Effectiveness and Safety of Turmeric for the Treatment of COVID-19: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials
et al., Complementary Therapies in Medicine, doi:10.1016/j.ctim.2025.103295, PROSPERO CRD42021274859, Oct 2025
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
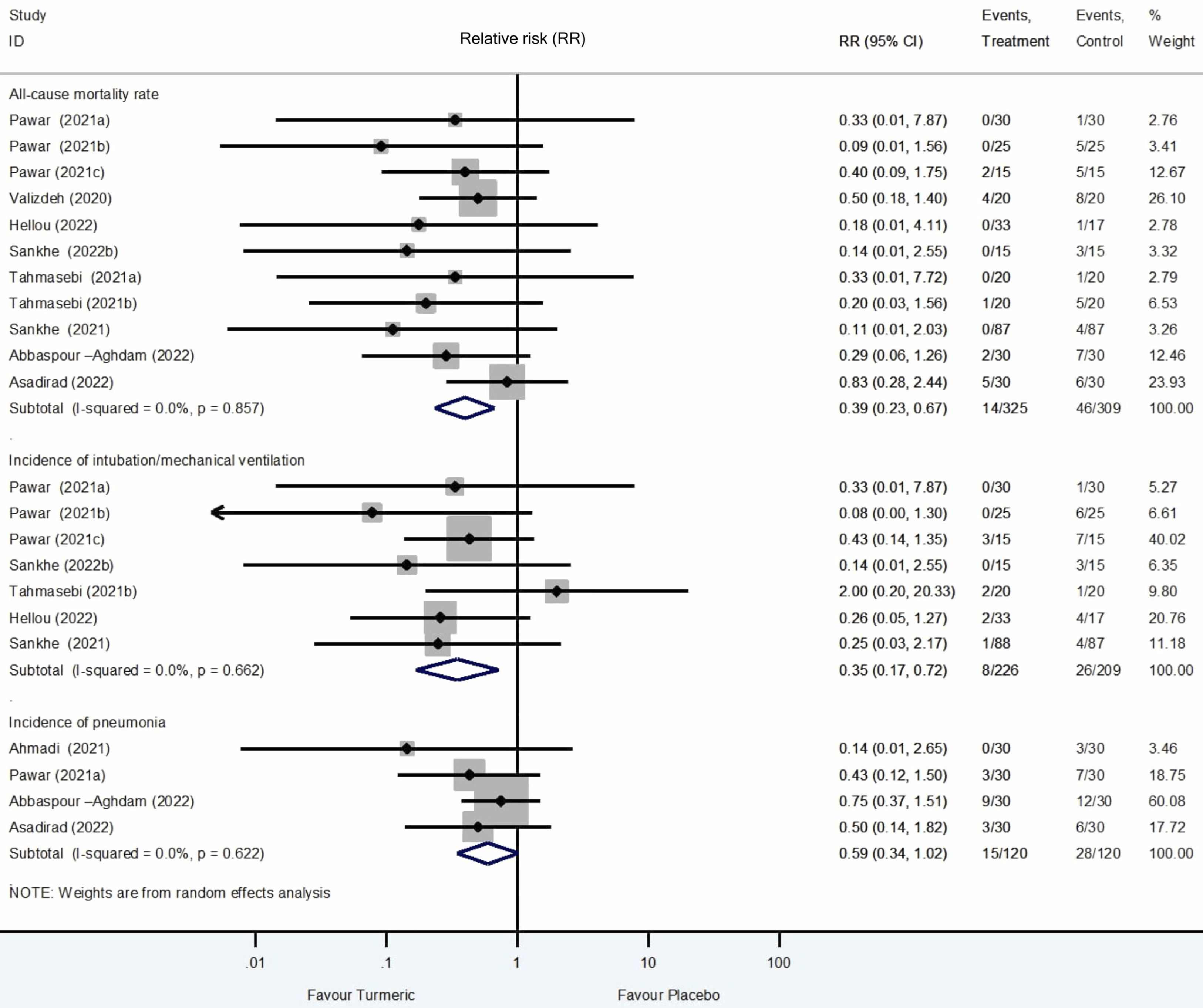
Systematic review and meta-analysis of 23 RCTs showing significantly lower mortality, ventilation, and progression, and improved recovery with turmeric treatment. Nano-curcumin was most commonly used for improved bioavailability and showed greater efficacy. Benefits were greater when administered early. The safety profile was favorable with adverse events comparable to placebo, mostly mild gastrointestinal symptoms. Effects remained robust after excluding high risk-of-bias studies.
6 meta-analyses show significant improvements with curcumin for mortality1-6,
mechanical ventilation6,
hospitalization1,4,
recovery3,6,
progression6, and
symptoms1.
Currently there are 28 curcumin for COVID-19 studies, showing 63% lower mortality [36‑78%], 80% lower ventilation [25‑95%], 78% lower ICU admission [-27‑96%], and 27% lower hospitalization [18‑34%].
|
risk of death, 61.0% lower, RR 0.39, p < 0.001.
|
|
risk of mechanical ventilation, 65.0% lower, RR 0.35, p = 0.004.
|
|
risk of progression, 64.0% lower, RR 0.36, p < 0.001.
|
|
risk of no recovery, 26.5% lower, RR 0.74, p < 0.001, inverted to make RR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Vahedian-Azimi et al., Effectiveness of Curcumin on Outcomes of Hospitalized COVID-19 Patients: A Systematic Review of Clinical Trials, Nutrients, doi:10.3390/nu14020256.
