Association of Remdesivir Treatment With Survival and Length of Hospital Stay Among US Veterans Hospitalized With COVID-19
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2021.14741, Jul 2021
Retrospective 5,898 hospitalized patients in the USA, 2,374 receiving remdesivir treatment, showing no significant difference in mortality, and a longer time to hospital discharge with treatment.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments15.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 6.0% higher, HR 1.06, p = 0.66, treatment 143 of 1,172 (12.2%), control 124 of 1,172 (10.6%), adjusted per study, PSM, Cox proportional hazards regression, day 30.
|
|
hospitalization time, 100% higher, relative time 2.00, p < 0.001, treatment 1,172, control 1,172, PSM, Cox proportional hazards regression.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
13.
Mohammed et al., Bradycardia associated with remdesivir treatment in coronavirus disease 2019 patients: A propensity score-matched analysis, Medicine, doi:10.1097/MD.0000000000044501.
Ohl et al., 15 Jul 2021, retrospective, propensity score matching, USA, peer-reviewed, 9 authors.
Association of Remdesivir Treatment With Survival and Length of Hospital Stay Among US Veterans Hospitalized With COVID-19
JAMA Network Open, doi:10.1001/jamanetworkopen.2021.14741
IMPORTANCE Randomized clinical trials have yielded conflicting results about the effects of remdesivir therapy on survival and length of hospital stay among people with COVID-19. OBJECTIVE To examine associations between remdesivir treatment and survival and length of hospital stay among people hospitalized with COVID-19 in routine care settings. DESIGN, SETTING, AND PARTICIPANTS This retrospective cohort study used data from the Veterans Health Administration (VHA) to identify adult patients in 123 VHA hospitals who had a first hospitalization with laboratory-confirmed COVID-19 from May 1 to October 8, 2020. Propensity score matching of patients initiating remdesivir treatment to control patients who had not initiated remdesivir treatment by the same hospital day was used to create the analytic cohort. EXPOSURES Remdesivir treatment. MAIN OUTCOMES AND MEASURES Time to death within 30 days of remdesivir treatment initiation (or corresponding hospital day for matched control individuals) and time to hospital discharge with time to death as a competing event. Associations between remdesivir treatment and these outcomes were assessed using Cox proportional hazards regression in the matched cohort.
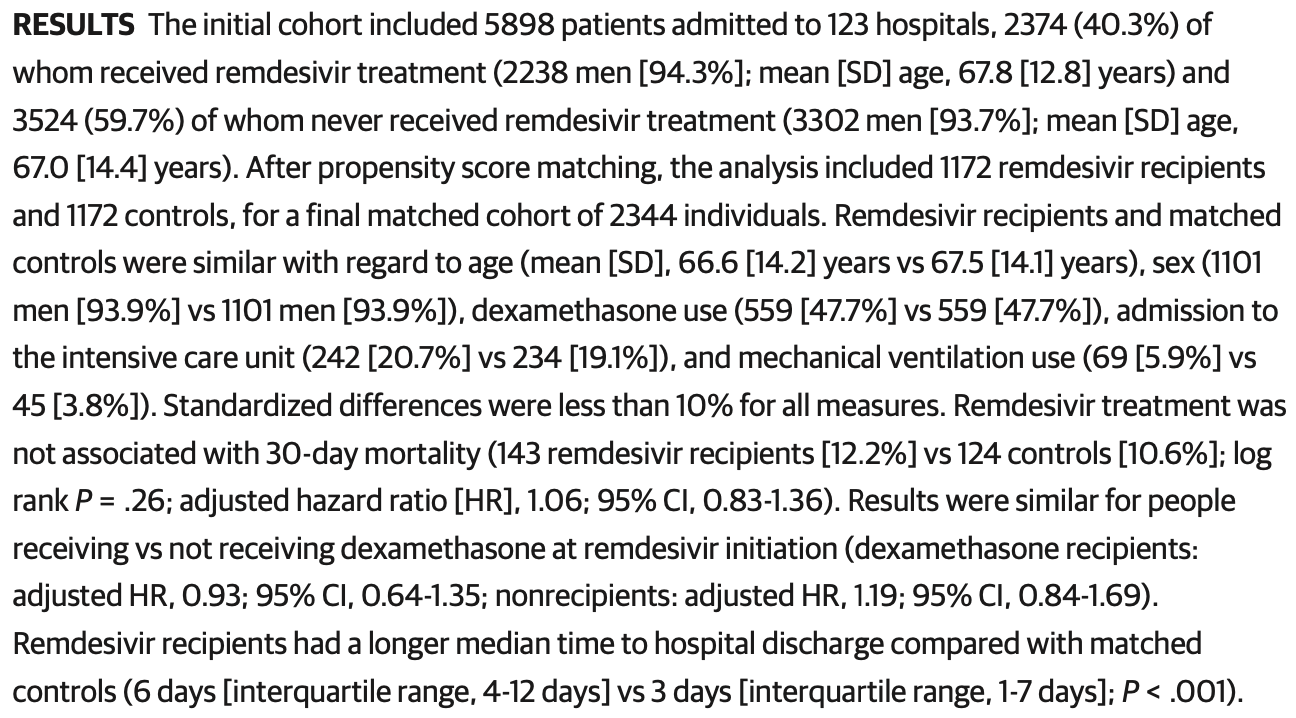
