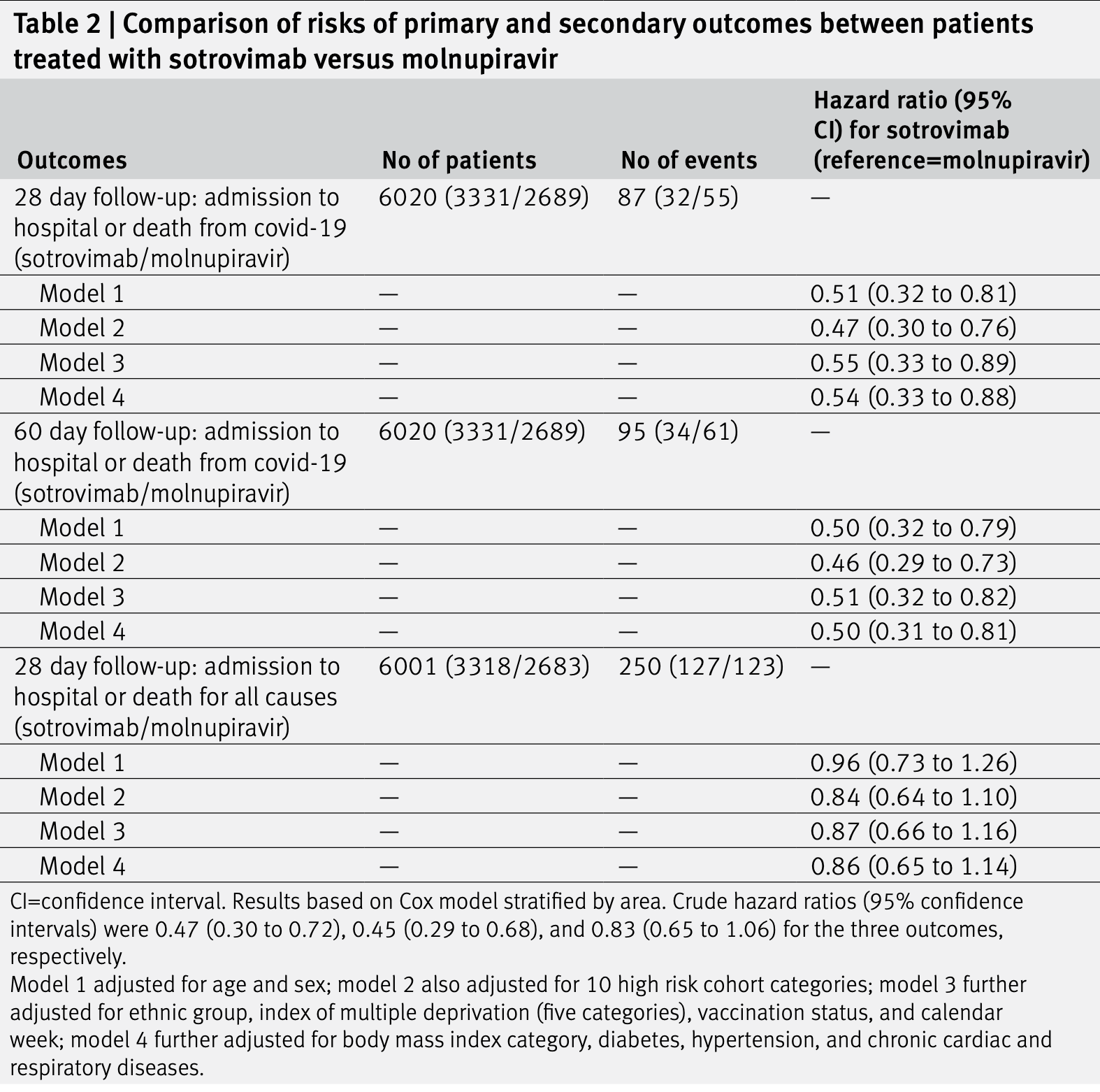
Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform
et al., BMJ, doi:10.1136/bmj-2022-071932, Nov 2022
Retrospective 3,331 sotrovimab and 2,689 molnupiravir patients in the UK, showing higher risk of combined hospitalization/death with molnupiravir.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments25.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
Study covers sotrovimab and molnupiravir.