2.
Kow et al., The effect of curcumin on the risk of mortality in patients with COVID-19: A systematic review and meta-analysis of randomized trials, Phytotherapy Research, doi:10.1002/ptr.7468.
3.
Shafiee et al., Curcumin for the treatment of COVID-19 patients: A meta-analysis of randomized controlled trials, Phytotherapy Research, doi:10.1002/ptr.7724.
4.
Shojaei et al., The effectiveness of nano‐curcumin on patients with COVID‐19: A systematic review of clinical trials, Phytotherapy Research, doi:10.1002/ptr.7778.
Sawangjit et al., 31 Oct 2025, peer-reviewed, 10 authors, trial PROSPERO CRD42021274859.
Contact: nathorn.chaiyakunapruk@utah.edu.
Effectiveness and Safety of Turmeric for the Treatment of COVID-19: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials
Complementary Therapies in Medicine, doi:10.1016/j.ctim.2025.103295
We conducted a comprehensive and updated systematic review and meta-analysis (SR-MA) to determine the effectiveness and safety of turmeric in patients with coronavirus disease 2019 (COVID-19). Multiple databases were searched from inception to July 31, 2024, for randomized controlled trials (RCTs) assessing turmeric in mild to severe COVID-19. This SR-MA uniquely includes recent trials conducted alongside modern antiviral-based regimens and explores effect modifiers by disease severity, comorbidity, formulation, and treatment duration. Twenty-three RCTs with 1,407 participants were included, making this the largest synthesis to date. Most studies (17/23, 73.9%) enrolled hospitalized patients; over half involved mild to moderate cases. The most common intervention was nano-curcumin 160-240 mg/day (39%), used as an adjunct to standard care. Nine studies were rated high risk of bias (ROB). Metaanalysis showed turmeric significantly reduced all-cause mortality (Relative risk (RR) = 0.39; 95% confidence interval (95%CI): 0.23-0.67; I² = 0%; n = 8 RCTs; moderate certainty), J o u r n a l P r e -p r o o f suggesting a 61% reduction in risk of death. It also reduced the need for intubation/mechanical ventilation (RR = 0.35; 95%CI: 0.17-0.72) and clinical deterioration (RR=0.36; 95%CI: 0.22-0.59), while improving overall symptom resolution (RR = 1.36; 95%CI: 1.16-1.59). These results remained robust after excluding high ROB studies. Adverse events, mostly mild gastrointestinal symptoms, were comparable to placebo. In conclusion, turmeric, particularly bioavailability-enhanced nano-curcumin, provides meaningful clinical benefits and favorable safety profile as adjunctive therapy for COVID-19. Further large-scale, high-quality, multicenter RCTs are warranted to confirm its therapeutic potential, particularly in resourcelimited settings.
Conflict of interest The authors declare no relevant conflicts of interest or financial relationships. J o u r n a l P r e -p r o o f 3 Other considerations for downgrading include publication bias. Other considerations for upgrading include a strong association with no plausible confounders, a dose response relationship, and if all plausible confounders or biases would decrease the size of the effect (if there is evidence of an effect), or increase it if there is evidence of no harmful effect (safety) 1 4 High = This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different** is low.
References
Abbaspour-Aghdam, Hazrati, Abdolmohammadi-Vahid, Immunomodulatory role of Nanocurcumin in COVID-19 patients with dropped natural killer cells frequency and function, Eur J Pharmacol, doi:10.1016/j.ejphar.2022.175267
Ahmadi, Mehrabi, Zare, Ghadir, Masoumi, Efficacy of Nanocurcumin as an Add-On Treatment for Patients Hospitalized with COVID-19: A Double-Blind, Randomized Clinical Trial, Int J Clin Pract, doi:10.1155/2023/5734675
Ahmadi, Salari, Sharifi, Reihani, Rostamiani et al., Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebocontrolled clinical trial, Food Sci Nutr, doi:10.1002/fsn3.2226
Asadirad, Nashibi, Khodadadi, Ghadiri, Sadeghi et al., Antiinflammatory potential of nano-curcumin as an alternative therapeutic agent for the treatment of mild-to-moderate hospitalized COVID-19 patients in a placebo-controlled clinical trial, Phytother Res, doi:10.1002/ptr.7375
Askari, Sahebkar, Soleimani, Mahdavi, Rafiee et al., The efficacy of curcumin-piperine cosupplementation on clinical symptoms, duration, severity, and inflammatory factors in COVID-19 outpatients: a randomized double-blind, placebo-controlled trial, Trials, doi:10.1186/s13063-022-06375-w
Atmar, Finch, New perspectives on antimicrobial agents: molnupiravir and nirmatrelvir/ritonavir for treatment of COVID-19, Antimicrob Agents Chemother, doi:10.1128/aac.02404-21
Borenstein, Hedges, Higgins, Hr, Introduction to meta-analysis
Chitre, Nadkarni, Jagtap, Tulle, Gitte et al., Phase III randomized clinical trial of BV-4051, an Ayurvedic polyherbal formulation in moderate SARS-J o u r n a l P r e -p r o o f CoV-2 infections and its impact on inflammatory biomarkers, Phytother Res, doi:10.1002/ptr.7683