RESULTS The initial cohort included 5898 patients admitted to 123 hospitals, 2374 (40.3%) of whom received remdesivir treatment (2238 men [94.3%]; mean [SD] age, 67.8 [12.8] years) and 3524 (59.7%) of whom never received remdesivir treatment (3302 men [93.7%]; mean [SD] age, 67.0 [14.4] years). After propensity score matching, the analysis included 1172 remdesivir recipients and 1172 controls, for a final matched cohort of 2344 individuals. Remdesivir recipients and matched controls were similar with regard to age (mean [SD], 66.6 [14.2] years vs 67.5 [14.1] years), sex (1101 men [93.9%] vs 1101 men [93.9%]), dexamethasone use (559 [47.7%] vs 559 [47.7%]), admission to the intensive care unit (242 [20.7%] vs 234 [19.1%]), and mechanical ventilation use (69 [5.9%] vs 45 [3.8%]). Standardized differences were less than 10% for all measures. Remdesivir treatment was not associated with 30-day mortality (143 remdesivir recipients [12.2%] vs 124 controls [10.6%]; log rank P = .26; adjusted hazard ratio [HR], 1.06; 95% CI, 0.83-1.36). Results were similar for people receiving vs not receiving dexamethasone at remdesivir initiation (dexamethasone recipients:
be extrapolated to patients who do not resemble those in the propensity score-matched cohort. In addition, this study of US veterans included a small number of women, which affects the generalizability of the findings to the overall population. Third, limitations in available data prevented us from identifying specific subgroups of patients who may have been more likely to benefit from remdesivir treatment and from precisely emulating clinical trials. Subgroup analyses in the ACTT-1 suggested that remdesivir was most effective when patients required supplemental oxygen but had not yet progressed to require mechanical ventilation. 4 It is biologically plausible that remdesivir treatment is most beneficial during the early, viral replication phase of COVID-19, when antiviral drugs can still alter the course of illness before severe lung injury occurs. 3 Although we had data on oxygen saturation levels for patients during hospitalization and the matched remdesivir recipients and controls were balanced based on these values, we lacked data on the time from symptom onset to remdesivir initiation and the amount of supplemental oxygen patients required during hospitalization. We were therefore not able to examine variation in the outcomes associated with remdesivir according to phase of illness.
Conclusions In this cohort study of US veterans hospitalized with COVID-19, remdesivir treatment was not associated with survival but was associated with longer hospitalization. These..
