The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19—Results from a pragmatic randomized clinical trial
et al., Frontiers in Nutrition, doi:10.3389/fnut.2022.1023997, NCT04603690, Jan 2023
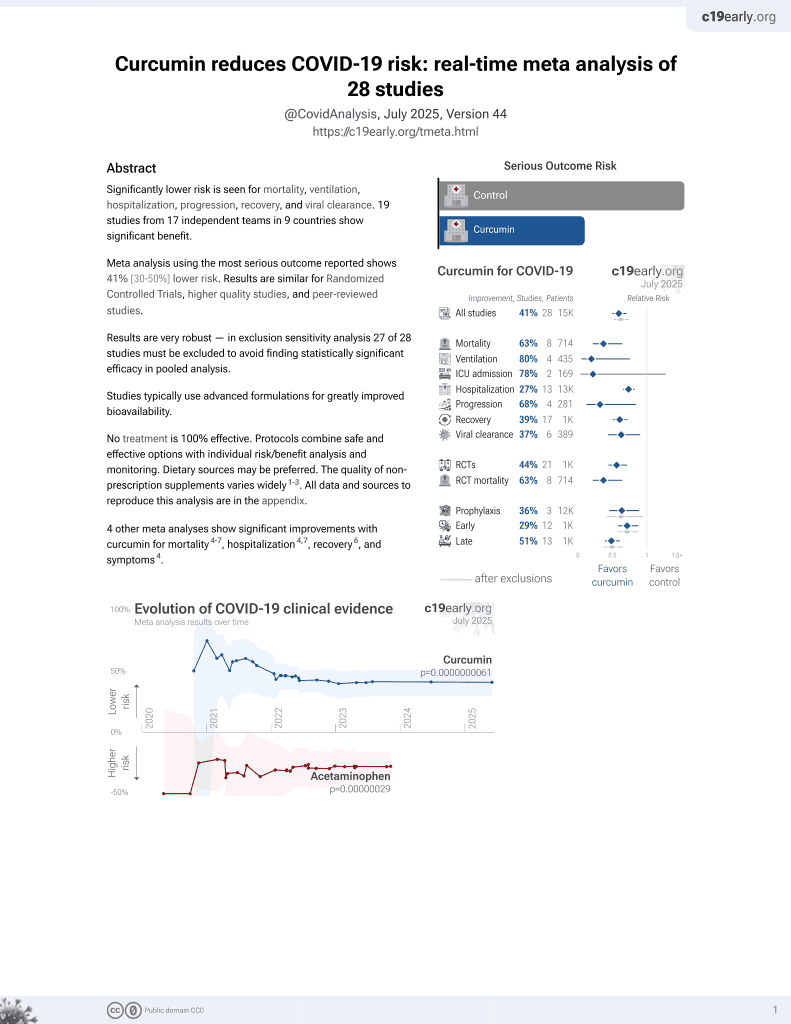
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Small RCT with 50 outpatients, 25 treated with curcumin, quercetin, and vitamin D, showing improved recovery and viral clearance with treatment. 168mg curcumin, 260mg, 360IU vitamin D3 daily for 14 days.
Unadjusted baseline differences: the treatment arm had significantly more comorbidities, but the control arm had significantly more myalgia and asthenia, suggesting poor randomization and potential selection bias. In Table 1, the control arm value for '>=5 symptoms' is listed as '15 (60.05)', where 60.05 is a typo for 60.0%.
This is the 17th of 21 COVID-19 RCTs for curcumin, which collectively show efficacy with p=0.0000022.
This is the 23rd of 28 COVID-19 controlled studies for curcumin, which collectively show efficacy with p=0.0000000061.
