Demographics and outcomes of initial phase of COVID-19 Medicines Delivery Units across 4 UK centres during peak B1.1.529 omicron epidemic: a service evaluation
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofac527, Oct 2022
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
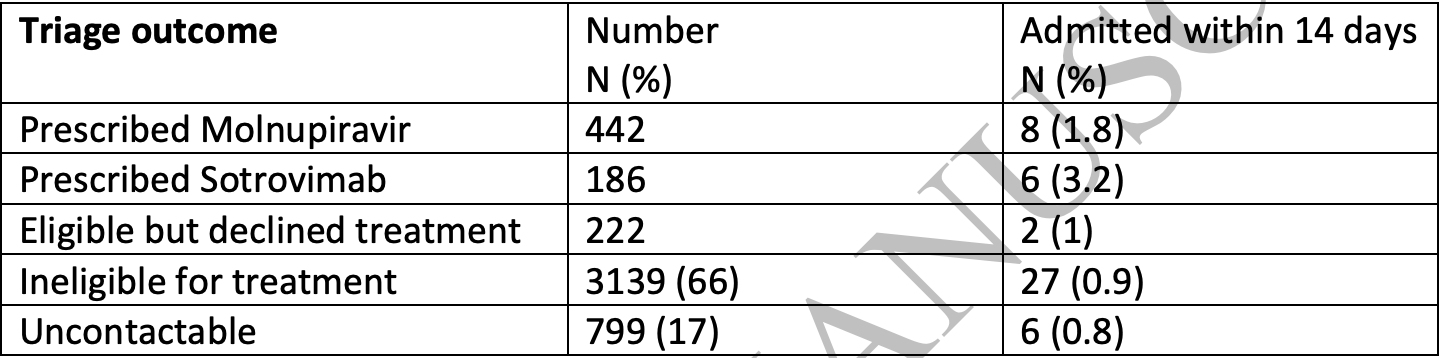
Retrospective 186 patients in the UK treated with sotrovimab, and 222 eligible but declining treatment, showing no significant difference in hospitalization. No group details are provided and the results are subject to confounding by indication.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments8.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
unadjusted results with no group details; significant unadjusted confounding possible.
Study covers sotrovimab and molnupiravir.
|
risk of hospitalization, 258.1% higher, RR 3.58, p = 0.15, treatment 6 of 186 (3.2%), control 2 of 222 (0.9%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Zhou et al., SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies, bioRxiv, doi:10.1101/2022.02.15.480166.
Brown et al., 6 Oct 2022, retrospective, United Kingdom, peer-reviewed, 17 authors.
Contact: michael.marks@lshtm.ac.uk, anna.goodman@gstt.nhs.uk.
doi:10.1093/ofid/ofac527/6750023
Title
Demographics and outcomes of initial phase of COVID-19 Medicines Delivery Units across 4 UK centres during peak B1.1.529 omicron epidemic: a service evaluation.
Funding No specific funding for this work. MM is supported by NIHR and the EDCTP.
Conflict of Interest The authors have no conflicts of interest to declare
References
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Carr, Wu, Harvey, Omicron neutralising antibodies after COVID-19 vaccination in haemodialysis patients, The Lancet
Cheng, Mok, Leung, Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination, Nat Med
Gottlieb, Vaca, Paredes, Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, N Engl J Med
Gupta, Gonzalez-Rojas, Juarez, Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, N Engl J Med
Menni, Valdes, Polidori, Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study, The Lancet
Nyberg, Ferguson, Nash, Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study, The Lancet
Planas, Saunders, Maes, Considerable escape of SARS-CoV-2 Omicron to antibody neutralization, Nature
DOI record:
{
"DOI": "10.1093/ofid/ofac527",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofac527",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Introduction</jats:title>\n <jats:p>COVID-19 Medicine Delivery Units (CMDU) were established in late December 2021 to deliver early antiviral therapy to patients classified as at risk with the aim of preventing hospitalisation.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We performed a service evaluation at four CMDUs in England. We assessed demographics and triage outcomes of CMDU referral, uptake of antiviral therapy and the rate of subsequent hospitalisations within two weeks of CMDU referral.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Over a three week period 4,788 patients were referred and 3,989 were ultimately assessed by a CMDU. Overall, 832 of the patients referred (17%) were judged eligible for treatment and 628 (13%) were ultimately prescribed an antiviral agent. The overall rate of admission within 14 days was 1%. Patients who were admitted were significantly older than those who did not require hospitalisation. Of patients prescribed molnupiravir and sotrovimab 1.8% and 3.2% were admitted respectively.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>There were a high volume of referrals to CMDU service during the the initial surge of the Omicron wave in the UK. A minority of patients were judged to be eligible for therapy. In a highly vaccinated population, the overall hospitalisation rate was low.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Division of Infection, University College London Hospitals NHS Foundation Trust , London , UK"
},
{
"name": "Clinical Research Dept, London School of Hygiene & Tropical Medicine , London , UK"
}
],
"family": "Brown",
"given": "M",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Division of Infection, University College London Hospitals NHS Foundation Trust , London , UK"
}
],
"family": "Saund",
"given": "J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infection, Guys and St Thomas’s NHS Foundation Trust , London , UK"
},
{
"name": "South East London Covid Prevention and Intervention Service, Guys and St Thomas’s NHS Foundation Trust , London , UK"
}
],
"family": "Qureshi",
