Efficacy of highly bioavailable oral curcumin in asymptomatic or mild COVID-19 patients: a double-blind, randomized, placebo-controlled trial
et al., Journal of Health, Population and Nutrition, doi:10.1186/s41043-024-00584-6, jRCTs051210176, Jun 2024
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 138 COVID-19 outpatients in Japan showing lower progression to fever and hypoxemia with curcuRouge, a highly bioavailable oral curcumin formulation, compared to placebo. The curcuRouge group also had a greater reduction in body temperature and took fewer antipyretic medications. The event rate was lower than expected and the difference in progression was not statistically significant.
This is the 21st COVID-19 RCT for curcumin, which collectively show efficacy with p=0.0000022.
This is the 27th of 28 COVID-19 controlled studies for curcumin, which collectively show efficacy with p=0.0000000061.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
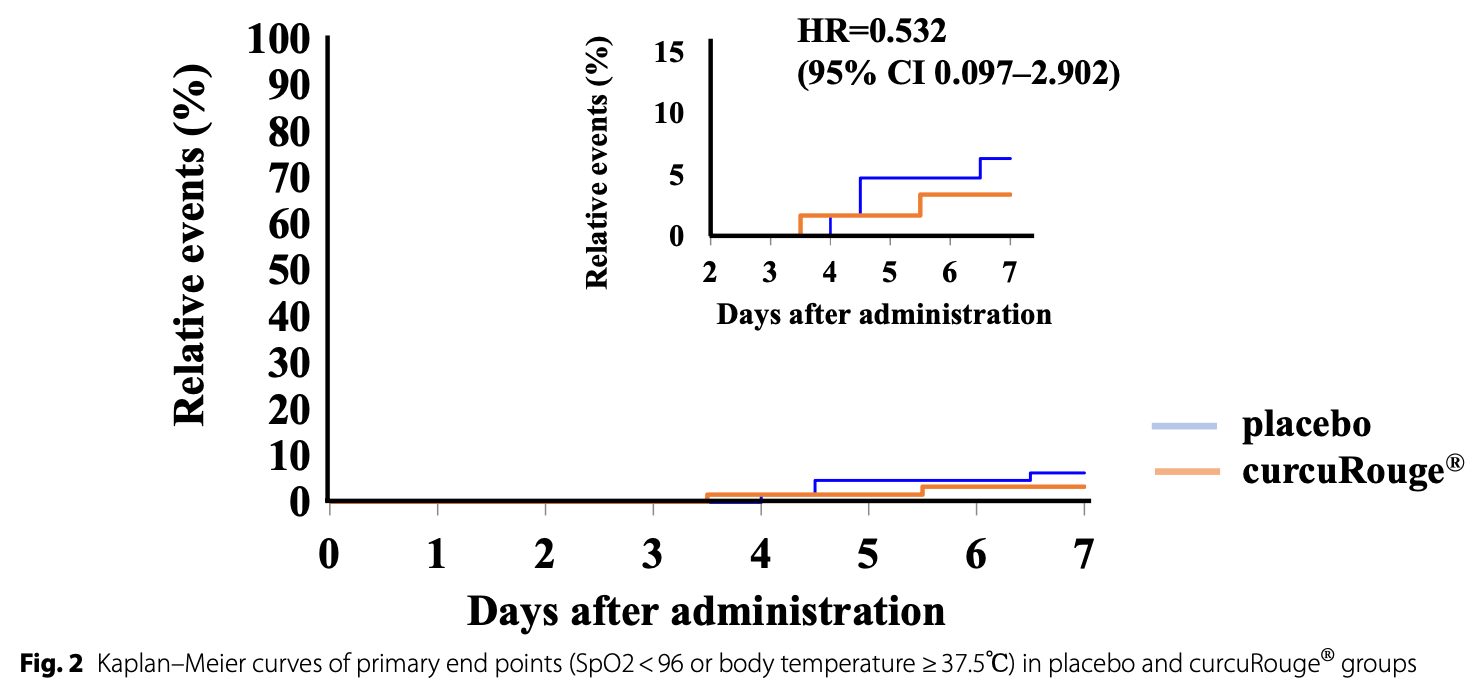
SpO2<96 or temperature≥37.5, 46.8% lower, HR 0.53, p = 0.48, treatment 2 of 71 (2.8%), control 4 of 67 (6.0%), NNT 32, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kishimoto et al., 24 Jun 2024, Double Blind Randomized Controlled Trial, placebo-controlled, Japan, peer-reviewed, 15 authors, study period February 2022 - January 2023, trial jRCTs051210176.