This study is excluded in the after exclusion results of meta-analysis:
combined treatments may contribute significantly to the effect seen; unadjusted differences between groups.
|
risk of no recovery, 28.6% lower, RR 0.71, p = 0.11, treatment 15 of 25 (60.0%), control 21 of 25 (84.0%), NNT 4.2, no symptoms, day 7.
|
|
risk of no recovery, 71.4% lower, RR 0.29, p < 0.001, treatment 6 of 25 (24.0%), control 21 of 25 (84.0%), NNT 1.7, ≤1 symptom, day 7.
|
|
risk of no recovery, 76.9% lower, RR 0.23, p = 0.005, treatment 3 of 25 (12.0%), control 13 of 25 (52.0%), NNT 2.5, ≤2 symptoms, day 7.
|
|
risk of no recovery, 85.7% lower, RR 0.14, p = 0.23, treatment 0 of 25 (0.0%), control 3 of 25 (12.0%), NNT 8.3, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), ≤3 symptoms, day 7.
|
|
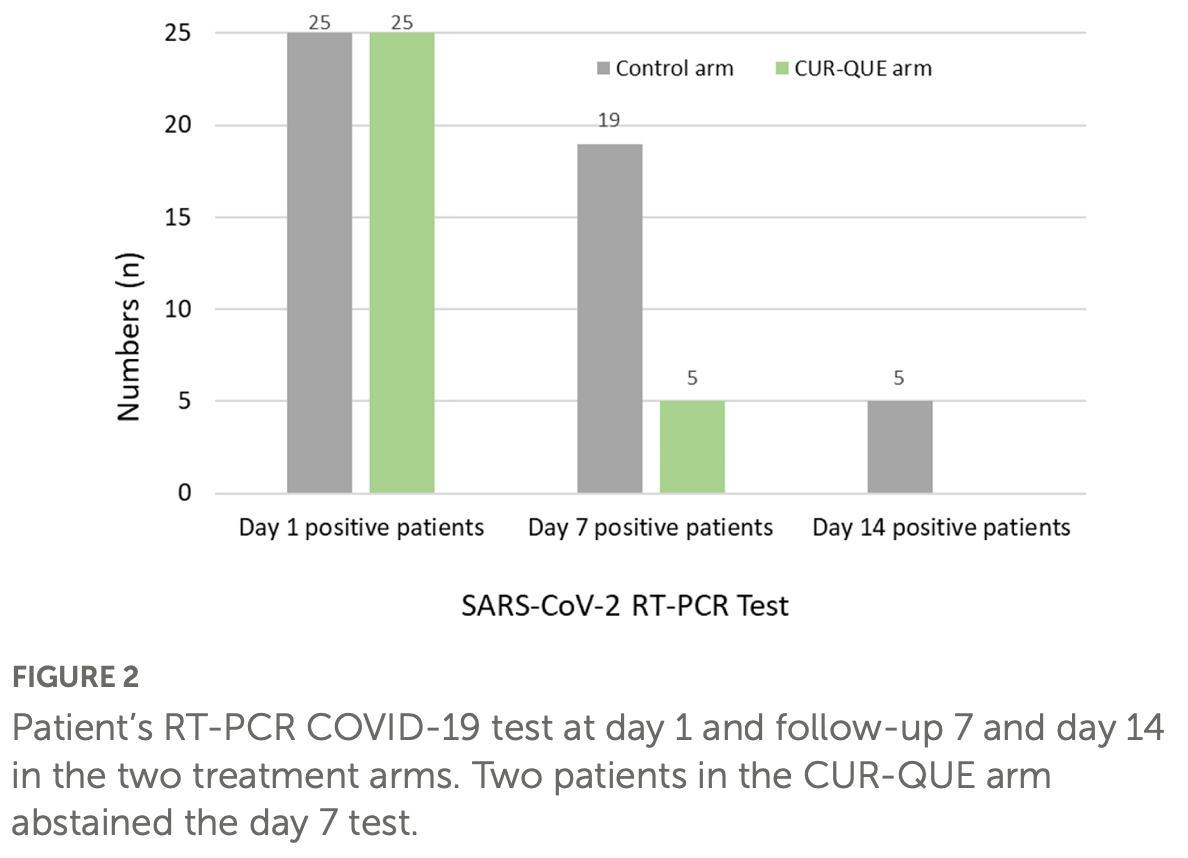
risk of no viral clearance, 90.9% lower, RR 0.09, p = 0.05, treatment 0 of 25 (0.0%), control 5 of 25 (20.0%), NNT 5.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 14.
|
|
risk of no viral clearance, 73.7% lower, RR 0.26, p < 0.001, treatment 5 of 25 (20.0%), control 19 of 25 (76.0%), NNT 1.8, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Din Ujjan et al., 18 Jan 2023, Randomized Controlled Trial, Pakistan, peer-reviewed, 6 authors, study period 21 September, 2021 - 21 January, 2022, this trial uses multiple treatments in the treatment arm (combined with quercetin and vitamin D) - results of individual treatments may vary, trial NCT04603690 (history).
