Effect of ArtemiC in patients with COVID-19: A Phase II prospective study
et al., Journal of Cellular and Molecular Medicine, doi:10.1111/jcmm.17337, NCT04382040, May 2022
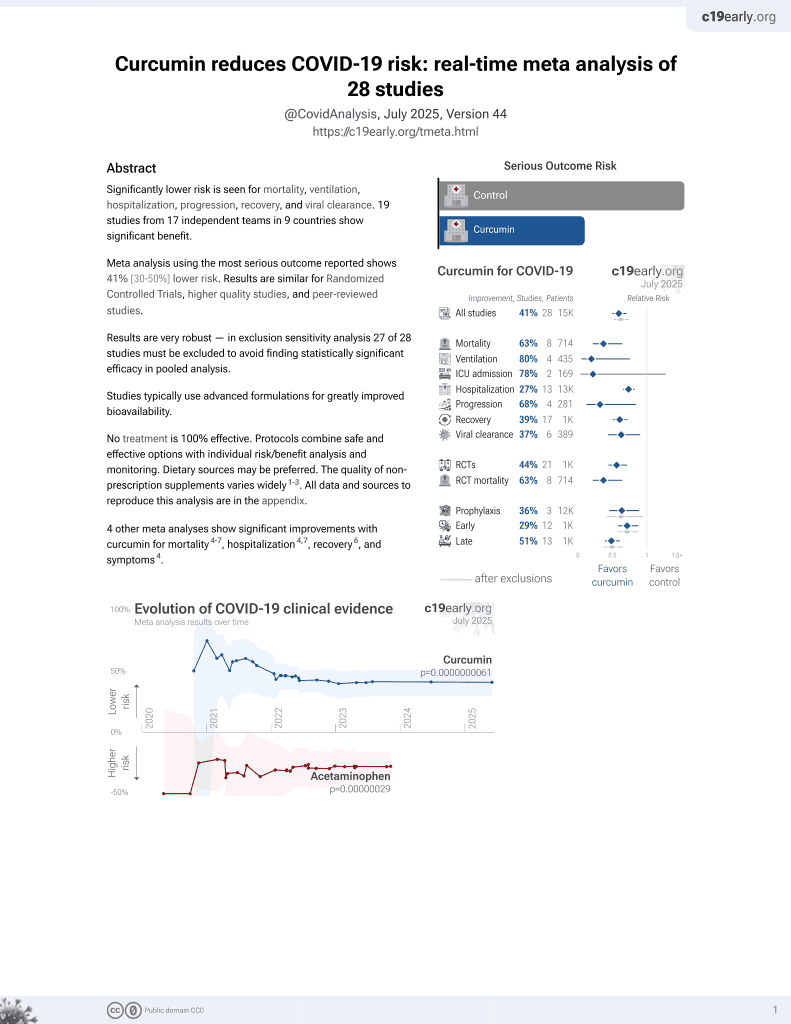
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 50 hospitalized patients in Israel, 33 treated with curcumin, vitamin C, artemisinin, and frankincense oral spray, showing improved recovery with treatment.
Viral load measured by PCR may not accurately reflect infectious virus measured by viral culture. Porter et al. show that viral load early in infection was correlated with infectious virus, but viral load late in infection could be high even with low or undetectable infectious virus. Assessing viral load later in infection may underestimate reductions in infectious virus with treatment.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects (early treatment may be more beneficial).
This is the 13th of 21 COVID-19 RCTs for curcumin, which collectively show efficacy with p=0.0000022.
This is the 18th of 28 COVID-19 controlled studies for curcumin, which collectively show efficacy with p=0.0000000061.
Study covers vitamin C and curcumin.