Contact: koj@kuhp.kyoto-u.ac.jp.
Efficacy of highly bioavailable oral curcumin in asymptomatic or mild COVID-19 patients: a double-blind, randomized, placebo-controlled trial
Journal of Health, Population and Nutrition, doi:10.1186/s41043-024-00584-6
Introduction Even after the peak of the COVID-19 pandemic, the number of mild cases remains high, requiring continuous control. Curcumin, owing to its anti-inflammatory properties, can suppress vital proliferation and cytokine secretion in animal models. We developed a highly absorbable curcumin, curcuRouge ® (cR), which is approximately 100 times more orally bioavailable than conventional curcumin. We evaluated the effect of cR on the inhibition of disease progression in asymptomatic or mildly symptomatic COVID-19 patients.
Methods This study evaluated the effect of 7-day oral intake of cR (360 mg twice daily). Patients within 5 days of COVID-19 diagnosis were randomly assigned to a placebo or cR group in a double-blind manner.
Results Primary endpoint events [body temperature (BT) ≥ 37.5 °C and saturation of percutaneous oxygen (SpO2) < 96%] were fewer than expected, and the rate of these events was 2.8% in the cR group (2/71) and 6.0% in the placebo group (4/67); hazard ratio (HR) = 0.532, 95% confidence interval (CI) 0.097-2.902. Patients receiving cR tended to take fewer antipyretic medications than those receiving placebo (HR = 0.716, 95% CI 0.374-1.372). Among patients with a normal range of BT at baseline, the BT change rate was significantly (p = 0.014) lower in the cR group (-0.34%) versus placebo (-0.01%).
Conclusion The relative suppression of event rates and antipyretic medications taken, and significant decrease of subclinical BT support the anti-inflammatory effects of cR in asymptomatic or mildly symptomatic patients with COVID-19. Trial registration: Japan Registry of Clinical Trials (CRB5200002).
Abbreviations
COVID-19 Coronavirus disease-2019 SARS-CoV-
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s41043-024-00584-6. Additional file 1: Table S1 . Change of parameters from Day-1 to Day-7 in curcuRouge ® and placebo groups. Additional file 2: Table S2 . Change of parameters from Day-1 to Day-7 in curcuRouge ® and placebo groups of patients who did not take antipyretics.
Author contributions This manuscript has been written by AK and KH. Research conception was designed by AK and KH. The data have been gathered by HA, YA and AI. The data have been analyzed by MKomiyama, HW and HY. The final manuscript has been reviewed by NSA, YK, TH, YS, TM, MKanai and HK.
Declarations Ethics approval and consent to participate The trial was approved by the Nara Medical University Ethics Committee and registered with the Japan Registry of Clinical Trials (CRB5200002).
Consent for publication Not applicable as no personal data were used in this article.
Competing interests This study was funded by a joint research agreement between NHO Kyoto Medical Center and Therabiopharma Inc. M. Kanai and H. Kakeya own equity and they are the scientific consultants of Therabiopharma Inc.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Abe, Horisawa, Kikuchi, Ozawa-Umeta, Kishimoto et al., Pharmacologic characterization of TBP1901, a prodrug form of aglycone curcumin, and CRISPR-Cas9 screen for therapeutic targets of aglycone curcumin, Eur J Pharmacol
Ahmadi, Mehrabi, Zare, Ghadir, Masoumi, Efficacy of nanocurcumin as an add-on treatment for patients hospitalized with COVID-19: a double-blind, randomized clinical trial, Int J Clin Pract
Aktas, Hematological predictors of novel Coronavirus infection, Rev Assoc Méd Bras
Bilgin, Kurtkulagi, Kahveci, Duman, Tel, Millennium pandemic: a review of coronavirus disease (COVID-19), Exp Biomed Res
De Matos, Da Silva, Mansano, Gancedo, Tonin et al., Bioactive compounds as potential angiotensin-converting enzyme II inhibitors against COVID-19: a scoping review, Inflamm Res
Demirkol, Bilgin, Kahveci, Kurtkulagi, Tel et al., C-reactive protein-to-lymphocyte ratio is a reliable marker in patients with COVID-19 infection: the CLEAR COVID study, Cir Cir
Hassaniazad, Eftekhar, Inchehsablagh, Kamali, Tousi et al., A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients, Phytother Res
Ijcpt, Comparative pharmacokinetics of Theracurmin, a highly bioavailable curcumin, in healthy adult subjects, Int J Clin Pharmacol Ther
Ijcpt, Comparative pharmacokinetics of new curcumin preparations and evidence for increased bioavailability in healthy adult participants, Int J Clin Pharmacol Ther
Kishimoto, Imaizumi, Wada, Yamakage, Satoh-Asahara et al., Newly developed highly bioavailable curcumin formulation, curcuRougeTM, reduces neutrophil/lymphocyte ratio in the elderly: a double-blind, placebo-controlled clinical trial, J Nutr Sci Vitaminol
