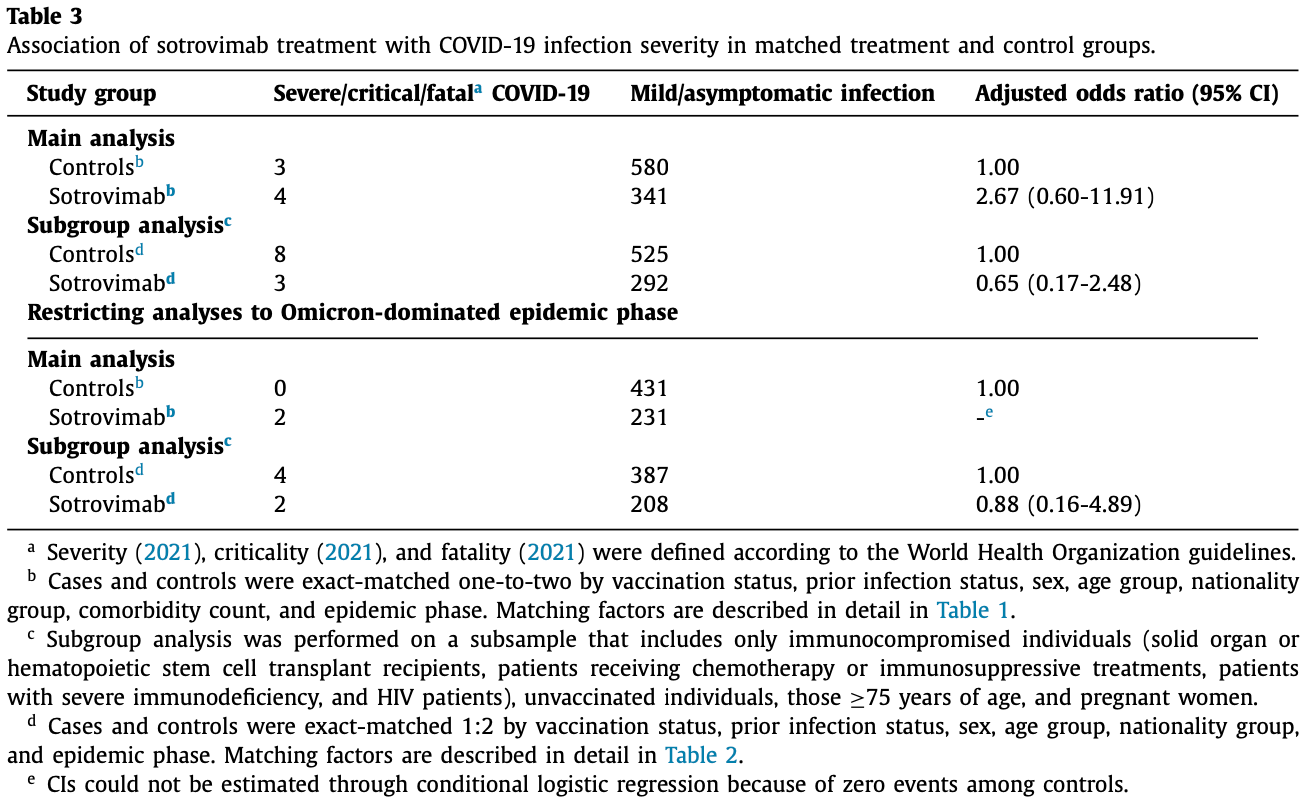
Effectiveness of the neutralizing antibody sotrovimab among high-risk patients with mild-to-moderate SARS-CoV-2 in Qatar
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.09.023, Apr 2022 (preprint)
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 345 sotrovimab treated patients in Qatar matched with 583 patients that opted not to receive treatment, showing higher progression with treatment, without statistical significance.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
|
risk of progression, 164.7% higher, RR 2.65, p = 0.19, treatment 4 of 345 (1.2%), control 3 of 583 (0.5%), adjusted per study, odds ratio converted to relative risk, progression to severe/critical disease or mortality.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Zaqout et al., 21 Apr 2022, retrospective, Qatar, peer-reviewed, median age 40.0, 17 authors, study period 20 October, 2021 - 28 February, 2022.
Contact: azaqout@hamad.qa, lja2002@qatarmed.cornell.edu.