|
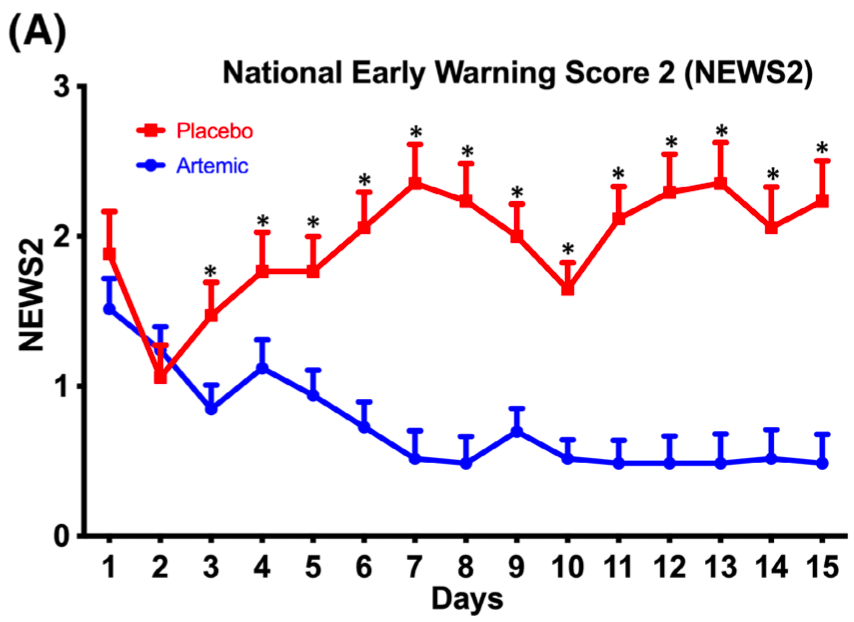
relative NEWS2 score, 76.7% better, RR 0.23, p = 0.04, treatment mean 0.52 (±0.67) n=33, control mean 2.23 (±3.2) n=17, day 15.
|
|
risk of oxygen therapy, 92.2% lower, RR 0.08, p = 0.01, treatment 0 of 33 (0.0%), control 4 of 17 (23.5%), NNT 4.2, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 15.
|
|
oxygen time, 69.7% lower, relative time 0.30, p = 0.17, treatment mean 2.3 (±1.4) n=33, control mean 7.6 (±4.6) n=17.
|
|
hospitalization time, 13.3% lower, relative time 0.87, p = 0.92, treatment mean 7.8 (±7.3) n=33, control mean 9.0 (±8.0) n=17.
|
|
risk of no viral clearance, 9.8% lower, RR 0.90, p = 0.77, treatment 14 of 33 (42.4%), control 8 of 17 (47.1%), NNT 22, day 15.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hellou et al., 19 May 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Israel, peer-reviewed, 6 authors, study period 8 May, 2020 - 21 December, 2020, this trial uses multiple treatments in the treatment arm (combined with vitamin C, artemisinin, and frankincense) - results of individual treatments may vary, trial NCT04382040 (history).
Effect of ArtemiC in patients with COVID‐19: A Phase II prospective study
Journal of Cellular and Molecular Medicine, doi:10.1111/jcmm.17337
The coronavirus disease 2019 (COVID-19) pandemic, which initially emerged in Wuhan-South-eastern China in 2019, is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is associated with significant morbidity and mortality among vulnerable patients. 1 This grim situation is mainly attributed to the poor understanding of the pathogenesis of SARS-CoV-2-induced injury to vital organs, particularly in aged patients with diabetes, obesity, hypertension, heart failure and respiratory diseases. 2, 3 Critically ill cases are characterized by acute respiratory distress syndrome (ARDS) and septic shock, as well as multiple organ dysfunction or failure. [2] [3] [4] Human angiotensin-converting enzyme 2 (ACE2) receptor serves as the binding domain of SARS-CoV-2 in human host cells, exploiting its high affinity to this enzyme to inflict remarkable damage to key target organs. [5] [6] [7]
AUTH O R CO NTR I B UTI O N S Elias Hellou involved in investigation, writing-original draft (lead), review and editing (lead). Jameel Mohsin, Fahed Hakim and Ameer Elemy involved in investigation (supporting). Mona Mustafa-Hellou involved in investigation (supporting), writing-review and editing (supporting). Shadi Hamou involved in writing-original draft, review and editing (equal).
CO N FLI C T O F I NTE R E S T The authors confirm that there are no conflicts of interest.