Kozlov, Ivanova, Grechko, Wu, Starodubova et al., Involvement of oxidative stress and the innate immune system in SARS-CoV-2 infection, Diseases
Nakagawa, Yamada, Mukai, Akiyoshi, Katsuura et al., Efficacy of high-and low-dose, highly bioavailable curcumin (CurcuRouge ® ) for treating knee osteoarthritis: a randomized, double-blind. Placebo-Control Prospect Study, Clin Med Insights
Pawar, Mastud, Pawar, Pawar, Bhoite et al., Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: a randomized clinical trial, Front Pharmacol
Richart, Li, Mizushina, Chang, Chung et al., Synergic effect of curcumin and its structural analogue (Monoacetylcurcumin) on anti-influenza virus infection, J Food Drug Anal
Roche, Media, Release, Phase III prevention trial showed subcutaneous administration of investigational antibody cocktail casirivimab and imdevimab reduced risk of symptomatic COVID-19 infections by 81%
Sunagawa, Miyazaki, Funamoto, Shimizu, Shimizu et al., A novel amorphous preparation improved curcumin bioavailability in healthy volunteers: a single-dose, double-blind, two-way crossover study, J Funct Foods
Tan, Knight, Regulation of body temperature by the nervous system, Neuron
Umadevi, Manivannan, Fayad, Shelvy, In silico analysis of phytochemicals as potential inhibitors of proteases involved in SARS-CoV-2 infection, J Biomol Struct Dyn
Wen, Kuo, Jan, Liang, Wang et al., Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome corona virus, J Med Chem
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the Chinese Center for disease control and prevention, JAMA
Xu, Liu, Curcumin alleviates macrophage activation and lung inflammation induced by influenza virus infection through inhibiting the NF-κB signaling pathway, Influenza Other Respir Viruses
DOI record:
{
"DOI": "10.1186/s41043-024-00584-6",
"ISSN": [
"2072-1315"
],
"URL": "http://dx.doi.org/10.1186/s41043-024-00584-6",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Introduction</jats:title>\n <jats:p>Even after the peak of the COVID-19 pandemic, the number of mild cases remains high, requiring continuous control. Curcumin, owing to its anti-inflammatory properties, can suppress vital proliferation and cytokine secretion in animal models. We developed a highly absorbable curcumin, curcuRouge<jats:sup>®</jats:sup> (cR), which is approximately 100 times more orally bioavailable than conventional curcumin. We evaluated the effect of cR on the inhibition of disease progression in asymptomatic or mildly symptomatic COVID-19 patients.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This study evaluated the effect of 7-day oral intake of cR (360 mg twice daily). Patients within 5 days of COVID-19 diagnosis were randomly assigned to a placebo or cR group in a double-blind manner.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Primary endpoint events [body temperature (BT) ≥ 37.5 °C and saturation of percutaneous oxygen (SpO2) < 96%] were fewer than expected, and the rate of these events was 2.8% in the cR group (2/71) and 6.0% in the placebo group (4/67); hazard ratio (HR) = 0.532, 95% confidence interval (CI) 0.097–2.902. Patients receiving cR tended to take fewer antipyretic medications than those receiving placebo (HR = 0.716, 95% CI 0.374–1.372). Among patients with a normal range of BT at baseline, the BT change rate was significantly (<jats:italic>p</jats:italic> = 0.014) lower in the cR group (− 0.34%) versus placebo (− 0.01%).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>The relative suppression of event rates and antipyretic medications taken, and significant decrease of subclinical BT support the anti-inflammatory effects of cR in asymptomatic or mildly symptomatic patients with COVID-19.</jats:p>\n <jats:p><jats:italic>Trial registration</jats:italic>: Japan Registry of Clinical Trials (CRB5200002).</jats:p>\n </jats:sec>",
"alternative-id": [
"584"
],
"article-number": "93",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "25 April 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "9 June 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "24 June 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The trial was approved by the Nara Medical University Ethics Committee and registered with the Japan Registry of Clinical Trials (CRB5200002)."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable as no personal data were used in this article."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "This study was funded by a joint research agreement between NHO Kyoto Medical Center and Therabiopharma Inc. M. Kanai and H. Kakeya own equity and they are the scientific consultants of Therabiopharma Inc."