"given": "A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Dept of Infectious Diseases & Tropical Medicine, Sheffield Teaching Hospitals NHS Foundation Trust , Sheffield , UK"
},
{
"name": "Dept Infection, Immunity & Cardiovascular Disease, University of Sheffield , UK"
}
],
"family": "Plowright",
"given": "M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infection Research Group, Hull University Teaching Hospitals NHS Foundation Trust , Hull , UK"
}
],
"family": "Drury",
"given": "K",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infection, University College London Hospitals NHS Foundation Trust , London , UK"
}
],
"family": "Gahir",
"given": "J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Lewisham Hospital , London , UK"
}
],
"family": "Simpson",
"given": "T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Dept of Infectious Diseases & Tropical Medicine, Sheffield Teaching Hospitals NHS Foundation Trust , Sheffield , UK"
},
{
"name": "Dept Infection, Immunity & Cardiovascular Disease, University of Sheffield , UK"
}
],
"family": "Newman",
"given": "T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infection Research Group, Hull University Teaching Hospitals NHS Foundation Trust , Hull , UK"
}
],
"family": "Adams",
"given": "K",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centre for Rheumatic Disease, Kings College London , UK"
}
],
"family": "Galloway",
"given": "J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infection, Guys and St Thomas’s NHS Foundation Trust , London , UK"
},
{
"name": "South East London Covid Prevention and Intervention Service, Guys and St Thomas’s NHS Foundation Trust , London , UK"
}
],
"family": "Durairaj",
"given": "K",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infection Research Group, Hull University Teaching Hospitals NHS Foundation Trust , Hull , UK"
}
],
"family": "Elgizouli",
"given": "K",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infection, University College London Hospitals NHS Foundation Trust , London , UK"
}
],
"family": "Rampling",
"given": "T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Dept of Infectious Diseases & Tropical Medicine, Sheffield Teaching Hospitals NHS Foundation Trust , Sheffield , UK"
},
{
"name": "Dept Infection, Immunity & Cardiovascular Disease, University of Sheffield , UK"
}
],
"family": "Cole",
"given": "J",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6413-919X",
"affiliation": [
{
"name": "Infection Research Group, Hull University Teaching Hospitals NHS Foundation Trust , Hull , UK"
}
],
"authenticated-orcid": false,
"family": "Easom",
"given": "N",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infection, Guys and St Thomas’s NHS Foundation Trust , London , UK"
},
{
"name": "South East London Covid Prevention and Intervention Service, Guys and St Thomas’s NHS Foundation Trust , London , UK"
},
{
"name": "MRC Clinical Trials Unit, University College London , London , UK"
}
],
"family": "Goodman",
"given": "A",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7585-4743",
"affiliation": [
{
"name": "Division of Infection, University College London Hospitals NHS Foundation Trust , London , UK"
},
{
"name": "Clinical Research Dept, London School of Hygiene & Tropical Medicine , London , UK"
},
{
"name": "Division of Infection and Immunity, University College London , London , UK"
}
],
"authenticated-orcid": false,
"family": "Marks",
"given": "M",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
10,
6
]
],
"date-time": "2022-10-06T20:13:46Z",
"timestamp": 1665087226000
},
"deposited": {
"date-parts": [
[
2022,
10,
6
]
],
"date-time": "2022-10-06T20:13:46Z",
"timestamp": 1665087226000
},
"indexed": {
"date-parts": [
[
2022,
10,
6
]
],
"date-time": "2022-10-06T20:43:50Z",
"timestamp": 1665089030312
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
10,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
6
]
],
"date-time": "2022-10-06T00:00:00Z",
"timestamp": 1665014400000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofac527/46354871/ofac527.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofac527/46354871/ofac527.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
10,
6
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
6
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/advance-article/doi/10.1093/ofid/ofac527/6750023"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Oncology"
],
"subtitle": [],
"title": "Demographics and outcomes of initial phase of COVID-19 Medicines Delivery Units across 4 UK centres during peak B1.1.529 omicron epidemic: a service evaluation.",
"type": "journal-article"
}
brown2