References
Armaly, Kinaneh, Skorecki, Renal manifestations of Covid-19: physiology and pathophysiology, J Clin Med, doi:10.3390/jcm10061216
Azzi, Bartash, Scalea, Loarte-Campos, Akalin, COVID-19 and solid organ transplantation: a review article, Transplantation, doi:10.1097/TP.0000000000003523
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 -final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Campbell, Cole, Bunditrutavorn, Vella, Ascorbic acid is a potent inhibitor of various forms of T cell apoptosis, Cell Immunol, doi:10.1006/cimm.1999.1485
Caricchio, Gallucci, Dass, Preliminary predictive criteria for COVID-19 cytokine storm, Ann Rheum Dis, doi:10.1136/annrheumdis-2020-218323
Cazzola, Rogliani, Salvi, Ora, Matera, Use of thiols in the treatment of COVID-19: current evidence, Lung, doi:10.1007/s00408-021-00465-3
Chernyak, Popova, Prikhodko, Grebenchikov, Zinovkina et al., COVID-19 and oxidative stress
Crawford, Dingens, Eguia, Dynamics of neutralizing antibody titers in the months after SARS-CoV-2 infection, J Infect Dis, doi:10.1093/infdis/jiaa618
Dagcioglu, Keskin, Guner, Thiol levels in mild or moderate Covid-19 patients: a comparison of variant and classic Covid-19 cases, Int J Clin Pract, doi:10.1111/ijcp.14753
Derouiche, Oxidative stress associated with SARS-Cov-2 (COVID-19) increases the severity of the lung disease -a systematic review, J Infect Dis Epidemiol, doi:10.23937/2474-3658/1510121
Efferth, Li, Konkimalla, Kaina, From traditional Chinese medicine to rational cancer therapy, Trends Mol Med, doi:10.1016/j.molmed.2007.07.001
Fajgenbaum, June, Cytokine storm, N Engl J Med, doi:10.1056/NEJMra2026131
Fo R M Ati O N ; Hellou, Mohsin, Elemy, Hakim, Mustafa-Hellou et al., Additional supporting information may be found in the online version of the article at the publisher's website, J Cell Mol Med
Fowler Aa 3rd, Truwit, Hite, Effect of Vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial, JAMA, doi:10.1001/jama.2019.11825
Gadoth, Halbrook, Blais, Cross-sectional assessment of COVID-19 vaccine acceptance among health care workers in Los Angeles, Ann Intern Med, doi:10.7326/M20-7580
Garibaldi, Wang, Robinson, Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalized patients with COVID-19, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.3071
Gavriatopoulou, Ntanasis-Stathopoulos, Korompoki, Emerging treatment strategies for COVID-19 infection, Clin Exp Med, doi:10.1007/s10238-020-00671-y
Group, Horby, Lim, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Group, Horby, Mafham, Effect of hydroxychloroquine in hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2022926
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med, doi:10.1056/NEJMoa2002032
Huang, Wang, Tan, Liu, Ni, High-dose vitamin C intravenous infusion in the treatment of patients with COVID-19: a protocol for systematic review and meta-analysis, Medicine, doi:10.1097/MD.0000000000025876
Huang, Wang, Yang, A review of severe acute respiratory syndrome coronavirus 2 infection in the reproductive system, J Chin Med Assoc, doi:10.1097/JCMA.0000000000000388
Iwai, Horiuchi, Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs. ACE2-angiotensin-(1-7)-Mas receptor axis, Hypertens Res, doi:10.1038/hr.2009.74
Kamidani, Rostad, Anderson, COVID-19 vaccine development: a pediatric perspective, Curr Opin Pediatr, doi:10.1097/MOP.0000000000000978
Li, Moore, Vasilieva, Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus, Nature, doi:10.1038/nature02145
Liu, Vanblargan, Bloyet, Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization, Cell Host Microbe, doi:10.1016/j.chom.2021.01.014
Mathew, Giles, Baxter, Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications, Science, doi:10.1126/science.abc8511
Moghadamtousi, Kadir, Hassandarvish, Tajik, Abubakar et al., A review on antibacterial, antiviral, and antifungal activity of curcumin, Biomed Res Int, doi:10.1155/2014/186864
Mp, N-acetylcysteine as a potential treatment for COVID-19, Future Microbiol, doi:10.2217/fmb-2020-0074
Myrstad, Ihle-Hansen, Tveita, National Early Warning Score 2 (NEWS2) on admission predicts severe disease and inhospital mortality from Covid-19 -a prospective cohort study, Scand J Trauma Resusc Emerg Med, doi:10.1186/s13049-020-00764-3
Ohl, Miller, Lund, Association of remdesivir treatment with survival and length of hospital stay among US veterans hospitalized with COVID-19, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.14741
Ou, Boyarsky, Zeiser, Kidney transplant recipient attitudes toward a SARS-CoV-2 vaccine, Transplant Direct, doi:10.1097/TXD.0000000000001171