References
Administrative, technical, or material support
Agostini, Andres, Sims, Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease, mBio, doi:10.1128/mBio.00221-18
Austin, Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples, Stat Med, doi:10.1002/sim.3697
Beigel, Tomashek, Dodd, ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19-final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Dolin, Hirsch, Remdesivir-an important first step, N Engl J Med, doi:10.1056/NEJMe2018715
Harrington, Baden, Hogan, A large, simple trial leading to complex questions, N Engl J Med, doi:10.1056/NEJMe2034294
Hernán, Brumback, Robins, Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men, Epidemiology, doi:10.1097/00001648-200009000-00012
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with COVID-19-preliminary report, N Engl J Med, doi:10.1056/NEJMoa2021436
Ohl, Kobayashi, Miell, Alexander, Sarrazin, Acquisition, analysis, or interpretation of data: All authors
Ohl, Md, Msph, None
Ohl, Sarrazin, the study and take responsibility for the integrity of the data and the accuracy of the data analysis
Pan, Peto, Henao-Restrepo, WHO Solidarity Trial Consortium. Repurposed antiviral drugs for Covid-19-interim WHO Solidarity Trial results, N Engl J Med, doi:10.1056/NEJMoa2023184
Quan, Sundararajan, Halfon, Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data, Med Care, doi:10.1097/01.mlr.0000182534.19832.83
Robins, Hernán, Brumback, Marginal structural models and causal inference in epidemiology, Epidemiology, doi:10.1097/00001648-200009000-00011
Rubin, Chan-Tack, Farley, Sherwat, FDA approval of remdesivir-a step in the right direction, N Engl J Med, doi:10.1056/NEJMp2032369
Spinner, Gottlieb, Criner, Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial, JAMA, doi:https://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2020.16349&utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamanetworkopen.2021.14741
Von Elm, Altman, Egger, Pocock, Gøtzsche et al., The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies, Lancet, doi:10.1016/S0140-6736(07)61602-X
Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebocontrolled, multicentre trial, Lancet, doi:10.1016/S0140-6736(20)31022-9
DOI record:
{
"DOI": "10.1001/jamanetworkopen.2021.14741",
"ISSN": [
"2574-3805"
],
"URL": "http://dx.doi.org/10.1001/jamanetworkopen.2021.14741",
"author": [
{
"affiliation": [
{
"name": "Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System, Iowa City"
},
{
"name": "Department of Internal Medicine, Carver College of Medicine, University of Iowa, Iowa City"
}
],
"family": "Ohl",
"given": "Michael E.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Center for Healthcare Organization & Implementation Research, VA Bedford Health Care System, Bedford, Massachusetts"
},
{
"name": "Center for Population Health, Department of Biomedical & Nutritional Sciences, University of Massachusetts, Lowell"
}
],
"family": "Miller",
"given": "Donald R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System, Iowa City"
}
],
"family": "Lund",
"given": "Brian C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System, Iowa City"
},
{
"name": "Department of Internal Medicine, Carver College of Medicine, University of Iowa, Iowa City"
}
],
"family": "Kobayashi",
"given": "Takaaki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System, Iowa City"
}
],
"family": "Richardson Miell",
"given": "Kelly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System, Iowa City"
}
],
"family": "Beck",
"given": "Brice F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System, Iowa City"
}
],
"family": "Alexander",
"given": "Bruce",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VA Puget Sound Health Care System, Seattle, Washington"
},
{
"name": "Department of Internal Medicine, University of Washington, Seattle"
}
],
"family": "Crothers",
"given": "Kristina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System, Iowa City"
},
{
"name": "Department of Internal Medicine, Carver College of Medicine, University of Iowa, Iowa City"
}
],
"family": "Vaughan Sarrazin",
"given": "Mary S.",
"sequence": "additional"
}
],
"container-title": "JAMA Network Open",
"container-title-short": "JAMA Netw Open",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
7,
15
]
],
"date-time": "2021-07-15T15:00:54Z",
"timestamp": 1626361254000
},
"deposited": {
"date-parts": [
[
2021,
7,
15
]
],
"date-time": "2021-07-15T15:01:03Z",
"timestamp": 1626361263000
},
"indexed": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T04:43:14Z",
"timestamp": 1711600994681
},
"is-referenced-by-count": 71,
"issue": "7",
"issued": {
"date-parts": [
[
2021,
7,
15
]
]
},
"journal-issue": {
"issue": "7",
"published-print": {
"date-parts": [
[
2021,
7,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamanetworkopen/articlepdf/2781959/ohl_2021_oi_210448_1625699934.47095.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "e2114741",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2021,
7,
15
]
]
},
"published-online": {
"date-parts": [
[
2021,
7,
15
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1128/mBio.00221-18",
"article-title": "Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease.",
"author": "Agostini",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "mBio",
"key": "zoi210448r1",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1056/NEJMe2018715",
"article-title": "Remdesivir—an important first step.",
"author": "Dolin",
"doi-asserted-by": "publisher",