Cucinotta, Vanelli, WHO declares COVID-19 a pandemic, Acta Biomed, doi:10.23750/abm.v91i1.9397
Dersimonian, Laird, Meta-analysis in clinical trials, Control Clin Trials, doi:10.1016/0197-2456(86)90046-2
Dhar, Bhattacharjee, Promising role of curcumin against viral diseases emphasizing COVID-19 management: A review on the mechanistic insights with reference to host-pathogen interaction and immunomodulation, J Funct Foods, doi:10.1016/j.jff.2021.104503
Egger, Smith, Schneider, Minder, Bias in meta-analysis detected by a simple, graphical test, BMJ, doi:10.1136/bmj.315.7109.629
Fatima, Azeem, Saeed, Shahid, Cheema, Efficacy and safety of molnupiravir for COVID-19 patients, Eur J Intern Med, doi:10.1016/j.ejim.2022.05.024
Fu, Chen, Weng, Huang, Lai et al., Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential, Biomed Pharmacother, doi:10.1016/j.biopha.2021.111888
Fu, Ho, Kang, Tsai, Wu et al., Pharmaceutical prospects of Curcuminoids for the remedy of COVID-19: Truth or Myth, Front Pharmacol, doi:10.3389/fphar.2022.863082
Ghasemian, Aliyali, Mehravaran, Maleki, Mohammadi et al., The Effect of Curcumex Supplement (Containing Turmeric, Ginger, and Black Pepper Extract) on Clinical Manifestations and Laboratory Findings of Patients with COVID-19, J Mazandaran univ med sci
Guyatt, Oxman, Vist, Kunz, Falck-Ytter et al., GRADE: an emerging consensus on rating quality of evidence and J o u r n a l P r e -p r o o f strength of recommendations, BMJ, doi:10.1136/bmj.39489.470347.AD
Hartono, Sari, Avicena, Sukmagautama, Apriningsih et al., The Effect of Curcumin and Virgin Coconut Oil Towards Cytokines Levels in COVID-19 Patients at Universitas Sebelas Maret Hospital, Surakarta, Indonesia, Pharmacogn J, doi:10.5530/pj.2022.14.27
Hassaniazad, Eftekhar, Inchehsablagh, Kamali, Tousi et al., A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients, Phytother Res, doi:10.1002/ptr.7294
Hellou, Mohsin, Elemy, Hakim, Mustafa-Hellou et al., Effect of ArtemiC in patients with COVID-19: A Phase II prospective study, J Cell Mol Med, doi:10.1111/jcmm.17337
Hewlings, Kalman, Curcumin: A Review of Its Effects on Human Health, Foods, doi:10.3390/foods6100092
Higgins, Altman, Gøtzsche, Jüni, Moher et al., The Cochrane Collaboration's tool for assessing risk of bias in randomised trials, BMJ, doi:10.1136/bmj.d5928
Higgins, Cochrane Handbook for Systematic Reviews of Interventions Version 5, doi:10.1016/j.lfs.2021.119437
Higgins, Thompson, Deeks, Altman, Measuring inconsistency in metaanalyses, BMJ, doi:10.1136/bmj.327.7414.557
J O U R N A L P R E, None, doi:10.3389/fnut.2022.1023997
J O U R N A L P R E, None, doi:10.1016/j.intimp.2020.107088
J O U R N A L P R E, None, doi:10.1016/j.ejim.2022.05.024
Khan, Iqtadar, Mumtaz, Heinrich, Pascual-Figal et al., Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19-Results From a Pilot Open-Label, Randomized Controlled Trial, Front Pharmacol, doi:10.3389/fphar.2022.898062
Kishimoto, Komiyama, Wada, Efficacy of highly bioavailable oral curcumin in asymptomatic or mild COVID-19 patients: a double-blind, randomized, placebocontrolled trial, J Health Popul Nutr, doi:10.1186/s41043-024-00584-6
Liu, Ying, The Inhibitory Effect of Curcumin on Virus-Induced Cytokine Storm and Its Potential Use in the Associated Severe Pneumonia, Front Cell Dev Biol, doi:10.3389/fcell.2020.00479
Majeed, Nagabhushanam, Shah, Mundkur, A Randomized, Double-Blind, Placebo-Controlled Study to Assess the Efficacy and Safety of a Nutritional Supplement (ImmuActive(TM)) for COVID-19 Patients, Evid Based Complement Alternat Med, doi:10.1155/2021/8447545
Merza, Combining nano-curcumin with catechin improves COVID-19-infected patient's inflammatory conditions, Hum Immunol, doi:10.1016/j.humimm.2023.05.003
Moghadamtousi, Kadir, Hassandarvish, Tajik, Abubakar et al., A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin, Biomed Res Int, doi:10.1155/2014/186864
Mollaamin, Physicochemical investigation of anti-COVID19 drugs using several medicinal plants, J Chil Chem Soc, doi:10.4067/S0717-97072022000205537
Mollaamin, Structural and functional characterization of medicinal plants as selective antibodies towards therapy of COVID-19 symptoms, Antibodies, doi:10.3390/antib13020038
Monajjemi, Mollaamin, Shojaei, An overview on coronaviruses family from past to COVID-19: Introduce some inhibitors as antiviruses from Gillan's plants, Biointerface Res Appl Chem, doi:10.33263/BRIAC103.575585
Nicoliche, Bartolomeo, Lemes, Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2, Sci Rep, doi:10.1038/s41598-024-61662-7
Page, Mckenzie, Bossuyt, The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, BMJ, doi:10.1136/bmj.n71
Pawar, Mastud, Pawar, Pawar, Bhoite et al., Oral Curcumin With Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial, Front Pharmacol, doi:10.3389/fphar.2021.669362