Ragab, Salah Eldin, Taeimah, Khattab, Salem, The COVID-19 cytokine storm; what we know so far, Front Immunol, doi:10.3389/fimmu.2020.01446
Rizk, Kalantar-Zadeh, Mehra, Lavie, Rizk et al., Pharmaco-immunomodulatory therapy in COVID-19, Drugs, doi:10.1007/s40265-020-01367-z
Romano, Chebabo, Levi, Past, present, and future of COVID-19: a review, Braz J Med Biol Res, doi:10.1590/1414-431x202010475
Russell, Millar, Baillie, Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury, Lancet, doi:10.1016/S0140-6736(20)30317-2
Santos, Ferreira, Silva, Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis, Exp Physiol, doi:10.1113/expphysiol.2008.042002
Siddiqui, Boswellia serrata, a potential antiinflammatory agent: an overview, Indian J Pharm Sci, doi:10.4103/0250-474X.93507
Sivaloganathan, Ladikou, Chevassut, COVID-19 mortality in patients on anticoagulants and antiplatelet agents, Br J Haematol, doi:10.1111/bjh.16968
Spagnolo, Balestro, Aliberti, Pulmonary fibrosis secondary to COVID-19: a call to arms?, Lancet Respir Med, doi:10.1016/S2213-2600(20)30222-8
Tang, Liu, Zhang, Xu, Wen, Cytokine storm in COVID-19: the current evidence and treatment strategies, Front Immunol, doi:10.3389/fimmu.2020.01708
Tsuda, Renin-Angiotensin system and sympathetic neurotransmitter release in the central nervous system of hypertension, Int J Hypertens, doi:10.1155/2012/474870
Tu, Artemisinin-A gift from traditional Chinese medicine to the world (Nobel Lecture), Angew Chem Int Ed Engl, doi:10.1002/anie.201601967
Valle, Kim-Schulze, Huang, An inflammatory cytokine signature predicts COVID-19 severity and survival, Nat Med, doi:10.1038/s41591-020-1051-9
Wan, Shang, Graham, Baric, Li, Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus, J Virol, doi:10.1128/JVI.00127-20
Wang, Nair, Liu, Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7, Nature, doi:10.1038/s41586-021-03398-2
Wang, Teo, Teo, Chai, Virtual reality as a bridge in palliative care during COVID-19, J Palliat Med, doi:10.1089/jpm.2020.0212
Weisblum, Schmidt, Zhang, Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants, Elife, doi:10.7554/eLife.61312
Wilson, Wiysonge, Social media and vaccine hesitancy, BMJ Glob Health, doi:10.1136/bmjgh-2020-004206
Yanay, Freiman, Shapira, Experience with SARS-CoV-2 BNT162b2 mRNA vaccine in dialysis patients, Kidney Int, doi:10.1016/j.kint.2021.04.006
Yang, Yu, Xu, Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a singlecentered, retrospective, observational study, Lancet Respir Med, doi:10.1016/S2213-2600(20)30079-5
Yedjou, Njiki, Enow, Pharmacological effects of selected medicinal plants and vitamins against COVID-19, J Food Nutr, doi:10.17303/jfn.2021.7.202
Yedjou, Njiki, Enow, Pharmacological effects of selected medicinal plants and vitamins against COVID-19, Jacobs J Food Nutr, doi:10.17303/jfn.2021.7.202
Zhang, Dong, Cao, Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China, Allergy, doi:10.1111/all.14238
Zhang, Zhao, Zhang, The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China, Clin Immunol, doi:10.1016/j.clim.2020.108393
Zhao, Zhao, Wang, Zhou, Ma et al., Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2, Am J Respir Crit Care Med, doi:10.1164/rccm.202001-0179LE
Zhu, Zhang, Wang, A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.1111/jcmm.17337",
"ISSN": [
"1582-1838",
"1582-4934"
],
"URL": "http://dx.doi.org/10.1111/jcmm.17337",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Despite intensive efforts, there is no effective remedy for COVID‐19. Moreover, vaccination efficacy declines over time and may be compromised against new SARS‐CoV‐2 lineages. Therefore, there remains an unmet need for simple, accessible, low‐cost and effective pharmacological anti‐SARS‐CoV‐2 agents. ArtemiC is a medical product comprising artemisinin, curcumin, frankincense and vitamin C, all of which possess anti‐inflammatory and anti‐oxidant properties. The present Phase II placebo‐controlled, double‐blinded, multi‐centred, prospective study evaluated the efficacy and safety of ArtemiC in patients with COVID‐19. The study included 50 hospitalized symptomatic COVID‐19 patients randomized (2:1) to receive ArtemiC or placebo oral spray, twice daily on Days 1 and 2, beside standard care. A physical examination was performed, and vital signs and blood tests were monitored daily until hospital discharge (or Day 15). A PCR assessment of SARS‐CoV‐2 carriage was performed at screening and on last visit. ArtemiC improved NEWS2 in 91% of patients and shortened durations of abnormal SpO<jats:sub>2</jats:sub> levels, oxygen supplementation and fever. No treatment‐related adverse events were reported. These findings suggest that ArtemiC curbed deterioration, possibly by limiting cytokine storm of COVID‐19, thus bearing great promise for COVID‐19 patients, particularly those with comorbidities.</jats:p>",