The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19—Results from a pragmatic randomized clinical trial
Frontiers in Nutrition, doi:10.3389/fnut.2022.1023997
A (2023) The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19-Results from a pragmatic randomized clinical trial.
Ethics statement The study was approved by the Research Ethics Committee (REC), Liaquat University of Medical and Health Sciences (LUMHS), Jamshoro, Pakistan via Ref. No. LUMHS/REC/-137. The patients/participants provided their written informed consent to participate in this study.
Author contributions IU: study design, data collection, data interpretation, supervision, and critical revision of the manuscript. SK: study design, data interpretation, and manuscript revision. RN: data collection, supervision, and manuscript revision. HA: study administration, data collection, and manuscript
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aggarwal, Harikumar, Potential therapeutic effects of curcumin, the antiinflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases, Int J Biochem Cell Biol, doi:10.1016/j.biocel.2008.06.010
Ahmadi, Salari, Sharifi, Reihani, Rostamiani et al., Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: a randomized triple-blind placebo-controlled clinical trial, Food Sci Nutr, doi:10.1002/fsn3.2226
Babaei, Nassiri-Asl, Hosseinzadeh, Curcumin (a constituent of turmeric): new treatment option against COVID-19, Food Sci Nutr, doi:10.1002/fsn3.1858
Bahun, Jukic, Oblak, Kranjc, Bajc et al., Inhibition of the SARS-CoV-2 3CL(pro) main protease by plant polyphenols, Food Chem, doi:10.1016/j.foodchem.2021.131594
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez, None
Biancatelli, Berrill, Catravas, Marik, Derosa et al., Antiviral, immunomodulatory, and anticoagulant effects of quercetin and its derivatives: potential role in prevention and management of COVID-19, Front Immunol, doi:10.1016/j.jpha.2021.09.009
Bormann, Alt, Schipper, Van De Sand, Le-Trilling et al., Turmeric root and its bioactive ingredient curcumin effectively neutralize SARS-CoV-2 in vitro, Viruses, doi:10.3390/v13101914
Bowman, Young, Graphical comparison of nonparametric curves, Appl Statist, doi:10.2307/2986225
Chabot, Huntwork, Honarkar ; Shafie, Taheri, Alijani et al., Effect of nanocurcumin supplementation on the severity of symptoms and length of hospital stay in patients with COVID-19: a randomized double-blind placebo-controlled trial, Phytother Res, doi:10.1002/ptr.7374
Chen, Li, Luo, Liu, Xu et al., Binding interaction of quercetin-3-beta-galactoside and its synthetic derivatives with SARS-CoV 3CL(pro): structure-activity relationship studies reveal salient pharmacophore features, Bioorg Med Chem, doi:10.1016/j.bmc.2006.09.014
Core, R: a language and environment for statistical computing
Docherty, Harrison, Green, Hardwick, Pius et al., Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study, BMJ, doi:10.1136/bmj.m1985
Dorward, Russell, Um, Elshani, Armstrong et al., Tissue-specific immunopathology in fatal COVID-19, Am J Respir Crit Care Med, doi:10.1164/rccm.202008-3265OC
Grasselli, Zangrillo, Zanella, Antonelli, Cabrini et al., Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy, JAMA, doi:10.1001/jama.2020.5394
Group, Dexamethasone in hospitalized patients with covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Group, Tocilizumab in patients admitted to hospital with COVID-19: a randomised, controlled, open-label, platform trial, Lancet, doi:10.1016/S0140-673600676-0
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med, doi:10.1056/NEJMoa2107934
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Harwood, Danielewska-Nikiel, Borzelleca, Flamm, Williams et al., A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties, Food Chem Toxicol, doi:10.1016/j.fct.2007.05.015
Hu, Huang, Yin, The cytokine storm and COVID-19, J Med Virol, doi:10.1002/jmv.26232
Jäger, Lowery, Calvanese, Joy, Purpura et al., Comparative absorption of curcumin formulations, Nutr J, doi:10.1186/1475-2891-13-11
Kalil, Patterson, Mehta, Tomashek, Wolfe et al., Baricitinib plus remdesivir for hospitalized adults with covid-19, N Engl J Med, doi:10.1056/NEJMoa2031994