}
],
"author": [
{
"affiliation": [],
"family": "Kishimoto",
"given": "Atsuhiro",
"sequence": "first"
},
{
"affiliation": [],
"family": "Komiyama",
"given": "Maki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wada",
"given": "Hiromichi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Satoh-Asahara",
"given": "Noriko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yamakage",
"given": "Hajime",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ajiro",
"given": "Yoichi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aoyama",
"given": "Hiroki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Katsuura",
"given": "Yasuhiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Imaizumi",
"given": "Atsushi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hashimoto",
"given": "Tadashi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sunagawa",
"given": "Yoichi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morimoto",
"given": "Tatsuya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kanai",
"given": "Masashi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kakeya",
"given": "Hideaki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hasegawa",
"given": "Koji",
"sequence": "additional"
}
],
"container-title": "Journal of Health, Population and Nutrition",
"container-title-short": "J Health Popul Nutr",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
6,
24
]
],
"date-time": "2024-06-24T15:01:42Z",
"timestamp": 1719241302000
},
"deposited": {
"date-parts": [
[
2024,
6,
24
]
],
"date-time": "2024-06-24T15:05:38Z",
"timestamp": 1719241538000
},
"indexed": {
"date-parts": [
[
2024,
6,
25
]
],
"date-time": "2024-06-25T00:30:08Z",
"timestamp": 1719275408875
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
6,
24
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
6,
24
]
],
"date-time": "2024-06-24T00:00:00Z",
"timestamp": 1719187200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
6,
24
]
],
"date-time": "2024-06-24T00:00:00Z",
"timestamp": 1719187200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s41043-024-00584-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s41043-024-00584-6/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s41043-024-00584-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
6,
24
]
]
},
"published-online": {
"date-parts": [
[
2024,
6,
24
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1001/jama.2020.2648",
"author": "Z Wu",
"doi-asserted-by": "publisher",
"first-page": "1239",
"issue": "13",
"journal-title": "JAMA",
"key": "584_CR1",
"unstructured": "Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the Chinese Center for disease control and prevention. JAMA. 2020;323(13):1239–42.",
"volume": "323",
"year": "2020"
},
{
"key": "584_CR2",
"unstructured": "Ministry of Health, Labor and Welfare (MHLW). New Coronavirus Infections COVID19 Guide to Clinical Practice, Version 10.0, Japan. 2023."
},
{
"DOI": "10.1016/j.ejphar.2022.175321",
"author": "T Abe",
"doi-asserted-by": "publisher",
"first-page": "17532",
"journal-title": "Eur J Pharmacol",
"key": "584_CR3",
"unstructured": "Abe T, Horisawa Y, Kikuchi O, Ozawa-Umeta H, Kishimoto A, Katsuura Y, Imaizumi A, Hashimoto T, Shirakawa K, Takaori-Kondo A, Yusa K, Asakura T, Kakeya H, Kanai M. Pharmacologic characterization of TBP1901, a prodrug form of aglycone curcumin, and CRISPR-Cas9 screen for therapeutic targets of aglycone curcumin. Eur J Pharmacol. 2022;935:17532.",
"volume": "935",
"year": "2022"
},
{
"DOI": "10.1111/irv.12459",
"author": "Y Xu",
"doi-asserted-by": "publisher",
"first-page": "457",
"issue": "5",
"journal-title": "Influenza Other Respir Viruses",
"key": "584_CR4",
"unstructured": "Xu Y, Liu L. Curcumin alleviates macrophage activation and lung inflammation induced by influenza virus infection through inhibiting the NF-κB signaling pathway. Influenza Other Respir Viruses. 2017;11(5):457–63.",