"alternative-id": [
"10.1111/jcmm.17337"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-09-12"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-04-05"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-05-19"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0705-9721",
"affiliation": [
{
"name": "Department of Cardiology E.M.M.S Hospital Nazareth Israel"
},
{
"name": "Department of Cardiology Hillel Yaffe Hospital Hadera Israel"
},
{
"name": "Rappaport Faculty of Medicine Technion‐Israel Institute of Technology Haifa Israel"
}
],
"authenticated-orcid": false,
"family": "Hellou",
"given": "Elias",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Cardiology Hillel Yaffe Hospital Hadera Israel"
},
{
"name": "Rappaport Faculty of Medicine Technion‐Israel Institute of Technology Haifa Israel"
}
],
"family": "Mohsin",
"given": "Jameel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Victory Department for COVID‐19 Patients E.M.M.S Hospital Nazareth Israel"
}
],
"family": "Elemy",
"given": "Ameer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Victory Department for COVID‐19 Patients E.M.M.S Hospital Nazareth Israel"
},
{
"name": "Azrieli Faculty of Medicine Bar‐Ilan University Zefat Israel"
}
],
"family": "Hakim",
"given": "Fahed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rappaport Faculty of Medicine Technion‐Israel Institute of Technology Haifa Israel"
},
{
"name": "Department of Internal Medicine E Rambam Health Care Campus Haifa Israel"
}
],
"family": "Mustafa‐Hellou",
"given": "Mona",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rappaport Faculty of Medicine Technion‐Israel Institute of Technology Haifa Israel"
},
{
"name": "Department of Internal Medicine E Rambam Health Care Campus Haifa Israel"
}
],
"family": "Hamoud",
"given": "Shadi",
"sequence": "additional"
}
],
"container-title": "Journal of Cellular and Molecular Medicine",
"container-title-short": "J Cellular Molecular Medi",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
5,
19
]
],
"date-time": "2022-05-19T09:45:02Z",
"timestamp": 1652953502000
},
"deposited": {
"date-parts": [
[
2023,
12,
29
]
],
"date-time": "2023-12-29T11:58:29Z",
"timestamp": 1703851109000
},
"indexed": {
"date-parts": [
[
2024,
3,
25
]
],
"date-time": "2024-03-25T01:07:32Z",
"timestamp": 1711328852953
},
"is-referenced-by-count": 13,
"issue": "11",
"issued": {
"date-parts": [
[
2022,
5,
19
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2022,
6
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
5,
19
]
],
"date-time": "2022-05-19T00:00:00Z",
"timestamp": 1652918400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/jcmm.17337",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/jcmm.17337",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/jcmm.17337",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "3281-3289",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2022,
5,
19
]
]
},
"published-online": {
"date-parts": [
[
2022,
5,
19
]
]
},
"published-print": {
"date-parts": [
[
2022,
6
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1590/1414‐431x202010475",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_2_1"
},
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_3_1"
},
{
"DOI": "10.1111/all.14238",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_4_1"
},
{
"DOI": "10.1016/S2213‐2600(20)30079‐5",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_5_1"
},
{
"DOI": "10.1038/nature02145",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_6_1"
},
{
"DOI": "10.1164/rccm.202001‐0179LE",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_7_1"
},
{
"DOI": "10.1128/JVI.00127‐20",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_8_1"
},
{
"DOI": "10.1155/2012/474870",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_9_1"
},
{
"DOI": "10.1113/expphysiol.2008.042002",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_10_1"
},
{
"DOI": "10.1038/hr.2009.74",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_11_1"
},
{
"DOI": "10.1007/s10238‐020‐00671‐y",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_12_1"
},
{
"DOI": "10.1056/NEJMoa2022926",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_13_1"
},
{
