The effect of curcumin on the risk of mortality in patients with COVID-19: A systematic review and meta-analysis of randomized trials
et al., Phytotherapy Research, doi:10.1002/ptr.7468, Apr 2022
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
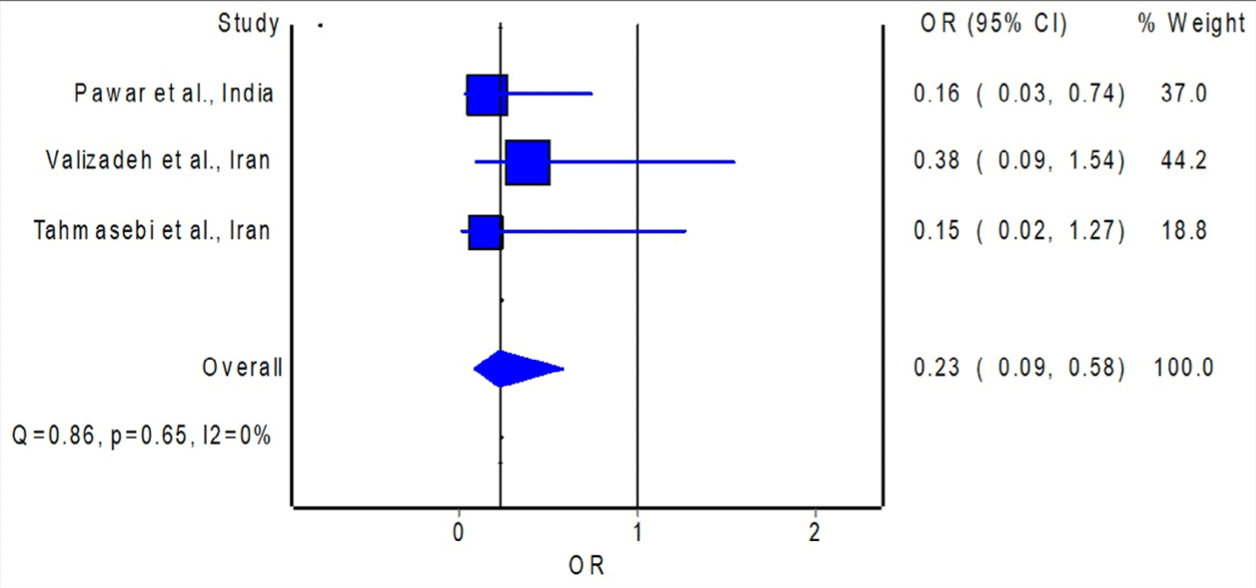
Meta analysis of 3 curcumin RCTs showing lower mortality with treatment. Author notes the small sample sizes of the included trials.
6 meta-analyses show significant improvements with curcumin for mortality1-6,
mechanical ventilation6,
hospitalization1,4,
recovery3,6,
progression6, and
symptoms1.
Currently there are 28 curcumin for COVID-19 studies, showing 63% lower mortality [36‑78%], 80% lower ventilation [25‑95%], 78% lower ICU admission [-27‑96%], and 27% lower hospitalization [18‑34%].
|
risk of death, 77.0% lower, OR 0.23, p = 0.002, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Vahedian-Azimi et al., Effectiveness of Curcumin on Outcomes of Hospitalized COVID-19 Patients: A Systematic Review of Clinical Trials, Nutrients, doi:10.3390/nu14020256.
2.
Kow et al., The effect of curcumin on the risk of mortality in patients with COVID-19: A systematic review and meta-analysis of randomized trials, Phytotherapy Research, doi:10.1002/ptr.7468.
3.
Shafiee et al., Curcumin for the treatment of COVID-19 patients: A meta-analysis of randomized controlled trials, Phytotherapy Research, doi:10.1002/ptr.7724.
4.
Shojaei et al., The effectiveness of nano‐curcumin on patients with COVID‐19: A systematic review of clinical trials, Phytotherapy Research, doi:10.1002/ptr.7778.
Kow et al., 28 Apr 2022, peer-reviewed, 3 authors.
Contact: chiasiang_93@hotmail.com.
The effect of curcumin on the risk of mortality in patients with COVID ‐19: A systematic review and meta‐analysis of randomized trials
Phytotherapy Research, doi:10.1002/ptr.7468
The coronavirus disease 2019 (COVID-19) pandemic is far from bygone, with the emergence of newer variations of concern of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). While the worldwide vaccine rollout has progressed at a breakneck pace, the hunt for new safe, effective, and targeted treatments should continue in parallel due to the frequent occurrence of breakthrough cases. Curcumin is one such natural polyphenolic compound with multiple benefits, including antiviral, anti-inflammatory, anticoagulant, antiplatelet,
CONFLICT OF INTEREST The authors declare no conflict of interest.