"volume": "11",
"year": "2017"
},
{
"DOI": "10.1021/jm070295s",
"author": "CC Wen",
"doi-asserted-by": "publisher",
"first-page": "4087",
"issue": "17",
"journal-title": "J Med Chem",
"key": "584_CR5",
"unstructured": "Wen CC, Kuo YH, Jan JT, Liang PH, Wang SY, Liu HG, Lee CK, Chang ST, Kuo CJ, Lee SS, Hou CC, Hsiao PW, Chien SH, Shyur LF, Yang NS. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome corona virus. J Med Chem. 2007;50(17):4087–95.",
"volume": "50",
"year": "2007"
},
{
"DOI": "10.1080/07391102.2020.1866669",
"author": "P Umadevi",
"doi-asserted-by": "publisher",
"first-page": "5053",
"issue": "11",
"journal-title": "J Biomol Struct Dyn",
"key": "584_CR6",
"unstructured": "Umadevi P, Manivannan S, Fayad AM, Shelvy S. In silico analysis of phytochemicals as potential inhibitors of proteases involved in SARS-CoV-2 infection. J Biomol Struct Dyn. 2020;40(11):5053–9.",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1016/j.jff.2021.104443",
"author": "Y Sunagawa",
"doi-asserted-by": "publisher",
"first-page": "104443",
"journal-title": "J Funct Foods",
"key": "584_CR7",
"unstructured": "Sunagawa Y, Miyazaki Y, Funamoto M, Shimizu K, Shimizu S, Nurmila S, Katanasaka Y, Ito M, Ogawa T, Ozawa-Umeta H, Hasegawa K, Morimoto T. A novel amorphous preparation improved curcumin bioavailability in healthy volunteers: a single-dose, double- blind, two-way crossover study. J Funct Foods. 2021;81:104443.",
"volume": "81",
"year": "2021"
},
{
"DOI": "10.5414/CP204058",
"doi-asserted-by": "crossref",
"key": "584_CR8",
"unstructured": "IJCPT. Comparative pharmacokinetics of Theracurmin, a highly bioavailable curcumin, in healthy adult subjects. Int J Clin Pharmacol Ther. 2021;59(10):684–90."
},
{
"DOI": "10.5414/CP204257",
"doi-asserted-by": "crossref",
"key": "584_CR9",
"unstructured": "IJCPT. Comparative pharmacokinetics of new curcumin preparations and evidence for increased bioavailability in healthy adult participants. Int J Clin Pharmacol Ther. 2022;60(12):530–8."
},
{
"DOI": "10.3177/jnsv.67.249",
"author": "A Kishimoto",
"doi-asserted-by": "publisher",
"first-page": "249",
"issue": "4",
"journal-title": "J Nutr Sci Vitaminol (Tokyo)",
"key": "584_CR10",
"unstructured": "Kishimoto A, Imaizumi A, Wada H, Yamakage H, Satoh-Asahara N, Hashimoto T, Hasegawa K. Newly developed highly bioavailable curcumin formulation, curcuRougeTM, reduces neutrophil/lymphocyte ratio in the elderly: a double-blind, placebo-controlled clinical trial. J Nutr Sci Vitaminol (Tokyo). 2021;67(4):249–52.",
"volume": "67",
"year": "2021"
},
{
"DOI": "10.30714/j-ebr.2020259176",
"author": "S Bilgin",
"doi-asserted-by": "publisher",
"first-page": "117",
"issue": "2",
"journal-title": "Exp Biomed Res",
"key": "584_CR11",
"unstructured": "Bilgin S, Kurtkulagi O, Kahveci GB, Duman TT, Atak Tel BM. Millennium pandemic: a review of coronavirus disease (COVID-19). Exp Biomed Res. 2020;3(2):117–25.",
"volume": "3",
"year": "2020"
},
{
"author": "ME Demirkol",
"first-page": "596",
"issue": "5",
"journal-title": "Cir Cir",
"key": "584_CR12",
"unstructured": "Demirkol ME, Bilgin S, Kahveci G, Kurtkulagi O, Atak Tel BM, Duman TT, Aktas G. C-reactive protein-to-lymphocyte ratio is a reliable marker in patients with COVID-19 infection: the CLEAR COVID study. Cir Cir. 2022;90(5):596–601.",
"volume": "90",
"year": "2022"
},
{
"DOI": "10.1590/1806-9282.67.01.001",
"author": "G Aktas",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "Rev Assoc Méd Bras",
"key": "584_CR13",
"unstructured": "Aktas G. Hematological predictors of novel Coronavirus infection. Rev Assoc Méd Bras. 2021;67(1):1–2.",
"volume": "67",
"year": "2021"
},
{
"author": "Y Nakagawa",
"first-page": "415",
"issue": "03",
"journal-title": "Placebo-Control Prospect Study Clin Med Insights",
"key": "584_CR14",