Kamel, Abdelseed, Albalawi, Aslsalameen, Almutairi et al., Treatment of COVID-19 patients with quercetin: a prospective, single center, randomized, controlled trial, Turk J Biol, doi:10.3906/biy-2104-16
Khursheed, Singh, Wadhwa, Gulati, Awasthi, Enhancing the potential preclinical and clinical benefits of quercetin through novel drug delivery systems, Drug Discovery Today, doi:10.1016/j.drudis.2019.11.001
Lee, Yu, Trimpert, Benthani, Mairhofer et al., Virus-induced senescence is a driver and therapeutic target in COVID-19, Nature, doi:10.1038/s41586-021-03995-1
Leka, Hamann, Desdemoustier, Frédérich, Garigliany et al., In vitro antiviral activity against SARS-CoV-2 of common herbal medicinal extracts and their bioactive compounds, Phytother Res, doi:10.3390/molecules26226900
Lelli, Sahebkar, Johnston, Pedone, Curcumin use in pulmonary diseases: state of the art and future perspectives, Pharmacol Res, doi:10.1016/j.phrs.2016.11.017
Lucas, Wong, Klein, Castro, Silva et al., Longitudinal analyses reveal immunological misfiring in severe COVID-19, Nature, doi:10.1038/s41586-020-2588-y
Majeed, Nagabhushanam, Shah, Mundkur, A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of a nutritional supplement (ImmuActive(TM)) for COVID-19 patients, Evid Based Complement Alternat Med, doi:10.1155/2021/8447545
Moderbacher, Ramirez, Grifoni, Hastie, Weiskopf, Antigen-specific adaptive immunity to SARS-CoV-2 in Acute COVID-19 and associations with age and disease severity, Cell, doi:10.1016/j.cell.2020.09.038
Moghadamtousi, Kadir, Hassandarvish, Tajik, Abubakar et al., Review on antibacterial, antiviral, and antifungal activity of curcumin, BioMed Res Int, doi:10.1155/2014/186864
Munafò, Donati, Brindani, Ottonello, Armirotti et al., Quercetin and luteolin are single-digit micromolar inhibitors of the SARS-CoV-2 RNA-dependent RNA polymerase, Sci Rep, doi:10.1038/s41598-022-14664-2
Nguyen, Woo, Kang, Nguyen, Kim et al., Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris, Biotechnol Lett, doi:10.1007/s10529-011-0845-8
Pan, Fang, Zhang, Pan, Liu et al., Chinese herbal compounds against SARS-CoV-2: puerarin and quercetin impair the binding of viral S-protein to ACE2 receptor, Comput Struct Biotechnol J, doi:10.1016/j.csbj.2020.11.010
Pawar, Mastud, Pawar, Pawar, Bhoite et al., A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients, Int Immunopharmacol, doi:10.1016/j.intimp.2020.107088
Petrillo, Orrù, Fais, Fantini, ; Abian et al., Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening, Int J Biol Macromol, doi:10.1016/j.ijbiomac.2020.07.235
Pierro, Derosa, Maffioli, Bertuccioli, Togni et al., Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: a prospective, randomized, controlled, and open-label study, Int J Gen Med, doi:10.2147/IJGM.S318949
Reyes, Molnupiravir for oral treatment of covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area, JAMA, doi:10.1001/jama.2020.6775
Rizzuti, Grande, Conforti, Jimenez-Alesanco, Ceballos-Laita et al., Rutin is a low micromolar inhibitor of SARS-CoV-2 main protease 3CLpro: implications for drug design of quercetin analogs, Biomedicines, doi:10.3390/biomedicines9040375
Rondanelli, Perna, Gasparri, Petrangolini, Allegrini et al., Promising effects of 3-month period of quercetin phytosome((R)) supplementation in the prevention of symptomatic COVID-19 disease in healthcare workers: a pilot study, Life, doi:10.3390/life12010066
Schultze, Aschenbrenner, COVID-19 and the human innate immune system, Cell, doi:10.1016/j.cell.2021.02.029
Sette, Crotty, Adaptive immunity to SARS-CoV-2 and COVID-19, Cell, doi:10.1016/j.cell.2021.01.007
Shohan, Nashibi, Mahmoudian-Sani, Abolnezhadian, Ghafourian et al., Oral co-supplementation of curcumin, quercetin, and vitamin D3 as an adjuvant therapy for mild to moderate symptoms of COVID-19-results from a pilot open-label, randomized controlled trial, Zaporizhia Med J, doi:10.3389/fphar.2022.898062
Tsai, Lai, Lin, Luo, Lin et al., Clinical manifestation and disease progression in COVID-19 infection, J Chin Med Assoc, doi:10.1097/jcma.0000000000000463
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002
Wen, Kuo, Jan, Liang, Wang et al., Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus, J Med Chem, doi:10.1021/jm070295s