References
Dourado, Freire, Pereira, Amaral-Machado, Alencar et al., Will curcumin nanosystems be the next promising antiviral alternatives in COVID-19 treatment trials?, Biomedicine & Pharmacotherapy
Han, The effects of black pepper on the intestinal absorption and hepatic metabolism of drugs, Expert Opinion on Drug Metabolism & Toxicology
Hassaniazad, Eftekhar, Inchehsablagh, Kamali, Tousi et al., A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients, Phytotherapy Research
Manoharan, Haridas, Vasanthakumar, Muthu, Thavoorullah et al., Curcumin: A wonder drug as a preventive measure for COVID19 management, Indian Journal of Clinical Biochemistry
Pawar, Mastud, Pawar, Pawar, Bhoite et al., Oral curcumin with Piperine as adjuvant therapy for the treatment of COVID-19: A randomized clinical trial, Frontiers in Pharmacology
Rattis, Ramos, Celes, Curcumin as a potential treatment for COVID-19, Frontiers in Pharmacology
Soni, Mehta, Ratre, Tiwari, Amit et al., Curcumin, a traditional spice component, can hold the promise against COVID-19?, European Journal of Pharmacology
Sterne, Savovi C, Page, Elbers, Blencowe et al., RoB 2: A revised tool for assessing risk of bias in randomised trials, BMJ (Clinical Research Ed)
Tahmasebi, El-Esawi, Mahmoud, Timoshin, Valizadeh et al., Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID-19 patients, Journal of Cellular Physiology
Valizadeh, Abdolmohammadi-Vahid, Danshina, Ziya Gencer, Ammari et al., Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients, International Immunopharmacology
Yin, Guo, Li, Tang, Li et al., Curcumin suppresses IL-1β secretion and prevents inflammation through inhibition of the NLRP3 inflammasome, Journal of Immunology
Zahedipour, Hosseini, Sathyapalan, Majeed, Jamialahmadi et al., Potential effects of curcumin in the treatment of COVID-19 infection, Phytotherapy Research
DOI record:
{
"DOI": "10.1002/ptr.7468",
"ISSN": [
"0951-418X",
"1099-1573"
],
"URL": "http://dx.doi.org/10.1002/ptr.7468",
"alternative-id": [
"10.1002/ptr.7468"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-02-04"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-03-30"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-04-28"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8186-2926",
"affiliation": [
{
"name": "School of Pharmacy International Medical University Kuala Lumpur Malaysia"
},
{
"name": "School of Pharmacy Monash University Malaysia Bandar Sunway Selangor Malaysia"
}
],
"authenticated-orcid": false,
"family": "Kow",
"given": "Chia Siang",
"sequence": "first"
},
{
"affiliation": [
{
"name": "School of Pharmacy Monash University Malaysia Bandar Sunway Selangor Malaysia"
}
],
"family": "Ramachandram",
"given": "Dinesh Sangarran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Applied Sciences University of Huddersfield Huddersfield UK"
},
{
"name": "School of Biomedical Sciences and Pharmacy University of Newcastle Callaghan Australia"
}
],
"family": "Hasan",
"given": "Syed Shahzad",
"sequence": "additional"
}
],
"container-title": "Phytotherapy Research",
"container-title-short": "Phytotherapy Research",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
4,
29
]
],
"date-time": "2022-04-29T04:53:57Z",
"timestamp": 1651208037000
},
"deposited": {
"date-parts": [
[
2022,
4,
29
]
],
"date-time": "2022-04-29T04:54:01Z",
"timestamp": 1651208041000
},
"indexed": {
"date-parts": [
[
2022,
4,
29
]
],
"date-time": "2022-04-29T05:15:14Z",
"timestamp": 1651209314580
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
4,
28
]
]
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
28
]
],
"date-time": "2022-04-28T00:00:00Z",
"timestamp": 1651104000000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
28
]
],
"date-time": "2022-04-28T00:00:00Z",
"timestamp": 1651104000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7468",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/ptr.7468",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7468",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2022,
4,
28
]
]
},
"published-online": {
"date-parts": [
[
2022,
4,
28
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/j.biopha.2021.111578",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_2_1"
},
{
"DOI": "10.1517/17425255.2011.570332",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_3_1"
},
{
"DOI": "10.1002/ptr.7294",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_4_1"
},
{
"DOI": "10.1007/s12291-020-00902-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_5_1"
},
{
"DOI": "10.3389/fphar.2021.669362",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_6_1"
},
{
"DOI": "10.3389/fphar.2021.675287",
"article-title": "Curcumin as a potential treatment for COVID‐19",
"author": "Rattis B.",
"doi-asserted-by": "crossref",
"first-page": "675287",
"journal-title": "Frontiers in Pharmacology",
"key": "e_1_2_1_4_7_1",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.ejphar.2020.173551",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_8_1"
},
{
"DOI": "10.1136/bmj.l4898",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_9_1"
},
{
"DOI": "10.1002/jcp.30233",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_10_1"
},
{
"DOI": "10.1016/j.intimp.2020.107088",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_11_1"
},
{
"DOI": "10.4049/jimmunol.1701495",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_12_1"
},
{
"DOI": "10.1002/ptr.6738",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_13_1"
}
],
"reference-count": 12,
"references-count": 12,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/ptr.7468"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology"
],
"subtitle": [],
"title": "The effect of curcumin on the risk of mortality in patients with\n <scp>COVID</scp>\n ‐19: A systematic review and meta‐analysis of randomized trials",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}