"unstructured": "Nakagawa Y, Yamada S, Mukai S, Akiyoshi K, Katsuura Y, Hashimoto T. Efficacy of high- and low-dose, highly bioavailable curcumin (CurcuRouge®) for treating knee osteoarthritis: a randomized, double-blind. Placebo-Control Prospect Study Clin Med Insights. 2023;04(03):415–29.",
"volume": "04",
"year": "2023"
},
{
"DOI": "10.1002/ptr.7294",
"author": "M Hassaniazad",
"doi-asserted-by": "publisher",
"first-page": "6417",
"issue": "11",
"journal-title": "Phytother Res",
"key": "584_CR15",
"unstructured": "Hassaniazad M, Eftekhar E, Inchehsablagh BR, Kamali H, Tousi A, Jaafari MR, Rafat M, Fathalipour M, Nikoofal-Sahlabadi S, Gouklani H, Alizade H, Nikpoor AM. A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients. Phytother Res. 2021;35(11):6417–27.",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.669362",
"author": "KS Pawar",
"doi-asserted-by": "publisher",
"first-page": "669362",
"journal-title": "Front Pharmacol",
"key": "584_CR16",
"unstructured": "Pawar KS, Mastud RN, Pawar SK, Pawar SS, Bhoite RR, Bhoite RR, Kulkarni MV, Deshpande AR. Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: a randomized clinical trial. Front Pharmacol. 2021;12:669362.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1155/2023/5734675",
"author": "S Ahmadi",
"doi-asserted-by": "publisher",
"first-page": "5734675",
"journal-title": "Int J Clin Pract",
"key": "584_CR17",
"unstructured": "Ahmadi S, Mehrabi Z, Zare M, Ghadir S, Masoumi SJ. Efficacy of nanocurcumin as an add-on treatment for patients hospitalized with COVID-19: a double-blind, randomized clinical trial. Int J Clin Pract. 2023;2023:5734675.",
"volume": "2023",
"year": "2023"
},
{
"key": "584_CR18",
"unstructured": "Roche. Media & Investor Release. Phase III prevention trial showed subcutaneous administration of investigational antibody cocktail casirivimab and imdevimab reduced risk of symptomatic COVID-19 infections by 81%. 2021."
},
{
"DOI": "10.1016/j.neuron.2018.02.022",
"author": "CL Tan",
"doi-asserted-by": "publisher",
"first-page": "31",
"issue": "1",
"journal-title": "Neuron",
"key": "584_CR19",
"unstructured": "Tan CL, Knight ZA. Regulation of body temperature by the nervous system. Neuron. 2018;98(1):31–48.",
"volume": "98",
"year": "2018"
},
{
"DOI": "10.3390/diseases9010017",
"author": "EM Kozlov",
"doi-asserted-by": "publisher",
"first-page": "17",
"issue": "1",
"journal-title": "Diseases",
"key": "584_CR20",
"unstructured": "Kozlov EM, Ivanova E, Grechko AV, Wu WK, Starodubova AV, Alexander N. Involvement of oxidative stress and the innate immune system in SARS-CoV-2 infection. Diseases. 2021;9(1):17.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.jfda.2017.12.006",
"author": "SM Richart",
"doi-asserted-by": "publisher",
"first-page": "1015",
"issue": "3",
"journal-title": "J Food Drug Anal",
"key": "584_CR21",
"unstructured": "Richart SM, Li YL, Mizushina Y, Chang YY, Chung TY, Chen GH, Tzen JTC, Shia KS, Hsu WL. Synergic effect of curcumin and its structural analogue (Monoacetylcurcumin) on anti-influenza virus infection. J Food Drug Anal. 2018;26(3):1015–23.",
"volume": "26",
"year": "2018"
},
{
"DOI": "10.1007/s00011-022-01642-7",
"doi-asserted-by": "crossref",
"key": "584_CR22",
"unstructured": "de Matos PH, da Silva TP, Mansano AB, Gancedo NC, Tonin FS, Pelloso FC, Petruco MV, de Melo EB, Fernandez-Llimos F, Sanches ACC, de Mello JCP, Chierrito D, de DCM Araújo. Bioactive compounds as potential angiotensin-converting enzyme II inhibitors against COVID-19: a scoping review. Inflamm Res. 2022;71(12):1489–500."
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://jhpn.biomedcentral.com/articles/10.1186/s41043-024-00584-6"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy of highly bioavailable oral curcumin in asymptomatic or mild COVID-19 patients: a double-blind, randomized, placebo-controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "43"
}