Effectiveness of the neutralizing antibody sotrovimab among high-risk patients with mild-to-moderate SARS-CoV-2 in Qatar
International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.09.023
Objectives: To estimate the real-world effectiveness of sotrovimab against severe, critical, or fatal COVID-19 in Qatar at a time in which most SARS-CoV-2 incidences occurred due to the BA.2 Omicron subvariant. Methods: We conducted a matched case-control study among all individuals eligible for sotrovimab treatment per United States Food and Drug Administration guidelines in the resident population of Qatar. The odds of progression to severe forms of COVID-19 were compared in cases (treatment group) versus controls (eligible patients who opted not to receive the treatment). Subgroup analyses were conducted. Results: A total of 3364 individuals were eligible for sotrovimab treatment during the study period, of whom 519 individuals received the treatment, whereas the remaining 2845 constituted the controls. The adjusted odds ratio of disease progression to severe, critical, or fatal COVID-19 comparing the treatment group to the control group was 2.67 (95% confidence interval 0.60-11.91). In the analysis including only the subgroup of patients at higher risk of severe forms of COVID-19, the adjusted odds ratio was 0.65 (95% confidence interval 0.17-2.48). Conclusion: There was no evidence for a protective effect of sotrovimab in reducing COVID-19 severity in a setting dominated by the BA.2 subvariant.
Funding The authors are grateful for institutional salary support from Hamad Medical Corporation, the Ministry of Public Health, and the Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core, both at Weill Cornell Medicine-Qatar. The authors are also grateful to the Qatar Genome Programme and Qatar University Biomedical Research Center for institutional support for the reagents needed for the viral genome sequencing. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the article. Statements made herein are solely the responsibility of the authors.
Ethical approval The Hamad Medical Corporation and Weill Cornell Medicine-Qatar Institutional Review Boards approved this retrospective study with a waiver of informed consent. The research was performed in accordance with relevant guidelines and regulations.
Author contributions AZ, MAA, and ASO co-designed the study, led the database development, and co-wrote the manuscript. HC co-designed the study, performed the statistical analyses, and co-wrote the first draft of the article. LJA co-designed the study, led the statistical analyses, and co-wrote the first draft of the article. All authors contributed to data collection and acquisition, database development, discussion and interpretation of the results, and the writing of the manuscript. All authors have read and approved the final manuscript.
Declaration of..
References
Abu-Raddad, Chemaitelly, Ayoub, Almukdad, Yassine et al., Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar, N Engl J Med
Abu-Raddad, Chemaitelly, Ayoub, Kanaani, Khal et al., Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic, Sci Rep
Abu-Raddad, Chemaitelly, Coyle, Malek, Ahmed et al., SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy, EClinicalmedicine
Aggarwal, Beaty, Bennett, Carlson, Davis et al., Realworld evidence of the neutralizing monoclonal antibody sotrovimab for preventing hospitalization and mortality in COVID-19 outpatients, J Infect Dis, doi:10.1093/infdis/jiac206
Altarawneh, Chemaitelly, Ayoub, Tang, Hasan et al., Effects of previous infection and vaccination on symptomatic omicron infections, N Engl J Med
Austin, Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research, Commun Stat Simul Comput
Butt, Dargham, Loka, Shaik, Chemaitelly et al., COVID-19 Disease Severity in Children Infected with the Omicron Variant, Clin Infect Dis 2022a, doi:10.1093/cid/ciac275
Butt, Dargham, Tang, Chemaitelly, Hasan et al., COVID-19 disease severity in persons infected with the Omicron variant compared with the Delta variant in Qatar, J Glob Health
Chemaitelly, Ayoub, Almukdad, Coyle, Tang et al., Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar, Nat Commun 2022b
Chemaitelly, Ayoub, Coyle, Tang, Yassine et al., Protection of Omicron sub-lineage infection against reinfection with another Omicron sub-lineage, Nat Commun 2022a
Chemaitelly, Tang, Hasan, Almukdad, Yassine et al., Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar, N Engl J Med
Feikin, Abu-Raddad, Andrews, Davies, Higdon et al., Assessing vaccine effectiveness against severe COVID-19 disease caused by omicron variant. Report from a meeting of the World Health Organization, Vaccine
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Huang, Mccreary, Bariola, Minnier, Wadas et al., Effectiveness of casirivimab-imdevimab and sotrovimab during a SARS-CoV-2 Delta variant surge: a cohort study and randomized comparative effectiveness trial, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.20957
Kojima, Shrestha, Klausner, A systematic review of the protective effect of prior SARS-CoV-2 infection on repeat infection, Eval Health Prof
Miguez-Rey, Choi, Kim, Yoon, Monoclonal antibody therapies in the management of SARS-CoV-2 infection, Expert Opin Investig Drugs
Ong, Ren, Lee, Sutjipto, Dugan et al., Real-world use of sotrovimab for pre-emptive treatment in high-risk hospitalized COVID-19 patients: an observational cross-sectional study, Antibiotics
