Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. Veterans: target trial emulation studies with one-month and six-month outcomes
et al., medRxiv, doi:10.1101/2022.12.05.22283134, Dec 2022
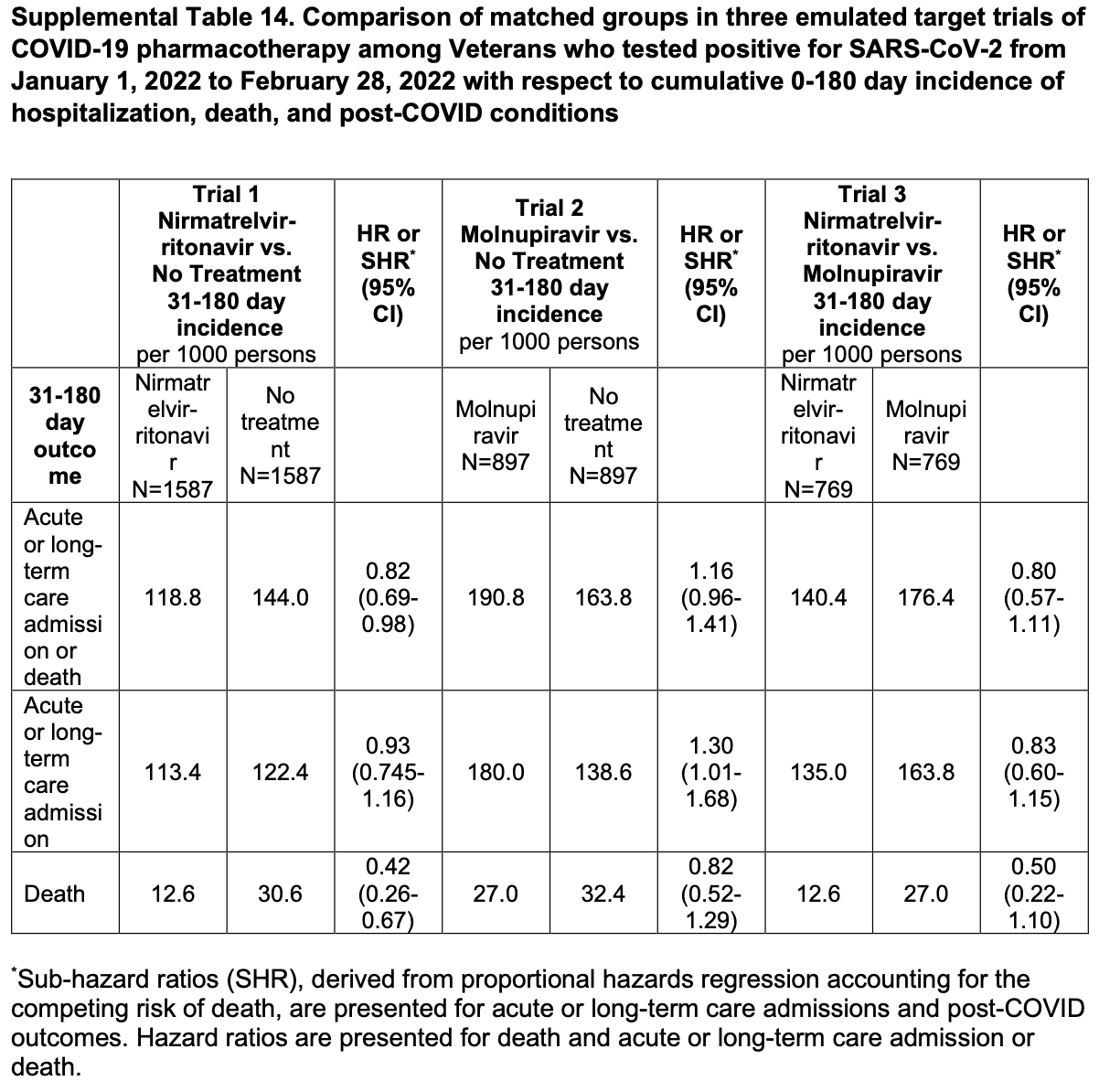
Retrospective 112,380 high-risk patients in the USA, showing significantly higher acute or long-term care admission at 180 days with molnupiravir treatment, and no significant difference for other outcomes. The title and headers of Table S14 are conflicting but the data appears to match be title.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments25.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers paxlovid and molnupiravir.