"DOI": "10.1016/j.clim.2020.108393",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_14_1"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_15_1"
},
{
"DOI": "10.1016/S0140‐6736(20)30317‐2",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_16_1"
},
{
"DOI": "10.1001/jama.2019.11825",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_17_1"
},
{
"DOI": "10.1097/MD.0000000000025876",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_18_1"
},
{
"DOI": "10.1038/s41586‐021‐03398‐2",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_19_1"
},
{
"DOI": "10.1016/j.chom.2021.01.014",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_20_1"
},
{
"DOI": "10.1097/MOP.0000000000000978",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_21_1"
},
{
"DOI": "10.7326/M20‐7580",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_22_1"
},
{
"DOI": "10.1136/bmjgh‐2020‐004206",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_23_1"
},
{
"DOI": "10.1097/TXD.0000000000001171",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_24_1"
},
{
"DOI": "10.1016/j.kint.2021.04.006",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_25_1"
},
{
"DOI": "10.1097/TP.0000000000003523",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_26_1"
},
{
"DOI": "10.1186/s13049‐020‐00764‐3",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_27_1"
},
{
"DOI": "10.17303/jfn.2021.7.202",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_28_1"
},
{
"DOI": "10.3390/jcm10061216",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_29_1"
},
{
"DOI": "10.7554/eLife.61312",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_30_1"
},
{
"DOI": "10.1093/infdis/jiaa618",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_31_1"
},
{
"DOI": "10.1002/anie.201601967",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_32_1"
},
{
"DOI": "10.1155/2014/186864",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_33_1"
},
{
"DOI": "10.4103/0250‐474X.93507",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_34_1"
},
{
"DOI": "10.1016/j.molmed.2007.07.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_35_1"
},
{
"DOI": "10.1136/annrheumdis‐2020‐218323",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_36_1"
},
{
"DOI": "10.1007/s40265‐020‐01367‐z",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_37_1"
},
{
"DOI": "10.3389/fimmu.2020.01446",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_38_1"
},
{
"DOI": "10.1111/bjh.16968",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_39_1"
},
{
"DOI": "10.1016/S2213‐2600(20)30222‐8",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_40_1"
},
{
"DOI": "10.1089/jpm.2020.0212",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_41_1"
},
{
"DOI": "10.1097/JCMA.0000000000000388",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_42_1"
},
{
"DOI": "10.1038/s41591‐020‐1051‐9",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_43_1"
},
{
"DOI": "10.1126/science.abc8511",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_44_1"
},
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_45_1"
},
{
"DOI": "10.1056/NEJMra2026131",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_46_1"
},
{
"DOI": "10.3389/fimmu.2020.01708",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_47_1"
},
{
"DOI": "10.1134/S0006297920120068",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_48_1"
},
{
"DOI": "10.23937/2474‐3658/1510121",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_49_1"
},
{
"DOI": "10.1111/ijcp.14753",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_50_1"
},
{
"DOI": "10.1007/s00408‐021‐00465‐3",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_51_1"
},
{
"DOI": "10.2217/fmb‐2020‐0074",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_52_1"
},
{
"DOI": "10.17303/jfn.2021.7.202",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_53_1"
},
{
"DOI": "10.1006/cimm.1999.1485",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_54_1"
},
{
"DOI": "10.1001/jamanetworkopen.2021.3071",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_55_1"
},
{
"DOI": "10.1001/jamanetworkopen.2021.14741",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_56_1"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_57_1"
}
],
"reference-count": 56,
"references-count": 56,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/jcmm.17337"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Cell Biology",
"Molecular Medicine"
],
"subtitle": [],
"title": "Effect of ArtemiC in patients with COVID‐19: A Phase II prospective study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "26"
}