Stowe, Andrews, Kirsebom, Ramsay, Bernal, Effectiveness of COVID-19 vaccines against Omicron and Delta hospitalisation, a test negative case-control study, Nat Comm, doi:10.1038/s41467-022-33378-7
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2, N Engl J Med 2022b, doi:10.1056/NEJMc2201933
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant, N Engl J Med 2022a
DOI record:
{
"DOI": "10.1016/j.ijid.2022.09.023",
"ISSN": [
"1201-9712"
],
"URL": "http://dx.doi.org/10.1016/j.ijid.2022.09.023",
"alternative-id": [
"S1201971222005227"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Effectiveness of the neutralizing antibody sotrovimab among high-risk patients with mild-to-moderate SARS-CoV-2 in Qatar"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijid.2022.09.023"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Author(s). Published by Elsevier Ltd on behalf of International Society for Infectious Diseases."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0173-9697",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zaqout",
"given": "Ahmed",
"sequence": "first"
},
{
"affiliation": [],
"family": "Almaslamani",
"given": "Muna A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8756-6968",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chemaitelly",
"given": "Hiam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hashim",
"given": "Samar A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ittaman",
"given": "Ajithkumar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alimam",
"given": "Abeir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rustom",
"given": "Fatma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daghfal",
"given": "Joanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abukhattab",
"given": "Mohammed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "AlMukdad",
"given": "Sawsan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kaleeckal",
"given": "Anvar Hassan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Latif",
"given": "Ali Nizar",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1118-1826",
"affiliation": [],
"authenticated-orcid": false,
"family": "Butt",
"given": "Adeel A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bertollini",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al-Khal",
"given": "Abdullatif",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Omrani",
"given": "Ali S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0790-0506",
"affiliation": [],
"authenticated-orcid": false,
"family": "Abu-Raddad",
"given": "Laith J.",
"sequence": "additional"
}
],
"container-title": "International Journal of Infectious Diseases",
"container-title-short": "International Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"ijidonline.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
9,
19
]
],
"date-time": "2022-09-19T16:07:00Z",
"timestamp": 1663603620000
},
"deposited": {
"date-parts": [
[
2022,
10,
11
]
],
"date-time": "2022-10-11T22:19:03Z",
"timestamp": 1665526743000
},
"indexed": {
"date-parts": [
[
2022,
10,
11
]
],
"date-time": "2022-10-11T22:44:18Z",
"timestamp": 1665528258404
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
11
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
1
]
],
"date-time": "2022-11-01T00:00:00Z",
"timestamp": 1667260800000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
14
]
],
"date-time": "2022-09-14T00:00:00Z",
"timestamp": 1663113600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971222005227?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971222005227?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "96-103",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
11
]
]
},
"published-print": {
"date-parts": [
[
2022,
11
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1038/s41598-021-85428-7",
"article-title": "Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic",
"author": "Abu-Raddad",
"doi-asserted-by": "crossref",
"first-page": "6233",
"journal-title": "Sci Rep",
"key": "10.1016/j.ijid.2022.09.023_bib0001",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.100861",
"article-title": "SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy",
"author": "Abu-Raddad",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalmedicine",
"key": "10.1016/j.ijid.2022.09.023_bib0002",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2200797",
"article-title": "Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar",
"author": "Abu-Raddad",
"doi-asserted-by": "crossref",
"first-page": "1804",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.09.023_bib0003",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2119407",
"article-title": "Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "995",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.09.023_bib0020",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2201933",
"article-title": "Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "1475",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.09.023_bib24",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(21)00751-9",
"article-title": "Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial",
"doi-asserted-by": "crossref",
"first-page": "622",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.ijid.2022.09.023_bib0004",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiac206",
"article-title": "Real-world evidence of the neutralizing monoclonal antibody sotrovimab for preventing hospitalization and mortality in COVID-19 outpatients",
"author": "Aggarwal",
"doi-asserted-by": "crossref",
"journal-title": "J Infect Dis",