|
risk of death, 18.0% lower, HR 0.82, p = 0.40, treatment 24 of 897 (2.7%), control 29.0 of 897 (3.2%), NNT 179, cumulative 0-180 days, propensity score matching, day 180, Table S14.
|
|
risk of death, 49.3% lower, RR 0.51, p = 0.19, treatment 7 of 897 (0.8%), control 13.8 of 897 (1.5%), NNT 132, propensity score matching, day 30.
|
|
risk of mechanical ventilation, 16.7% lower, RR 0.83, p = 1.00, treatment 3 of 897 (0.3%), control 3.6 of 897 (0.4%), NNT 1495, propensity score matching, day 30.
|
|
risk of ICU admission, 1.0% lower, RR 0.99, p = 1.00, treatment 10 of 897 (1.1%), control 10.1 of 897 (1.1%), NNT 8970, propensity score matching, day 30.
|
|
risk of hospitalization, 30.0% higher, HR 1.30, p = 0.04, treatment 162 of 897 (18.1%), control 124.3 of 897 (13.9%), cumulative 0-180 days, propensity score matching, day 180, Table S14.
|
|
risk of hospitalization, 8.6% lower, RR 0.91, p = 0.73, treatment 37 of 897 (4.1%), control 40.5 of 897 (4.5%), NNT 256, propensity score matching, day 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
23.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Bajema et al., 6 Dec 2022, retrospective, USA, preprint, median age 67.0, 18 authors, study period 1 January, 2022 - 28 February, 2022.
Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. Veterans: target trial emulation studies with one-month and six-month outcomes
doi:10.1101/2022.12.05.22283134
Background: Information about the effectiveness of oral antivirals in preventing short-and longterm COVID-19-related outcomes during the Omicron surge is limited. We sought to determine the effectiveness of nirmatrelvir-ritonavir and molnupiravir for the outpatient treatment of COVID-19. Methods: We conducted three retrospective target trial emulation studies comparing matched patient cohorts who received nirmatrelvir-ritonavir versus no treatment, molnupiravir versus no treatment, and nirmatrelvir-ritonavir versus molnupiravir in the Veterans Health Administration (VHA). Participants were Veterans in VHA care at risk for severe COVID-19 who tested positive for SARS-CoV-2 in the outpatient setting during January and February 2022. Primary outcomes included all-cause 30-day hospitalization or death and 31-180-day incidence of acute or longterm care admission, death, or post-COVID-19 conditions. For 30-day outcomes, we calculated unadjusted risk rates, risk differences, and risk ratios. For 31-180-day outcomes, we used unadjusted time-to-event analyses. Results: Participants were 90% male with median age 67 years and 26% unvaccinated. Compared to matched untreated controls, nirmatrelvir-ritonavir-treated participants (N=1,587) had a lower 30-day risk of hospitalization (27.10/1000 versus 41.06/1000, risk difference [RD] -13.97, 95% CI -23.85 to -4.09) and death (3.15/1000 versus 14.86/1000, ). Among persons who were alive at day 31, further significant reductions in 31-180-day incidence of hospitalization (sub-hazard ratio 1.07, 95% CI 0.83 to 1.37) or death (hazard ratio 0.61, 95% CI 0.35 to 1.08) were not observed. Molnupiravir-treated participants aged ≥65 years (n=543) had a lower combined 30-day risk of hospitalization or death (55.25/1000 versus 82.35/1000, . A statistically significant difference in 30-day or 31-180-day risk of hospitalization or death was not observed between matched nirmatrelvir-or molnupiravir-treated participants. Incidence of most post-COVID conditions was similar across comparison groups. Conclusions: Nirmatrelvir-ritonavir was highly effective in preventing 30-day hospitalization and death. Short-term benefit from molnupiravir was observed in older groups. Significant reductions in adverse outcomes from 31-180 days were not observed with either antiviral. for use under a CC0 license.
