Demographics and outcomes of initial phase of COVID-19 Medicines Delivery Units across 4 UK centres during peak B1.1.529 omicron epidemic: a service evaluation
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofac527, Oct 2022
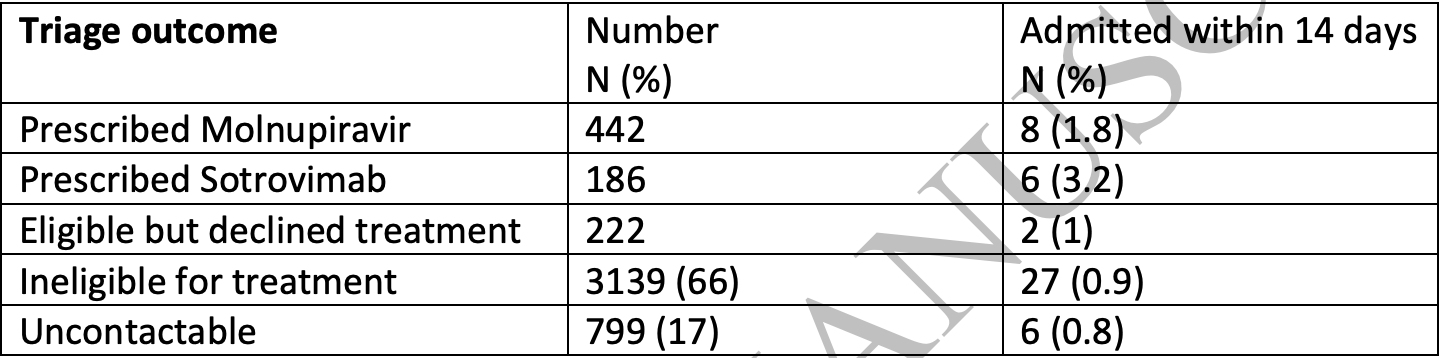
Retrospective 442 patients in the UK treated with molnupiravir, and 222 eligible but declining treatment, showing no significant difference in hospitalization. No group details are provided and the results are subject to confounding by indication.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments25.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
unadjusted results with no group details; significant unadjusted confounding possible.
Study covers sotrovimab and molnupiravir.
|
risk of hospitalization, 100.9% higher, RR 2.01, p = 0.51, treatment 8 of 442 (1.8%), control 2 of 222 (0.9%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
23.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Brown et al., 6 Oct 2022, retrospective, United Kingdom, peer-reviewed, 17 authors.
Contact: michael.marks@lshtm.ac.uk, anna.goodman@gstt.nhs.uk.
doi:10.1093/ofid/ofac527/6750023
Title
Demographics and outcomes of initial phase of COVID-19 Medicines Delivery Units across 4 UK centres during peak B1.1.529 omicron epidemic: a service evaluation.
Funding No specific funding for this work. MM is supported by NIHR and the EDCTP.
Conflict of Interest The authors have no conflicts of interest to declare
References
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Carr, Wu, Harvey, Omicron neutralising antibodies after COVID-19 vaccination in haemodialysis patients, The Lancet
Cheng, Mok, Leung, Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination, Nat Med
Gottlieb, Vaca, Paredes, Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, N Engl J Med
Gupta, Gonzalez-Rojas, Juarez, Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, N Engl J Med
Menni, Valdes, Polidori, Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study, The Lancet
Nyberg, Ferguson, Nash, Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study, The Lancet
Planas, Saunders, Maes, Considerable escape of SARS-CoV-2 Omicron to antibody neutralization, Nature
DOI record:
{
"DOI": "10.1093/ofid/ofac527",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofac527",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Introduction</jats:title>\n <jats:p>COVID-19 Medicine Delivery Units (CMDU) were established in late December 2021 to deliver early antiviral therapy to patients classified as at risk with the aim of preventing hospitalisation.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We performed a service evaluation at four CMDUs in England. We assessed demographics and triage outcomes of CMDU referral, uptake of antiviral therapy and the rate of subsequent hospitalisations within two weeks of CMDU referral.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Over a three week period 4,788 patients were referred and 3,989 were ultimately assessed by a CMDU. Overall, 832 of the patients referred (17%) were judged eligible for treatment and 628 (13%) were ultimately prescribed an antiviral agent. The overall rate of admission within 14 days was 1%. Patients who were admitted were significantly older than those who did not require hospitalisation. Of patients prescribed molnupiravir and sotrovimab 1.8% and 3.2% were admitted respectively.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>There were a high volume of referrals to CMDU service during the the initial surge of the Omicron wave in the UK. A minority of patients were judged to be eligible for therapy. In a highly vaccinated population, the overall hospitalisation rate was low.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Division of Infection, University College London Hospitals NHS Foundation Trust , London , UK"
},
{
"name": "Clinical Research Dept, London School of Hygiene & Tropical Medicine , London , UK"
}
],
"family": "Brown",
"given": "M",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Division of Infection, University College London Hospitals NHS Foundation Trust , London , UK"
}
],
"family": "Saund",
"given": "J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infection, Guys and St Thomas’s NHS Foundation Trust , London , UK"
},
{
"name": "South East London Covid Prevention and Intervention Service, Guys and St Thomas’s NHS Foundation Trust , London , UK"
}
],
"family": "Qureshi",