Peluso, Deeks, Mechanisms of long COVID and the path toward therapeutics, Cell, doi:10.1016/j.cell.2024.07.054
Peters, Sutton, Jones, Abrams, Rushton, Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity, Stat Med, doi:10.1002/sim.2889
Rao, Ranganatha, Vikneswaran, Sagar, Mathu et al., AYUSH medicine as add-on therapy for mild category COVID-19; an open label randomised, controlled clinical trial, medRxiv, doi:10.1101/2020.12.06.20245019
Rethlefsen, Kirtley, Waffenschmidt, Ayala, Moher et al., PRISMA-S: an extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews, Syst Rev, doi:10.1186/s13643-020-01542-z
Roberts, Brown, Buja, Weerasinghe, Molecular Mechanisms of Curcumin in COVID-19 Treatment and Prevention: A Global Health Perspective, Med Res Arch, doi:10.18103/mra.v8i10.2248
Sadeghizadeh, Asadollahi, Jahangiri, Yadollahzadeh, Mohajeri et al., Promising clinical outcomes of nano-curcumin treatment as an adjunct therapy in hospitalized J o u r n a l P r e -p r o o f COVID-19 patients: A randomized, double-blinded, placebo-controlled trial, Phytother Res, doi:10.1002/ptr.7844
Sankhe, Memane, Gawali, Memane, Ramakrishnan et al., A prospective, multi center, single blind, randomized controlled study evaluating "AyurCoro3" as an adjuvant in the treatment of mild to moderate COVID-19 patients, J Ayurveda Integr Med, doi:10.21760/jaims.6.4.6
Sankhe, Memane, Gawali, Memane, Ramakrishnan et al., A randomized, controlled, blinded, parallel group, clinical trial to study the role of Ayurcov (AyurCoro3), one day regimen as an adjuvant therapy for COVID-19 disease management, at dedicated Covid Hospital (DCH) in India, Complement Ther Med, doi:10.1016/j.ctim.2022.102824
Shafie, Taheri, Alijani, Okhovvat, Goudarzi et al., Effect of nanocurcumin supplementation on the severity of symptoms and length of hospital stay in J o u r n a l P r e -p r o o f patients with COVID-19: A randomized double-blind placebo-controlled trial, Phytother Res, doi:10.1002/ptr.7374
Shafiee, Athar, Shahid, Ghafoor, Ayyan et al., Curcumin for the treatment of COVID-19 patients: A meta-analysis of randomized controlled trials, Phytother Res, doi:10.1002/ptr.7724
Sharifi-Rad, Rayess, Rizk, Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications, Front Pharmacol, doi:10.3389/fphar.2020.01021
Sohrabi, Alsafi, Neill, Khan, Kerwan et al., World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19), Int J Surg, doi:10.1016/j.ijsu.2020.02.034
Tahmasebi, Saeed, Temirgalieva, Nanocurcumin improves Treg cell responses in patients with mild and severe SARS-CoV-2, Life Sci, doi:10.1016/j.lfs.2021.119437
Ujjan, Khan, Nigar, Ahmed, Ahmad et al., The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19-Results from a pragmatic randomized clinical trial, Original Research, doi:10.3389/fnut.2022.1023997
Vahedian-Azimi, Abbasifard, Rahimi-Bashar, Guest, Majeed et al., Effectiveness of Curcumin on Outcomes of Hospitalized COVID-19 Patients: A Systematic Review of Clinical Trials, Nutrients, doi:10.3390/nu14020256
Valizadeh, Abdolmohammadi-Vahid, Danshina, Gencer, Ammari et al., Nanocurcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients, Int Immunopharmacol, doi:10.1016/j.intimp.2020.107088
Yazdanpanah, Hamblin, Rezaei, The immune system and COVID-19: Friend or foe?, Life Sci
Zahedipour, Hosseini, Sathyapalan, Majeed, Jamialahmadi et al., Potential effects of curcumin in the treatment of COVID-19 infection, Phytother Res, doi:10.1002/ptr.6738
DOI record:
{
"DOI": "10.1016/j.ctim.2025.103295",
"ISSN": [
"0965-2299"
],
"URL": "http://dx.doi.org/10.1016/j.ctim.2025.103295",
"alternative-id": [
"S0965229925001712"
],
"article-number": "103295",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Effectiveness and Safety of Turmeric for the Treatment of COVID-19: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Complementary Therapies in Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ctim.2025.103295"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2025 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Sawangjit",
"given": "Ratree",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sadoyu",
"given": "Saranrat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Manosanthipaibul",
"given": "Siripong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teerawattanapong",
"given": "Nattawat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Puttarak",
"given": "Panupong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wanaratna",
"given": "Kulthanit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Charoensup",
"given": "Rawiwan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hiransai",
"given": "Poonsit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meetam",
"given": "Thunyaluk",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4572-8794",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chaiyakunapruk",