Yang, Wang, Long, Li, Quercetin: its main pharmacological activity and potential application in clinical medicine, Oxid Med Cell Longev, doi:10.1155/2020/8825387
Yang, Xie, Tu, Fu, Xu et al., The signal pathways and treatment of cytokine storm in COVID-19, Signal Trans Target Ther, doi:10.1038/s41392-021-00679-0
Young, Bowman, Non-parametric analysis of covariance, Biometrics, doi:10.2307/2532993
Zahedipour, Hosseini, Sathyapalan, Majeed, Jamialahmadi et al., Antiviral and immunomodulatory activity of curcumin: a case for prophylactic therapy for COVID-19, Front Pharmacol, doi:10.1016/j.heliyon.2021.e06350
DOI record:
{
"DOI": "10.3389/fnut.2022.1023997",
"ISSN": [
"2296-861X"
],
"URL": "http://dx.doi.org/10.3389/fnut.2022.1023997",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>Curcumin (CUR) and quercetin (QUE), two natural polyphenols, possess diverse biological activities including broad-spectrum antiviral, antioxidant, and immunomodulatory effects. Both CUR and QUE have shown inhibition of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in <jats:italic>in vitro</jats:italic> assays.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>In the present study we aimed to assess the possible treatment benefits of a combined curcumin and quercetin (CUR-QUE) oral supplement, alongside standard of care (SOC), in the <jats:italic>early-stage</jats:italic> COVID-19 infection.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>This was an exploratory, pragmatic, open-label, randomized controlled clinical trial, conducted at the Department of Pathology, Liaquat University of Medical and Health Sciences, Jamshoro, PK. The study compared the treatment effect of an oral CUR-QUE supplement <jats:italic>plus</jats:italic> SOC vs. SOC alone, in the <jats:italic>early-stage</jats:italic>/mild to moderately symptomatic COVID-19 outpatients. Patients were randomized in a 1:1 ratio to CUR-QUE (<jats:italic>n</jats:italic> = 25) and control (<jats:italic>n</jats:italic> = 25) treatment groups. The CUR-QUE supplementation consisted of a daily intake of 168 mg curcumin and 260 mg quercetin, as two soft capsules, to be taken twice a day at home for 14 days.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>After one-week of treatment, most of the patients in the CUR-QUE group showed an expedited clearance of the viral infection i.e., 18 (72.0%) vs. 6 (24.0%) patients in the control group tested negative for SARS-CoV-2 in the nasal-oropharyngeal swab reverse transcription-polymerase chain reaction (RT-PCR) analysis (<jats:italic>p</jats:italic> = 0.0002). In addition, COVID-19-associated acute symptoms were also speedily resolved in the CUR-QUE treated patients, i.e., 10 (40.0%) vs. 4 (16.0%) patients in the control group (<jats:italic>p</jats:italic> = 0.061). The CUR-QUE supplementation therapy was well-tolerated by all 25 patients and no treatment-emergent effects or serious adverse events were reported.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>The results revealed in this exploratory study suggest a possible therapeutic role of curcumin and quercetin in the <jats:italic>early-stage</jats:italic> of COVID-19. It is proposed that the two agents possibly acting in synergy, interfere the SARS-CoV-2 replication, and thus help a speedy recovery in the <jats:italic>early-stage</jats:italic> of COVID-19. Further research is highly encouraged.</jats:p></jats:sec><jats:sec><jats:title>Clinical trial registration</jats:title><jats:p><jats:ext-link>Clinicaltrials.gov</jats:ext-link>, Identifier NCT04603690.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fnut.2022.1023997"
],
"author": [
{
"affiliation": [],
"family": "Ujjan",
"given": "Ikram Din",
"sequence": "first"
},
{
"affiliation": [],
"family": "Khan",
"given": "Saeed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nigar",
"given": "Roohi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Hammad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmad",
"given": "Sagheer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "Amjad",
"sequence": "additional"
}
],
"container-title": "Frontiers in Nutrition",
"container-title-short": "Front. Nutr.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
