Drug Administration",
"key": "2023052402435640300_jiac206-B30"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jid/article/226/12/2129/6586521"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Immunology and Allergy"
],
"subtitle": [],
"title": "Real-World Evidence of the Neutralizing Monoclonal Antibody Sotrovimab for Preventing Hospitalization and Mortality in COVID-19 Outpatients",
"type": "journal-article",
"volume": "226"
}