"given": "Nathorn",
"sequence": "additional"
}
],
"container-title": "Complementary Therapies in Medicine",
"container-title-short": "Complementary Therapies in Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.com",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.fr",
"clinicalkey.jp",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2025,
10,
31
]
],
"date-time": "2025-10-31T08:08:11Z",
"timestamp": 1761898091000
},
"deposited": {
"date-parts": [
[
2025,
10,
31
]
],
"date-time": "2025-10-31T08:08:29Z",
"timestamp": 1761898109000
},
"funder": [
{
"DOI": "10.13039/501100007288",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100007288",
"id-type": "DOI"
}
],
"name": "Mahasarakham University"
}
],
"indexed": {
"date-parts": [
[
2025,
10,
31
]
],
"date-time": "2025-10-31T08:40:12Z",
"timestamp": 1761900012918,
"version": "build-2065373602"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
1
]
],
"date-time": "2025-10-01T00:00:00Z",
"timestamp": 1759276800000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
1
]
],
"date-time": "2025-10-01T00:00:00Z",
"timestamp": 1759276800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 28,
"start": {
"date-parts": [
[
2025,
10,
29
]
],
"date-time": "2025-10-29T00:00:00Z",
"timestamp": 1761696000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0965229925001712?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0965229925001712?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "103295",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2025,
10
]
]
},
"published-print": {
"date-parts": [
[
2025,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1128/aac.02404-21",
"article-title": "New perspectives on antimicrobial agents: molnupiravir and nirmatrelvir/ritonavir for treatment of COVID-19",
"author": "Atmar",
"doi-asserted-by": "crossref",
"first-page": "e02404",
"issue": "8",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.ctim.2025.103295_bib1",
"volume": "66",
"year": "2022"
},
{
"article-title": "WHO declares COVID-19 a pandemic",
"author": "Cucinotta",
"first-page": "157",
"issue": "1",
"journal-title": "Acta Biomed",
"key": "10.1016/j.ctim.2025.103295_bib2",
"volume": "91",
"year": "2020"
},
{
"DOI": "10.1016/j.ijsu.2020.02.034",
"article-title": "World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19)",
"author": "Sohrabi",
"doi-asserted-by": "crossref",
"first-page": "71",
"journal-title": "Int J Surg",
"key": "10.1016/j.ctim.2025.103295_bib3",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2024.07.054",
"article-title": "Mechanisms of long COVID and the path toward therapeutics",
"author": "Peluso",
"doi-asserted-by": "crossref",
"first-page": "5500",
"issue": "20",
"journal-title": "Cell",
"key": "10.1016/j.ctim.2025.103295_bib4",
"volume": "187",
"year": "2024"
},
{
"DOI": "10.1016/j.ejim.2022.05.024",
"article-title": "Efficacy and safety of molnupiravir for COVID-19 patients",
"author": "Fatima",
"doi-asserted-by": "crossref",
"first-page": "118",
"journal-title": "Eur J Intern Med",
"key": "10.1016/j.ctim.2025.103295_bib5",
"volume": "102",
"year": "2022"
},
{
"DOI": "10.1016/j.lfs.2020.117900",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ctim.2025.103295_bib6",
"unstructured": "Yazdanpanah F., Hamblin M.R., Rezaei N. The immune system and COVID-19: Friend or foe? Life Sci. https://doi.org/2020;256:117900."
},
{
"DOI": "10.3390/foods6100092",
"article-title": "Curcumin: A Review of Its Effects on Human Health",
"author": "Hewlings",
"doi-asserted-by": "crossref",
"first-page": "92",
"issue": "10",
"journal-title": "Foods",
"key": "10.1016/j.ctim.2025.103295_bib7",
"volume": "6",
"year": "2017"
},
{
"article-title": "A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin",
"author": "Zorofchian Moghadamtousi",
"issue": "1",
"journal-title": "Biomed Res Int",
"key": "10.1016/j.ctim.2025.103295_bib8",
"volume": "2014",
"year": "2014"
},
{
"DOI": "10.1002/ptr.6738",
"article-title": "Potential effects of curcumin in the treatment of COVID-19 infection",
"author": "Zahedipour",
"doi-asserted-by": "crossref",
"first-page": "2911",
"issue": "11",
"journal-title": "Phytother Res",
"key": "10.1016/j.ctim.2025.103295_bib9",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2020.01021",
"article-title": "Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications",
"author": "Sharifi-Rad",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol",
"key": "10.1016/j.ctim.2025.103295_bib10",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.jff.2021.104503",
"article-title": "Promising role of curcumin against viral diseases emphasizing COVID-19 management: A review on the mechanistic insights with reference to host-pathogen interaction and immunomodulation",
"author": "Dhar",
"doi-asserted-by": "crossref",
"journal-title": "J Funct Foods",
"key": "10.1016/j.ctim.2025.103295_bib11",