1,
18
]
],
"date-time": "2023-01-18T06:41:35Z",
"timestamp": 1674024095000
},
"deposited": {
"date-parts": [
[
2023,
1,
18
]
],
"date-time": "2023-01-18T06:41:39Z",
"timestamp": 1674024099000
},
"indexed": {
"date-parts": [
[
2024,
3,
4
]
],
"date-time": "2024-03-04T18:59:12Z",
"timestamp": 1709578752533
},
"is-referenced-by-count": 2,
"issued": {
"date-parts": [
[
2023,
1,
18
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
18
]
],
"date-time": "2023-01-18T00:00:00Z",
"timestamp": 1674000000000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fnut.2022.1023997/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
1,
18
]
]
},
"published-online": {
"date-parts": [
[
2023,
1,
18
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1097/jcma.0000000000000463",
"article-title": "Clinical manifestation and disease progression in COVID-19 infection.",
"author": "Tsai",
"doi-asserted-by": "publisher",
"first-page": "3",
"journal-title": "J Chin Med Assoc.",
"key": "B1",
"volume": "84",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2588-y",
"article-title": "Longitudinal analyses reveal immunological misfiring in severe COVID-19.",
"author": "Lucas",
"doi-asserted-by": "publisher",
"first-page": "463",
"journal-title": "Nature.",
"key": "B2",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.09.038",
"article-title": "Antigen-specific adaptive immunity to SARS-CoV-2 in Acute COVID-19 and associations with age and disease severity.",
"author": "Rydyznski Moderbacher",
"doi-asserted-by": "publisher",
"first-page": "996",
"journal-title": "Cell.",
"key": "B3",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2021.02.029",
"article-title": "COVID-19 and the human innate immune system.",
"author": "Schultze",
"doi-asserted-by": "publisher",
"first-page": "1671",
"journal-title": "Cell.",
"key": "B4",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2021.01.007",
"article-title": "Adaptive immunity to SARS-CoV-2 and COVID-19.",
"author": "Sette",
"doi-asserted-by": "publisher",
"first-page": "861",
"journal-title": "Cell.",
"key": "B5",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m1985",
"article-title": "Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study.",
"author": "Docherty",
"doi-asserted-by": "publisher",
"journal-title": "BMJ.",
"key": "B6",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.5394",
"article-title": "Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy.",
"author": "Grasselli",
"doi-asserted-by": "publisher",
"first-page": "1574",
"journal-title": "JAMA.",
"key": "B7",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area.",
"author": "Richardson",
"doi-asserted-by": "publisher",
"first-page": "2052",
"journal-title": "JAMA.",
"key": "B8",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26232",
"article-title": "The cytokine storm and COVID-19.",
"author": "Hu",
"doi-asserted-by": "publisher",
"first-page": "250",
"journal-title": "J Med Virol.",
"key": "B9",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1038/s41392-021-00679-0",
"article-title": "The signal pathways and treatment of cytokine storm in COVID-19.",
"author": "Yang",
"doi-asserted-by": "publisher",
"journal-title": "Signal Trans Target Ther.",
"key": "B10",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1164/rccm.202008-3265OC",
"article-title": "Tissue-specific immunopathology in fatal COVID-19.",
"author": "Dorward",
"doi-asserted-by": "publisher",
"first-page": "192",
"journal-title": "Am J Respir Crit Care Med.",
"key": "B11",
"volume": "203",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of covid-19 in nonhospitalized patients.",
"author": "Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"journal-title": "N Engl J Med.",
"key": "B12",
"volume": "386",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab.",
"author": "Gupta",
"doi-asserted-by": "publisher",
"first-page": "1941",
"journal-title": "N Engl J Med.",
"key": "B13",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19.",
"author": "Weinreich",
"doi-asserted-by": "publisher",
"first-page": "238",
"journal-title": "N Engl J Med.",
"key": "B14",
"volume": "384",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19.",