"key": "10.1016/j.ijid.2022.09.023_bib0005",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2203965",
"article-title": "Effects of previous infection and vaccination on symptomatic omicron infections",
"author": "Altarawneh",
"doi-asserted-by": "crossref",
"first-page": "21",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.09.023_bib0006",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1080/03610910902859574",
"article-title": "Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research",
"author": "Austin",
"doi-asserted-by": "crossref",
"first-page": "1228",
"journal-title": "Commun Stat Simul Comput",
"key": "10.1016/j.ijid.2022.09.023_bib0007",
"volume": "38",
"year": "2009"
},
{
"DOI": "10.1093/cid/ciac275",
"article-title": "COVID-19 Disease Severity in Children Infected with the Omicron Variant",
"author": "Butt",
"doi-asserted-by": "crossref",
"first-page": "e361",
"issue": "1",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ijid.2022.09.023_bib23",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.7189/jogh.12.05032",
"article-title": "COVID-19 disease severity in persons infected with the Omicron variant compared with the Delta variant in Qatar",
"author": "Butt",
"doi-asserted-by": "crossref",
"first-page": "05032",
"journal-title": "J Glob Health",
"key": "10.1016/j.ijid.2022.09.023_bib0008",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2114114",
"article-title": "Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar",
"author": "Chemaitelly",
"doi-asserted-by": "crossref",
"first-page": "e83",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.09.023_bib0009",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1038/s41467-022-32363-4",
"article-title": "Protection of Omicron sub-lineage infection against reinfection with another Omicron sub-lineage",
"author": "Chemaitelly",
"doi-asserted-by": "crossref",
"first-page": "4675",
"journal-title": "Nat Commun",
"key": "10.1016/j.ijid.2022.09.023_bib0010",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-30895-3",
"article-title": "Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar",
"author": "Chemaitelly",
"doi-asserted-by": "crossref",
"first-page": "3082",
"journal-title": "Nat Commun",
"key": "10.1016/j.ijid.2022.09.023_bib0011",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.vaccine.2022.04.069",
"article-title": "Assessing vaccine effectiveness against severe COVID-19 disease caused by omicron variant. Report from a meeting of the World Health Organization",
"author": "Feikin",
"doi-asserted-by": "crossref",
"first-page": "3516",
"journal-title": "Vaccine",
"key": "10.1016/j.ijid.2022.09.023_bib0012",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.09.023_bib0013",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1001/jama.2022.2832",
"article-title": "Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1236",
"journal-title": "JAMA",
"key": "10.1016/j.ijid.2022.09.023_bib0014",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2022.20957",
"article-title": "Effectiveness of casirivimab-imdevimab and sotrovimab during a SARS-CoV-2 Delta variant surge: a cohort study and randomized comparative effectiveness trial",
"author": "Huang",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.ijid.2022.09.023_bib0015",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1177/01632787211047932",
"article-title": "A systematic review of the protective effect of prior SARS-CoV-2 infection on repeat infection",
"author": "Kojima",
"doi-asserted-by": "crossref",
"first-page": "327",
"journal-title": "Eval Health Prof",
"key": "10.1016/j.ijid.2022.09.023_bib0016",
"volume": "44",
"year": "2021"
},
{
"DOI": "10.1080/13543784.2022.2030310",
"article-title": "Monoclonal antibody therapies in the management of SARS-CoV-2 infection",
"author": "Miguez-Rey",
"doi-asserted-by": "crossref",
"first-page": "41",
"journal-title": "Expert Opin Investig Drugs",
"key": "10.1016/j.ijid.2022.09.023_bib0017",
"volume": "31",
"year": "2022"
},
{
"DOI": "10.3390/antibiotics11030345",
"article-title": "Real-world use of sotrovimab for pre-emptive treatment in high-risk hospitalized COVID-19 patients: an observational cross-sectional study",
"author": "Ong",
"doi-asserted-by": "crossref",
"first-page": "345",
"journal-title": "Antibiotics (Basel)",
"key": "10.1016/j.ijid.2022.09.023_bib0018",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-33378-7",
"article-title": "Effectiveness of COVID-19 vaccines against Omicron and Delta hospitalisation, a test negative case-control study",
"author": "Stowe",
"doi-asserted-by": "crossref",
"first-page": "5736",
"journal-title": "Nat Comm",
"key": "10.1016/j.ijid.2022.09.023_bib0019",
"volume": "13",
"year": "2022"
},
{
"key": "10.1016/j.ijid.2022.09.023_bib0021",
"series-title": "Emergency use authorization (EUA) of sotrovimab, 2022",
"year": "2022"
},
{
"key": "10.1016/j.ijid.2022.09.023_bib0022",
"unstructured": "World Health Organization. International guidelines for certification and classification (coding) of COVID-19 as cause of death. 2020. Available at: https://www.who.int/publications/m/item/international-guidelines-for-certification-and-classification-(coding)-of-covid-19-as-cause-of-death. Accessed May 31, 2021."
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1201971222005227"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "Effectiveness of the neutralizing antibody sotrovimab among high-risk patients with mild-to-moderate SARS-CoV-2 in Qatar",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "124"
}