"volume": "82",
"year": "2021"
},
{
"article-title": "Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients",
"author": "Valizadeh",
"issue": "Pt B",
"journal-title": "Int Immunopharmacol",
"key": "10.1016/j.ctim.2025.103295_bib12",
"volume": "89",
"year": "2020"
},
{
"DOI": "10.4067/S0717-97072022000205537",
"article-title": "Physicochemical investigation of anti-COVID19 drugs using several medicinal plants",
"author": "Mollaamin",
"doi-asserted-by": "crossref",
"first-page": "5537",
"issue": "2",
"journal-title": "J Chil Chem Soc",
"key": "10.1016/j.ctim.2025.103295_bib13",
"volume": "67",
"year": "2022"
},
{
"DOI": "10.33263/BRIAC103.575585",
"article-title": "An overview on coronaviruses family from past to COVID-19: Introduce some inhibitors as antiviruses from Gillan’s plants",
"author": "Monajjemi",
"doi-asserted-by": "crossref",
"first-page": "5575",
"issue": "3",
"journal-title": "Biointerface Res Appl Chem",
"key": "10.1016/j.ctim.2025.103295_bib14",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.3390/antib13020038",
"article-title": "Structural and functional characterization of medicinal plants as selective antibodies towards therapy of COVID-19 symptoms",
"author": "Mollaamin",
"doi-asserted-by": "crossref",
"first-page": "38",
"issue": "2",
"journal-title": "Antibodies (Basel)",
"key": "10.1016/j.ctim.2025.103295_bib15",
"volume": "13",
"year": "2024"
},
{
"DOI": "10.1002/fsn3.2226",
"article-title": "Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebo-controlled clinical trial",
"author": "Ahmadi",
"doi-asserted-by": "crossref",
"first-page": "4068",
"issue": "8",
"journal-title": "Food Sci Nutr",
"key": "10.1016/j.ctim.2025.103295_bib16",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.669362",
"article-title": "Oral Curcumin With Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial",
"author": "Pawar",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol",
"key": "10.1016/j.ctim.2025.103295_bib17",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.lfs.2021.119437",
"article-title": "Nanocurcumin improves Treg cell responses in patients with mild and severe SARS-CoV-2",
"author": "Tahmasebi",
"doi-asserted-by": "crossref",
"journal-title": "Life Sci",
"key": "10.1016/j.ctim.2025.103295_bib18",
"volume": "276",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7724",
"article-title": "Curcumin for the treatment of COVID-19 patients: A meta-analysis of randomized controlled trials",
"author": "Shafiee",
"doi-asserted-by": "crossref",
"first-page": "1167",
"issue": "3",
"journal-title": "Phytother Res",
"key": "10.1016/j.ctim.2025.103295_bib19",
"volume": "37",
"year": "2023"
},
{
"DOI": "10.3390/nu14020256",
"article-title": "Effectiveness of Curcumin on Outcomes of Hospitalized COVID-19 Patients: A Systematic Review of Clinical Trials",
"author": "Vahedian-Azimi",
"doi-asserted-by": "crossref",
"first-page": "256",
"issue": "2",
"journal-title": "Nutrients",
"key": "10.1016/j.ctim.2025.103295_bib20",
"volume": "14",
"year": "2022"
},
{
"key": "10.1016/j.ctim.2025.103295_bib21",
"unstructured": "Higgins J.P.T. GSe. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. March 2011 ed. 2011."
},
{
"DOI": "10.1136/bmj.n71",
"article-title": "The PRISMA 2020 statement: an updated guideline for reporting systematic reviews",
"author": "Page",
"doi-asserted-by": "crossref",
"first-page": "n71",
"journal-title": "BMJ",
"key": "10.1016/j.ctim.2025.103295_bib22",
"volume": "372",
"year": "2021"
},
{
"DOI": "10.1186/s13643-020-01542-z",
"article-title": "PRISMA-S Group. PRISMA-S: an extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews",
"author": "Rethlefsen",
"doi-asserted-by": "crossref",
"first-page": "39",
"issue": "1",
"journal-title": "Syst Rev",
"key": "10.1016/j.ctim.2025.103295_bib23",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1136/bmj.d5928",
"article-title": "Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials",
"author": "Higgins",
"doi-asserted-by": "crossref",
"first-page": "d5928",
"journal-title": "BMJ",
"key": "10.1016/j.ctim.2025.103295_bib24",
"volume": "343",
"year": "2011"
},
{
"DOI": "10.1136/bmj.327.7414.557",
"article-title": "Measuring inconsistency in meta-analyses",
"author": "Higgins",
"doi-asserted-by": "crossref",
"first-page": "557",
"issue": "7414",
"journal-title": "BMJ",
"key": "10.1016/j.ctim.2025.103295_bib25",
"volume": "327",
"year": "2003"
},
{
"DOI": "10.1016/0197-2456(86)90046-2",
"article-title": "Meta-analysis in clinical trials",
"author": "DerSimonian",
"doi-asserted-by": "crossref",
"first-page": "177",
"issue": "3",
"journal-title": "Control Clin Trials",
"key": "10.1016/j.ctim.2025.103295_bib26",
"volume": "7",
"year": "1986"
},
{
"author": "Borenstein",
"key": "10.1016/j.ctim.2025.103295_bib27",
"series-title": "Introduction to meta-analysis",
"year": "2021"
},
{
"DOI": "10.1136/bmj.315.7109.629",