References
Adjei, Hong, Molinari, Bull-Otterson, Ajani et al., Mortality Risk Among Patients Hospitalized Primarily for COVID-19 During the Omicron and Delta Variant Pandemic Periods -United States, April 2020, MMWR Morb Mortal Wkly Rep
Aggarwal, Molina, Beaty, Bennett, Carlson et al., Real-world Use of Nirmatrelvir-Ritonavir in COVID-19 Outpatients During the Emergence of Omicron Variants BA, BA2
Al-Aly, Xie, Bowe, High-dimensional characterization of post-acute sequelae of COVID-19, Nature
Arbel, Sagy, Hoshen, Battat, Lavie et al., Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge, N Engl J Med
Austin, Cafri, Variance estimation when using propensity-score matching with replacement with survival or time-to-event outcomes, Stat Med
Bajema, Wang, Hynes, Rowneki, Hickok et al., Early Adoption of Anti-SARS-CoV-2 Pharmacotherapies Among US Veterans With Mild to Moderate COVID-19, January and February, JAMA Network Open
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Butler, None
Dickerman, Gerlovin, Madenci, Kurgansky, Ferolito et al., Comparative Effectiveness of BNT162b2 and mRNA-1273 Vaccines in U.S. Veterans, N Engl J Med
Dryden-Peterson, Kim, Kim, Caniglia, Lennes et al., Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med
Hernán, Robins, Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available, Am J Epidemiol
Ioannou, Locke, Green, Berry, Comparison of Moderna versus Pfizer-BioNTech COVID-19 vaccine outcomes: A target trial emulation study in the U.S. Veterans Affairs healthcare system, EClinicalMedicine
Ioannou, Locke, Green, Berry, Hare et al., Risk Factors for Hospitalization, Mechanical Ventilation, or Death Among 10 131 US Veterans With SARS-CoV-2 Infection, JAMA Netw Open
Ioannou, Locke, Hare, Bohnert, Boyko et al., COVID-19 Vaccination Effectiveness Against Infection or Death in a National U.S. Health Care System : A Target Trial Emulation Study, Ann Intern Med
Labrecque, Swanson, Target trial emulation: teaching epidemiology and beyond, Eur J Epidemiol
Lance, Csp-Cerc, VA Connecticut Healthcare System
Lewnard, Malden, Hong, Puzniak, Kim et al., Effectiveness of nirmatrelvir-ritonavir against hospital admission: a matched cohort study in a large US healthcare system
Maffucci, Va, Nicholas, Smith, Mph, VA Connecticut Healthcare System
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients, Clin Infect Dis
Osborne, Veigulis, Arreola, Röösli, Curtin, Automated EHR score to predict COVID-19 outcomes at US Department of Veterans Affairs, PLoS One
Randall, Stern, Su, Pageshttps
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet
Xie, Bowe, Al-Aly, Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status, Nat Commun
Xie, Choi, Al-Aly, Nirmatrelvir and the Risk of Post-Acute Sequelae of COVID-19, medRxiv
Xie, Xu, Al-Aly, Risks of mental health outcomes in people with covid-19: cohort study, BMJ
Xie, Xu, Bowe, Al-Aly, Long-term cardiovascular outcomes of COVID-19, Nat Med
Yip, Lui, Lai, Wong, Tse et al., Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients, Clin Infect Dis
DOI record:
{
"DOI": "10.1101/2022.12.05.22283134",
"URL": "http://dx.doi.org/10.1101/2022.12.05.22283134",
"abstract": "<jats:p>Background: Information about the effectiveness of oral antivirals in preventing short- and long-term COVID-19-related outcomes during the Omicron surge is limited. We sought to determine the effectiveness of nirmatrelvir-ritonavir and molnupiravir for the outpatient treatment of COVID-19. Methods: We conducted three retrospective target trial emulation studies comparing matched patient cohorts who received nirmatrelvir-ritonavir versus no treatment, molnupiravir versus no treatment, and nirmatrelvir-ritonavir versus molnupiravir in the Veterans Health Administration (VHA). Participants were Veterans in VHA care at risk for severe COVID-19 who tested positive for SARS-CoV-2 in the outpatient setting during January and February 2022. Primary outcomes included all-cause 30-day hospitalization or death and 31-180-day incidence of acute or long-term care admission, death, or post-COVID-19 conditions. For 30-day outcomes, we calculated unadjusted risk rates, risk differences, and risk ratios. For 31-180-day outcomes, we used unadjusted time-to-event analyses. Results: Participants were 90% male with median age 67 years and 26% unvaccinated. Compared