|
risk of death/hospitalization, 100% higher, HR 2.00, p = 0.005, treatment 61 of 2,689 (2.3%), control 34 of 3,331 (1.0%), adjusted per study, inverted to make HR<1 favor treatment, multivariable, Cox proportional hazards, day 60, model 4.
|
|
risk of death/hospitalization, 85.2% higher, HR 1.85, p = 0.01, treatment 55 of 2,689 (2.0%), control 32 of 3,331 (1.0%), adjusted per study, inverted to make HR<1 favor treatment, multivariable, Cox proportional hazards, day 28, model 4.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
23.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Zheng et al., 16 Nov 2022, retrospective, United Kingdom, peer-reviewed, mean age 52.0, 33 authors, study period 16 December, 2021 - 10 February, 2022, this trial compares with another treatment - results may be better when compared to placebo.
Contact: laurie.tomlinson@lshtm.ac.uk.
Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform
BMJ, doi:10.1136/bmj-2022-071932
Objective To compare the effectiveness of sotrovimab (a neutralising monoclonal antibody) with molnupiravir (an antiviral) in preventing severe outcomes of covid-19 in adult patients infected with SARS-CoV-2 in the community and at high risk of severe outcomes from covid-19.
Design Observational cohort study with the OpenSAFELY platform. setting With the approval of NHS England, a real world cohort study was conducted with the OpenSAFELY-TPP platform (a secure, transparent, open source software platform for analysis of NHS electronic health records), and patient level electronic health record data were obtained from 24 million people registered with a general practice in England that uses TPP software. The primary care data were securely linked with data on SARS-CoV-2 infection and treatments, hospital admission, and death, over a period when both drug treatments were frequently prescribed in community settings.
ParticiPants Adult patients with covid-19 in the community at high risk of severe outcomes from covid-19, treated with sotrovimab or molnupiravir from 16 December 2021. interventiOns Sotrovimab or molnupiravir given in the community by covid-19 medicine delivery units.
Main OutcOMe Measures Admission to hospital with covid-19 (ie, with covid-19 as the primary diagnosis) or death from covid-19 (ie, with covid-19 as the underlying or contributing cause of death) within 28 days of the start of treatment.
results Between 16 December 2021 and 10 February 2022, 3331 and 2689 patients were treated with sotrovimab and molnupiravir, respectively, with no substantial differences in baseline characteristics. Mean age of all 6020 patients was 52 (standard deviation 16) years; 59% were women, 89% were white, and 88% had received three or more covid-19 vaccinations. Within 28 days of the start of treatment, 87 (1.4%) patients were admitted to hospital or died of infection from SARS-CoV-2 (32 treated with sotrovimab and 55 with molnupiravir). Cox proportional hazards models stratified by area showed that after adjusting for demographic information, high risk cohort categories, vaccination status, calendar time, body mass index, and other comorbidities, treatment with sotrovimab was associated with a substantially lower risk than treatment with molnupiravir (hazard ratio 0.54, 95% confidence interval 0.33 to 0.88, P=0.01). Consistent results were found from propensity score weighted Cox models (0.50, 0.31 to 0.81, P=0.005) and when restricted to people who were fully vaccinated (0.53, 0.31 to 0.90, P=0.02). No substantial effect modifications by other characteristics were detected (all P values for interaction >0.10). The findings were
References
Agarwal, Rochwerg, Lamontagne, A living WHO guideline on drugs for covid-19, BMJ, doi:10.1136/bmj.m3379
Austin, An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies, Multivariate Behav Res, doi:10.1080/00273171.2011.568786
Bager, Wohlfahrt, Bhatt, study group. Risk of hospitalisation associated with infection with SARS-CoV-2 omicron variant versus delta variant in Denmark: an observational cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00154-2
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Brophy, Molnupiravir's authorisation was premature, BMJ, doi:10.1136/bmj.o443
Bruel, Hadjadj, Maes, Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies, Nat Med, doi:10.1038/s41591-022-01792-5
Cameroni, Bowen, Rosen, Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift, Nature, doi:10.1038/s41586-021-04386-2
Dal-Ré, Becker, Bottieau, Holm, Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00119-0