"first-page": "1886",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "zoi210448r2",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMe2034294",
"article-title": "A large, simple trial leading to complex questions.",
"author": "Harrington",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med.",
"key": "zoi210448r3",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19—final report.",
"author": "Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "zoi210448r4",
"volume": "383",
"year": "2020"
},
{
"article-title": "Repurposed antiviral drugs for Covid-19—interim WHO Solidarity Trial results.",
"author": "Pan",
"journal-title": "N Engl J Med.",
"key": "zoi210448r5",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.16349",
"article-title": "Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial.",
"author": "Spinner",
"doi-asserted-by": "publisher",
"first-page": "1048",
"issue": "11",
"journal-title": "JAMA",
"key": "zoi210448r6",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial.",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "1569",
"issue": "10236",
"journal-title": "Lancet",
"key": "zoi210448r7",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056/NEJMp2032369",
"article-title": "FDA approval of remdesivir—a step in the right direction.",
"author": "Rubin",
"doi-asserted-by": "publisher",
"first-page": "2598",
"issue": "27",
"journal-title": "N Engl J Med",
"key": "zoi210448r9",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(07)61602-X",
"article-title": "The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies.",
"author": "von Elm",
"doi-asserted-by": "publisher",
"first-page": "1453",
"issue": "9596",
"journal-title": "Lancet",
"key": "zoi210448r15",
"volume": "370",
"year": "2007"
},
{
"DOI": "10.1097/01.mlr.0000182534.19832.83",
"article-title": "Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data.",
"author": "Quan",
"doi-asserted-by": "publisher",
"first-page": "1130",
"issue": "11",
"journal-title": "Med Care",
"key": "zoi210448r17",
"volume": "43",
"year": "2005"
},
{
"DOI": "10.1097/00001648-200009000-00012",
"article-title": "Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men.",
"author": "Hernán",
"doi-asserted-by": "publisher",
"first-page": "561",
"issue": "5",
"journal-title": "Epidemiology",
"key": "zoi210448r18",
"volume": "11",
"year": "2000"
},
{
"DOI": "10.1097/00001648-200009000-00011",
"article-title": "Marginal structural models and causal inference in epidemiology.",
"author": "Robins",
"doi-asserted-by": "publisher",
"first-page": "550",
"issue": "5",
"journal-title": "Epidemiology",
"key": "zoi210448r19",
"volume": "11",
"year": "2000"
},
{
"article-title": "Dexamethasone in hospitalized patients with COVID-19—preliminary report.",
"author": "Horby",
"journal-title": "N Engl J Med",
"key": "zoi210448r20",
"year": "2021"
},
{
"DOI": "10.1002/sim.3697",
"article-title": "Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples.",
"author": "Austin",
"doi-asserted-by": "publisher",
"first-page": "3083",
"issue": "25",
"journal-title": "Stat Med",
"key": "zoi210448r21",
"volume": "28",
"year": "2009"
},
{
"DOI": "10.1002/sim.v36.27",
"article-title": "Practical recommendations for reporting Fine-Gray model analyses for competing risk data.",
"author": "Austin",
"doi-asserted-by": "publisher",
"first-page": "4391",
"issue": "27",
"journal-title": "Stat Med",
"key": "zoi210448r22",
"volume": "36",
"year": "2017"
},
{
"author": "Anderson",
"key": "zoi210448r23"
},
{
"key": "zoi210448r8",
"unstructured": "United States Food and Drug Administration. Remdesivir emergency use authorization letter. Accessed May 30, 2020. https://www.fda.gov/media/137564/download"
},
{
"key": "zoi210448r10",
"unstructured": "Infectious Diseases Society of America. ISDA guidelines on the treatment and management of patients with COVID-19. Updated December 2, 2020. Accessed December 3, 2020. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#toc-8"
},
{
"key": "zoi210448r11",
"unstructured": "National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. Accessed December 3, 2020. https://www.covid19treatmentguidelines.nih.gov/"
},
{
"key": "zoi210448r12",
"unstructured": "World Health Organization. Therapeutics and COVID-19: living guideline. Updated November 20, 2020. Accessed December 3, 2020. https://www.who.int/publications/i/item/therapeutics-and-covid-19-living-guideline"
},
{
"key": "zoi210448r13",
"unstructured": "US Department of Veterans Affairs. Veterans Health Administration. Accessed December 6, 2020. https://www.va.gov/health/"
},
{
"key": "zoi210448r14",
"unstructured": "VA Pharmacy Benefits Management Services. Remdesivir emergency use authorization (EUA) requirements May 2020. Accessed June 18, 2020. https://www.va.gov/covidtraining/docs/20200618_Dynamic_Drugs_in_the_Battle_of_COVID_19/Remdesivir_Emergency_Use_Authorization_Requirements.pdf"
},
{
"key": "zoi210448r16",
"unstructured": "VA Informatics and Computing Infrastructure. VA COVID-19 shared data resource: update. US Department of Veterans Affairs. Accessed May 29, 2021. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3834-notes.pdf"
},
{
"key": "zoi210448r24",
"unstructured": "Griffin? D, Racaniello? V. COVID-19 clinical update #41 with Dr Daniel Griffin. This Week in Virology. December 18, 2020. Accessed December 26, 2020. https://www.microbe.tv/twiv/twiv-695/"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2781959"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Association of Remdesivir Treatment With Survival and Length of Hospital Stay Among US Veterans Hospitalized With COVID-19",
"type": "journal-article",
"volume": "4"
}