"article-title": "Bias in meta-analysis detected by a simple, graphical test",
"author": "Egger",
"doi-asserted-by": "crossref",
"first-page": "629",
"issue": "7109",
"journal-title": "BMJ",
"key": "10.1016/j.ctim.2025.103295_bib28",
"volume": "315",
"year": "1997"
},
{
"DOI": "10.1002/sim.2889",
"article-title": "Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity",
"author": "Peters",
"doi-asserted-by": "crossref",
"first-page": "4544",
"issue": "25",
"journal-title": "Stat Med",
"key": "10.1016/j.ctim.2025.103295_bib29",
"volume": "26",
"year": "2007"
},
{
"DOI": "10.1136/bmj.39489.470347.AD",
"article-title": "GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations",
"author": "Guyatt",
"doi-asserted-by": "crossref",
"first-page": "924",
"issue": "7650",
"journal-title": "BMJ",
"key": "10.1016/j.ctim.2025.103295_bib30",
"volume": "336",
"year": "2008"
},
{
"DOI": "10.1016/j.ejphar.2022.175267",
"article-title": "Immunomodulatory role of Nanocurcumin in COVID-19 patients with dropped natural killer cells frequency and function",
"author": "Abbaspour-Aghdam",
"doi-asserted-by": "crossref",
"journal-title": "Eur J Pharmacol",
"key": "10.1016/j.ctim.2025.103295_bib31",
"volume": "933",
"year": "2022"
},
{
"DOI": "10.1016/j.humimm.2023.05.003",
"article-title": "Combining nano-curcumin with catechin improves COVID-19-infected patient's inflammatory conditions",
"author": "Ahmad Merza Mohammad",
"doi-asserted-by": "crossref",
"first-page": "471",
"issue": "9",
"journal-title": "Hum Immunol",
"key": "10.1016/j.ctim.2025.103295_bib32",
"volume": "84",
"year": "2023"
},
{
"DOI": "10.1155/2023/5734675",
"article-title": "Efficacy of Nanocurcumin as an Add-On Treatment for Patients Hospitalized with COVID-19: A Double-Blind, Randomized Clinical Trial",
"author": "Ahmadi",
"doi-asserted-by": "crossref",
"journal-title": "Int J Clin Pract",
"key": "10.1016/j.ctim.2025.103295_bib33",
"volume": "2023",
"year": "2023"
},
{
"DOI": "10.1002/ptr.7375",
"article-title": "Antiinflammatory potential of nano-curcumin as an alternative therapeutic agent for the treatment of mild-to-moderate hospitalized COVID-19 patients in a placebo-controlled clinical trial",
"author": "Asadirad",
"doi-asserted-by": "crossref",
"first-page": "1023",
"issue": "2",
"journal-title": "Phytother Res",
"key": "10.1016/j.ctim.2025.103295_bib34",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.1186/s13063-022-06375-w",
"article-title": "The efficacy of curcumin-piperine co-supplementation on clinical symptoms, duration, severity, and inflammatory factors in COVID-19 outpatients: a randomized double-blind, placebo-controlled trial",
"author": "Askari",
"doi-asserted-by": "crossref",
"first-page": "472",
"issue": "1",
"journal-title": "Trials",
"key": "10.1016/j.ctim.2025.103295_bib35",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1002/ptr.7683",
"article-title": "Phase III randomized clinical trial of BV-4051, an Ayurvedic polyherbal formulation in moderate SARS-CoV-2 infections and its impact on inflammatory biomarkers",
"author": "Chitre",
"doi-asserted-by": "crossref",
"first-page": "1232",
"issue": "4",
"journal-title": "Phytother Res",
"key": "10.1016/j.ctim.2025.103295_bib36",
"volume": "37",
"year": "2023"
},
{
"article-title": "The Effect of Curcumex Supplement (Containing Turmeric, Ginger, and Black Pepper Extract) on Clinical Manifestations and Laboratory Findings of Patients with COVID-19",
"author": "Ghasemian",
"first-page": "70",
"issue": "229",
"journal-title": "J Mazandaran univ med sci",
"key": "10.1016/j.ctim.2025.103295_bib37",
"volume": "33",
"year": "2024"
},
{
"DOI": "10.5530/pj.2022.14.27",
"article-title": "The Effect of Curcumin and Virgin Coconut Oil Towards Cytokines Levels in COVID-19 Patients at Universitas Sebelas Maret Hospital, Surakarta, Indonesia",
"author": "Hartono",
"doi-asserted-by": "crossref",
"first-page": "216",
"issue": "1",
"journal-title": "Pharmacogn J",
"key": "10.1016/j.ctim.2025.103295_bib38",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1002/ptr.7294",
"article-title": "A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients",
"author": "Hassaniazad",
"doi-asserted-by": "crossref",
"first-page": "6417",
"issue": "11",
"journal-title": "Phytother Res",
"key": "10.1016/j.ctim.2025.103295_bib39",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1111/jcmm.17337",
"article-title": "Effect of ArtemiC in patients with COVID-19: A Phase II prospective study",
"author": "Hellou",
"doi-asserted-by": "crossref",
"first-page": "3281",
"issue": "11",
"journal-title": "J Cell Mol Med",
"key": "10.1016/j.ctim.2025.103295_bib40",
"volume": "26",
"year": "2022"
},
{
"DOI": "10.1002/ptr.7374",
"article-title": "Effect of nanocurcumin supplementation on the severity of symptoms and length of hospital stay in patients with COVID-19: A randomized double-blind placebo-controlled trial",
"author": "Honarkar Shafie",