Grange, Buelo, Sullivan, Characteristics and risk of COVID-19-related death in fully vaccinated people in Scotland, Lancet, doi:10.1016/S0140-6736(21)02316-3
Grint, Wing, Gibbs, Accident and emergency (AE) attendance in England following infection with SARS-CoV-2 Omicron or Delta, medRxiv, doi:10.1101/2022.05.03.22274602
Gupta, Gonzalez-Rojas, Juarez, Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, N Engl J Med, doi:10.1056/NEJMoa2107934
Gupta, Gonzalez-Rojas, Juarez, Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2022.2832
Hoffmann, Krüger, Schulz, The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic, Cell, doi:10.1016/j.cell.2021.12.032
Iketani, Liu, Guo, Antibody evasion properties of SARS-CoV-2 Omicron sublineages, Nature, doi:10.1038/s41586-022-04594-4
Jo, Kim, Radnaabaatar, Model-based cost-effectiveness analysis of oral antivirals against SARS-CoV-2 in Korea, Epidemiol Health, doi:10.4178/epih.e2022034
Nhs, Neutralising monoclonal antibodies (nMABs) or antivirals for non-hospitalised patients with COVID-19
Nyberg, Ferguson, Nash, Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron, Lancet, doi:10.1016/S0140-6736(22)00462-7
Tada, Zhou, Dcosta, Increased resistance of SARS-CoV-2 Omicron variant to neutralization by vaccine-elicited and therapeutic antibodies, EBioMedicine, doi:10.1016/j.ebiom.2022.103944
Takashita, Yamayoshi, Simon, Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants, N Engl J Med, doi:10.1056/NEJMc2207519
Taylor, Adams, Hufford, De La Torre, Winthrop et al., Neutralizing monoclonal antibodies for treatment of COVID-19, Nat Rev Immunol, doi:10.1038/s41577-021-00542-x
Uraki, Kiso, Iida, Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2, Nature, doi:10.1038/s41586-022-04856-1
Vangeel, Chiu, Jonghe, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern, Antiviral Res, doi:10.1016/j.antiviral.2022.105252
DOI record:
{
"DOI": "10.1136/bmj-2022-071932",
"ISSN": [
"1756-1833"
],
"URL": "http://dx.doi.org/10.1136/bmj-2022-071932",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Objective</jats:title>\n <jats:p>To compare the effectiveness of sotrovimab (a neutralising monoclonal antibody) with molnupiravir (an antiviral) in preventing severe outcomes of covid-19 in adult patients infected with SARS-CoV-2 in the community and at high risk of severe outcomes from covid-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Design</jats:title>\n <jats:p>Observational cohort study with the OpenSAFELY platform.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Setting</jats:title>\n <jats:p>With the approval of NHS England, a real world cohort study was conducted with the OpenSAFELY-TPP platform (a secure, transparent, open source software platform for analysis of NHS electronic health records), and patient level electronic health record data were obtained from 24 million people registered with a general practice in England that uses TPP software. The primary care data were securely linked with data on SARS-CoV-2 infection and treatments, hospital admission, and death, over a period when both drug treatments were frequently prescribed in community settings.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Participants</jats:title>\n <jats:p>Adult patients with covid-19 in the community at high risk of severe outcomes from covid-19, treated with sotrovimab or molnupiravir from 16 December 2021.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Interventions</jats:title>\n <jats:p>Sotrovimab or molnupiravir given in the community by covid-19 medicine delivery units.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Main outcome measures</jats:title>\n <jats:p>Admission to hospital with covid-19 (ie, with covid-19 as the primary diagnosis) or death from covid-19 (ie, with covid-19 as the underlying or contributing cause of death) within 28 days of the start of treatment.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Between 16 December 2021 and 10 February 2022, 3331 and 2689 patients were treated with sotrovimab and molnupiravir, respectively, with no substantial differences in baseline characteristics. Mean age of all 6020 patients was 52 (standard deviation 16) years; 59% were women, 89% were white, and 88% had received three or more covid-19 vaccinations. Within 28 days of the start of treatment, 87 (1.4%) patients were admitted to hospital or died of infection from SARS-CoV-2 (32 treated with sotrovimab and 55 with molnupiravir). Cox proportional hazards models stratified by area showed that after adjusting for demographic information, high risk cohort categories, vaccination status, calendar time, body mass index, and other comorbidities, treatment with sotrovimab was associated with a substantially lower risk than treatment with molnupiravir (hazard ratio 0.54, 95% confidence interval 0.33 to 0.88, P=0.01). Consistent results were found from propensity score weighted Cox models (0.50, 0.31 to 0.81, P=0.005) and when restricted to people who were fully vaccinated (0.53, 0.31 to 0.90, P=0.02). No substantial effect modifications by other characteristics were detected (all P values for interaction >0.10). The findings were similar in an exploratory analysis of patients treated between 16 February and 1 May 2022 when omicron BA.2 was the predominant variant in England.