to matched untreated controls, nirmatrelvir-ritonavir-treated participants (N=1,587) had a lower 30-day risk of hospitalization (27.10/1000 versus 41.06/1000, risk difference [RD] -13.97, 95% CI -23.85 to -4.09) and death (3.15/1000 versus 14.86/1000, RD -11.71, 95% CI -16.07 to -7.35). Among persons who were alive at day 31, further significant reductions in 31-180-day incidence of hospitalization (sub-hazard ratio 1.07, 95% CI 0.83 to 1.37) or death (hazard ratio 0.61, 95% CI 0.35 to 1.08) were not observed. Molnupiravir-treated participants aged ≥65 years (n=543) had a lower combined 30-day risk of hospitalization or death (55.25/1000 versus 82.35/1000, RD -27.10, 95% CI -50.63 to -3.58). A statistically significant difference in 30-day or 31-180-day risk of hospitalization or death was not observed between matched nirmatrelvir- or molnupiravir-treated participants. Incidence of most post-COVID conditions was similar across comparison groups. Conclusions: Nirmatrelvir-ritonavir was highly effective in preventing 30-day hospitalization and death. Short-term benefit from molnupiravir was observed in older groups. Significant reductions in adverse outcomes from 31-180 days were not observed with either antiviral.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
12,
6
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-3229-5590",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bajema",
"given": "Kristina L.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Berry",
"given": "Kristin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1261-0098",
"affiliation": [],
"authenticated-orcid": false,
"family": "Streja",
"given": "Elani",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rajeevan",
"given": "Nallakkandi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Yuli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yan",
"given": "Lei",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7924-8372",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cunningham",
"given": "Francesca",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6436-7157",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hynes",
"given": "Denise M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9213-5134",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rowneki",
"given": "Mazhgan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bohnert",
"given": "Amy",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3695-192X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Boyko",
"given": "Edward J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4226-9310",
"affiliation": [],
"authenticated-orcid": false,
"family": "Iwashyna",
"given": "Theodore J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1765-5938",
"affiliation": [],
"authenticated-orcid": false,
"family": "Maciejewski",
"given": "Matthew L.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8896-2487",
"affiliation": [],
"authenticated-orcid": false,
"family": "Osborne",
"given": "Thomas F.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7439-6322",
"affiliation": [],
"authenticated-orcid": false,
"family": "Viglianti",
"given": "Elizabeth M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aslan",
"given": "Mihaela",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1217-0002",
"affiliation": [],
"authenticated-orcid": false,
"family": "Huang",
"given": "Grant D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ioannou",
"given": "George N.",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
12,
7
]
],
"date-time": "2022-12-07T05:15:22Z",
"timestamp": 1670390122000
},
"deposited": {
"date-parts": [
[
2022,
12,
7
]
],
"date-time": "2022-12-07T05:15:22Z",
"timestamp": 1670390122000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
12,
7
]
],
"date-time": "2022-12-07T06:15:11Z",
"timestamp": 1670393711776
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
12,
6
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.12.05.22283134",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
12,
6
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
12,
6
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2022.12.05.22283134"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. Veterans: target trial emulation studies with one-month and six-month outcomes",
"type": "posted-content"
}
bajema