"doi-asserted-by": "crossref",
"first-page": "1013",
"issue": "2",
"journal-title": "Phytother Res",
"key": "10.1016/j.ctim.2025.103295_bib41",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2022.898062",
"article-title": "Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19-Results From a Pilot Open-Label, Randomized Controlled Trial",
"author": "Khan",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol",
"key": "10.1016/j.ctim.2025.103295_bib42",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1186/s41043-024-00584-6",
"article-title": "Efficacy of highly bioavailable oral curcumin in asymptomatic or mild COVID-19 patients: a double-blind, randomized, placebo-controlled trial",
"author": "Kishimoto",
"doi-asserted-by": "crossref",
"first-page": "93",
"issue": "1",
"journal-title": "J Health Popul Nutr",
"key": "10.1016/j.ctim.2025.103295_bib43",
"volume": "43",
"year": "2024"
},
{
"DOI": "10.1155/2021/8447545",
"article-title": "A Randomized, Double-Blind, Placebo-Controlled Study to Assess the Efficacy and Safety of a Nutritional Supplement (ImmuActive(TM)) for COVID-19 Patients",
"author": "Majeed",
"doi-asserted-by": "crossref",
"journal-title": "Evid Based Complement Alternat Med",
"key": "10.1016/j.ctim.2025.103295_bib44",
"volume": "2021",
"year": "2021"
},
{
"article-title": "AYUSH medicine as add-on therapy for mild category COVID-19; an open label randomised, controlled clinical trial",
"author": "Rao",
"journal-title": "medRxiv",
"key": "10.1016/j.ctim.2025.103295_bib45",
"year": "2020"
},
{
"DOI": "10.1002/ptr.7844",
"article-title": "Promising clinical outcomes of nano-curcumin treatment as an adjunct therapy in hospitalized COVID-19 patients: A randomized, double-blinded, placebo-controlled trial",
"author": "Sadeghizadeh",
"doi-asserted-by": "crossref",
"first-page": "3631",
"issue": "8",
"journal-title": "Phytother Res",
"key": "10.1016/j.ctim.2025.103295_bib46",
"volume": "37",
"year": "2023"
},
{
"article-title": "A prospective, multi center, single blind, randomized controlled study evaluating \"AyurCoro3\" as an adjuvant in the treatment of mild to moderate COVID-19 patients",
"author": "Sankhe",
"first-page": "31",
"issue": "4",
"journal-title": "J Ayurveda Integr Med",
"key": "10.1016/j.ctim.2025.103295_bib47",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/j.ctim.2022.102824",
"article-title": "A randomized, controlled, blinded, parallel group, clinical trial to study the role of Ayurcov (AyurCoro3), one day regimen as an adjuvant therapy for COVID-19 disease management, at dedicated Covid Hospital (DCH) in India",
"author": "Sankhe",
"doi-asserted-by": "crossref",
"journal-title": "Complement Ther Med",
"key": "10.1016/j.ctim.2025.103295_bib48",
"volume": "67",
"year": "2022"
},
{
"article-title": "The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19—Results from a pragmatic randomized clinical trial",
"author": "Ujjan",
"journal-title": "Original Research. Front Nutr",
"key": "10.1016/j.ctim.2025.103295_bib49",
"volume": "9",
"year": "2023"
},
{
"DOI": "10.3389/fphar.2022.863082",
"article-title": "Pharmaceutical prospects of Curcuminoids for the remedy of COVID-19: Truth or Myth",
"author": "Fu",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol",
"key": "10.1016/j.ctim.2025.103295_bib50",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.biopha.2021.111888",
"article-title": "Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential",
"author": "Fu",
"doi-asserted-by": "crossref",
"journal-title": "Biomed Pharmacother",
"key": "10.1016/j.ctim.2025.103295_bib51",
"volume": "141",
"year": "2021"
},
{
"DOI": "10.1038/s41598-024-61662-7",
"article-title": "Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2",
"author": "Nicoliche",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Sci Rep",
"key": "10.1016/j.ctim.2025.103295_bib52",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.18103/mra.v8i10.2248",
"article-title": "Molecular Mechanisms of Curcumin in COVID-19 Treatment and Prevention: A Global Health Perspective",
"author": "Roberts",
"doi-asserted-by": "crossref",
"issue": "10",
"journal-title": "Med Res Arch",
"key": "10.1016/j.ctim.2025.103295_bib53",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.3389/fcell.2020.00479",
"article-title": "The Inhibitory Effect of Curcumin on Virus-Induced Cytokine Storm and Its Potential Use in the Associated Severe Pneumonia",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "479",
"journal-title": "Front Cell Dev Biol",
"key": "10.1016/j.ctim.2025.103295_bib54",
"volume": "8",
"year": "2020"
}
],
"reference-count": 54,
"references-count": 54,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0965229925001712"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effectiveness and Safety of Turmeric for the Treatment of COVID-19: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy"
}