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>In routine care of adult patients in England with covid-19 in the community, at high risk of severe outcomes from covid-19, those who received sotrovimab were at lower risk of severe outcomes of covid-19 than those treated with molnupiravir.</jats:p>\n </jats:sec>",
"alternative-id": [
"10.1136/bmj-2022-071932"
],
"author": [
{
"affiliation": [],
"family": "Zheng",
"given": "Bang",
"sequence": "first"
},
{
"affiliation": [],
"family": "Green",
"given": "Amelia C A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tazare",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Curtis",
"given": "Helen J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fisher",
"given": "Louis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nab",
"given": "Linda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schultze",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahalingasivam",
"given": "Viyaasan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parker",
"given": "Edward P K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hulme",
"given": "William J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bacon",
"given": "Sebastian C J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DeVito",
"given": "Nicholas J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bates",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evans",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Inglesby",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Drysdale",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davy",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cockburn",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morton",
"given": "Caroline E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hickman",
"given": "George",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ward",
"given": "Tom",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Rebecca M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parry",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hester",
"given": "Frank",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harper",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mehrkar",
"given": "Amir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eggo",
"given": "Rosalind M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Walker",
"given": "Alex J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evans",
"given": "Stephen J W",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Douglas",
"given": "Ian J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "MacKenna",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goldacre",
"given": "Ben",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8848-9493",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tomlinson",
"given": "Laurie A",
"sequence": "additional"
}
],
"container-title": "BMJ",
"container-title-short": "BMJ",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2022,
11,
17
]
],
"date-time": "2022-11-17T01:12:28Z",
"timestamp": 1668647548000
},
"deposited": {
"date-parts": [
[
2022,
11,
17
]
],
"date-time": "2022-11-17T05:00:56Z",
"timestamp": 1668661256000
},
"funder": [
{
"DOI": "10.13039/100010269",
"doi-asserted-by": "publisher",
"name": "Wellcome Trust"
}
],
"indexed": {
"date-parts": [
[
2022,
11,
18
]
],
"date-time": "2022-11-18T06:01:26Z",
"timestamp": 1668751286696
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
11,
16
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
16
]
],
"date-time": "2022-11-16T00:00:00Z",
"timestamp": 1668556800000
}
}
],
"link": [
{
"URL": "http://data.bmj.org/tdm/10.1136/bmj-2022-071932",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmj-2022-071932",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e071932",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2022,
11,
16
]
]
},
"published-online": {
"date-parts": [
[
2022,
11,
16
]
]
},
"publisher": "BMJ",
"reference": [
{
"DOI": "10.1136/bmj.m3379",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.1"
},