"given": "A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Dept of Infectious Diseases & Tropical Medicine, Sheffield Teaching Hospitals NHS Foundation Trust , Sheffield , UK"
},
{
"name": "Dept Infection, Immunity & Cardiovascular Disease, University of Sheffield , UK"
}
],
"family": "Plowright",
"given": "M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infection Research Group, Hull University Teaching Hospitals NHS Foundation Trust , Hull , UK"
}
],
"family": "Drury",
"given": "K",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infection, University College London Hospitals NHS Foundation Trust , London , UK"
}
],
"family": "Gahir",
"given": "J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Lewisham Hospital , London , UK"
}
],
"family": "Simpson",
"given": "T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Dept of Infectious Diseases & Tropical Medicine, Sheffield Teaching Hospitals NHS Foundation Trust , Sheffield , UK"
},
{
"name": "Dept Infection, Immunity & Cardiovascular Disease, University of Sheffield , UK"
}
],
"family": "Newman",
"given": "T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infection Research Group, Hull University Teaching Hospitals NHS Foundation Trust , Hull , UK"
}
],
"family": "Adams",
"given": "K",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centre for Rheumatic Disease, Kings College London , UK"
}
],
"family": "Galloway",
"given": "J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infection, Guys and St Thomas’s NHS Foundation Trust , London , UK"
},
{
"name": "South East London Covid Prevention and Intervention Service, Guys and St Thomas’s NHS Foundation Trust , London , UK"
}
],
"family": "Durairaj",
"given": "K",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infection Research Group, Hull University Teaching Hospitals NHS Foundation Trust , Hull , UK"
}
],
"family": "Elgizouli",
"given": "K",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infection, University College London Hospitals NHS Foundation Trust , London , UK"
}
],
"family": "Rampling",
"given": "T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Dept of Infectious Diseases & Tropical Medicine, Sheffield Teaching Hospitals NHS Foundation Trust , Sheffield , UK"
},
{
"name": "Dept Infection, Immunity & Cardiovascular Disease, University of Sheffield , UK"
}
],
"family": "Cole",
"given": "J",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6413-919X",
"affiliation": [
{
"name": "Infection Research Group, Hull University Teaching Hospitals NHS Foundation Trust , Hull , UK"
}
],
"authenticated-orcid": false,
"family": "Easom",
"given": "N",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infection, Guys and St Thomas’s NHS Foundation Trust , London , UK"
},
{
"name": "South East London Covid Prevention and Intervention Service, Guys and St Thomas’s NHS Foundation Trust , London , UK"
},
{
"name": "MRC Clinical Trials Unit, University College London , London , UK"
}
],
"family": "Goodman",
"given": "A",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7585-4743",
"affiliation": [
{
"name": "Division of Infection, University College London Hospitals NHS Foundation Trust , London , UK"
},
{
"name": "Clinical Research Dept, London School of Hygiene & Tropical Medicine , London , UK"
},
{
"name": "Division of Infection and Immunity, University College London , London , UK"
}
],
"authenticated-orcid": false,
"family": "Marks",
"given": "M",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
10,
6
]
],
"date-time": "2022-10-06T20:13:46Z",
"timestamp": 1665087226000
},
"deposited": {
"date-parts": [
[
2022,
10,
6
]
],
"date-time": "2022-10-06T20:13:46Z",
"timestamp": 1665087226000
},
"indexed": {
"date-parts": [
[
2022,
10,
6
]
],
"date-time": "2022-10-06T20:43:50Z",
"timestamp": 1665089030312
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
10,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
6
]
],
"date-time": "2022-10-06T00:00:00Z",
"timestamp": 1665014400000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofac527/46354871/ofac527.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofac527/46354871/ofac527.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
10,
6
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
6
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/advance-article/doi/10.1093/ofid/ofac527/6750023"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Oncology"
],
"subtitle": [],
"title": "Demographics and outcomes of initial phase of COVID-19 Medicines Delivery Units across 4 UK centres during peak B1.1.529 omicron epidemic: a service evaluation.",
"type": "journal-article"
}