{
"DOI": "10.1101/2022.03.07.22272026",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.2"
},
{
"key": "2022111621001198000_379.nov16_9.e071932.3",
"unstructured": "NHS. Neutralising monoclonal antibodies (nMABs) or antivirals for non-hospitalised patients with COVID-19. 2021. https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAlert.aspx?AlertID=103186"
},
{
"DOI": "10.1056/NEJMoa2107934",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.4"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.5"
},
{
"DOI": "10.1136/bmj.o443",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.6"
},
{
"key": "2022111621001198000_379.nov16_9.e071932.7",
"unstructured": "NHS. Interim Clinical Commissioning Policy: Antivirals or neutralising monoclonal antibodies for non-hospitalised patients with COVID-19. 2022. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2021/12/C1650-interim-ccp-antivirals-or-neutralising-monoclonal-antibodies-non-hospitalised-patients-with-covid19-v6.pdf"
},
{
"key": "2022111621001198000_379.nov16_9.e071932.8",
"unstructured": "UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England: technical briefing 43. 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1086494/Technical-Briefing-43-28.06.22.pdf"
},
{
"DOI": "10.1080/00273171.2011.568786",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.9"
},
{
"DOI": "10.1001/jama.2022.2832",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.10"
},
{
"DOI": "10.1038/s41586-021-04386-2",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.11"
},
{
"DOI": "10.1016/j.antiviral.2022.105252",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.12"
},
{
"DOI": "10.1016/j.cell.2021.12.032",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.13"
},
{
"DOI": "10.1016/j.ebiom.2022.103944",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.14"
},
{
"key": "2022111621001198000_379.nov16_9.e071932.15",
"unstructured": "National Institutes of Health COVID-19 Treatment Guidelines Panel. Therapeutic Management of Nonhospitalized Adults With COVID-19. 2022. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management/"
},
{
"DOI": "10.1038/s41591-022-01792-5",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.16"
},
{
"DOI": "10.1038/s41586-022-04594-4",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.17"
},
{
"DOI": "10.1056/NEJMc2207519",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.18"
},
{
"DOI": "10.1038/s41586-022-04856-1",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.19"
},
{
"key": "2022111621001198000_379.nov16_9.e071932.20",
"unstructured": "UK Health Security Agency. SARS-CoV-2 therapeutics technical briefing 3. 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1074186/therapeutics-programme-technical-briefing-3.pdf"
},
{
"DOI": "10.1136/dtb.2022.000008",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.21"
},
{
"DOI": "10.1038/s41577-021-00542-x",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.22"
},
{
"DOI": "10.1016/S1473-3099(22)00119-0",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.23"
},
{
"key": "2022111621001198000_379.nov16_9.e071932.24",
"unstructured": "European Medicines Agency. Sotrovimab (also known as VIR-7831 and GSK4182136) - COVID19 - Article-5(3) procedure: Conditions of use, conditions for distribution and patients targeted and conditions for safety monitoring. 2021. https://www.ema.europa.eu/en/documents/referral/sotrovimab-also-known-vir-7831-gsk4182136-covid19-article-53-procedure-assessment-report_en.pdf"
},
{
"key": "2022111621001198000_379.nov16_9.e071932.25",
"unstructured": "European Medicines Agency. Lagevrio (also known as molnupiravir or MK 4482) - COVID-19 - Article-5(3) procedure: Assessment report. 2021. https://www.ema.europa.eu/en/documents/referral/lagevrio-also-known-molnupiravir-mk-4482-covid-19-article-53-procedure-assessment-report_en.pdf"
},
{
"DOI": "10.1016/S1473-3099(22)00154-2",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.26"
},
{
"DOI": "10.1016/S0140-6736(22)00462-7",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.27"
},
{
"DOI": "10.1101/2022.05.03.22274602",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.28"
},
{
"DOI": "10.1016/S0140-6736(21)02316-3",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.29"
},
{
"DOI": "10.4178/epih.e2022034",
"doi-asserted-by": "publisher",
"key": "2022111621001198000_379.nov16_9.e071932.30"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.bmj.com/lookup/doi/10.1136/bmj-2022-071932"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Engineering"
],
"subtitle": [],
"title": "Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy"
}