"author": "Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "N Engl J Med.",
"key": "B15",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with covid-19.",
"author": "Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "693",
"journal-title": "N Engl J Med.",
"key": "B16",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-673600676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19: a randomised, controlled, open-label, platform trial.",
"author": "Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "1637",
"journal-title": "Lancet.",
"key": "B17",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031994",
"article-title": "Baricitinib plus remdesivir for hospitalized adults with covid-19.",
"author": "Kalil",
"doi-asserted-by": "publisher",
"first-page": "795",
"journal-title": "N Engl J Med.",
"key": "B18",
"volume": "384",
"year": "2020"
},
{
"DOI": "10.1002/ptr.6738",
"article-title": "Potential effects of curcumin in the treatment of COVID-19 infection.",
"author": "Zahedipour",
"doi-asserted-by": "publisher",
"first-page": "2911",
"journal-title": "Phytother Res.",
"key": "B19",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2021.675287",
"article-title": "Curcumin as a potential treatment for COVID-19.",
"author": "Rattis",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol.",
"key": "B20",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.heliyon.2021.e06350",
"article-title": "Antiviral and immunomodulatory activity of curcumin: a case for prophylactic therapy for COVID-19.",
"author": "Thimmulappa",
"doi-asserted-by": "publisher",
"journal-title": "Heliyon.",
"key": "B21",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2020.01451",
"article-title": "Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19).",
"author": "Colunga Biancatelli",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol.",
"key": "B22",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1002/ptr.6887",
"article-title": "A role for quercetin in coronavirus disease 2019 (COVID-19).",
"author": "Derosa",
"doi-asserted-by": "publisher",
"first-page": "1230",
"journal-title": "Phytother Res.",
"key": "B23",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.jpha.2021.09.009",
"article-title": "Antiviral, immunomodulatory, and anticoagulant effects of quercetin and its derivatives: potential role in prevention and management of COVID-19.",
"author": "Manjunath",
"doi-asserted-by": "publisher",
"first-page": "29",
"journal-title": "J Pharm Analy.",
"key": "B24",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1002/fsn3.1858",
"article-title": "Curcumin (a constituent of turmeric): new treatment option against COVID-19.",
"author": "Babaei",
"doi-asserted-by": "publisher",
"first-page": "5215",
"journal-title": "Food Sci Nutr.",
"key": "B25",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.biocel.2008.06.010",
"article-title": "Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases.",
"author": "Aggarwal",
"doi-asserted-by": "publisher",
"first-page": "40",
"journal-title": "Int J Biochem Cell Biol.",
"key": "B26",
"volume": "41",
"year": "2009"
},
{
"DOI": "10.1186/1475-2891-13-11",
"article-title": "Comparative absorption of curcumin formulations.",
"author": "Jäger",
"doi-asserted-by": "publisher",
"journal-title": "Nutr J.",
"key": "B27",
"volume": "13",
"year": "2014"
},
{
"DOI": "10.1016/j.phrs.2016.11.017",
"article-title": "Curcumin use in pulmonary diseases: state of the art and future perspectives.",
"author": "Lelli",
"doi-asserted-by": "publisher",
"first-page": "133",
"journal-title": "Pharmacol Res.",
"key": "B28",
"volume": "115",
"year": "2017"
},
{
"DOI": "10.1021/jm070295s",
"article-title": "Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus.",
"author": "Wen",
"doi-asserted-by": "publisher",
"first-page": "4087",
"journal-title": "J Med Chem.",
"key": "B29",
"volume": "50",
"year": "2007"
},
{
"DOI": "10.1155/2014/186864",
"article-title": "Review on antibacterial, antiviral, and antifungal activity of curcumin.",
"author": "Zorofchian Moghadamtousi",
"doi-asserted-by": "publisher",
"journal-title": "BioMed Res Int.",
"key": "B30",
"volume": "2014",
"year": "2014"
},
{
"DOI": "10.1016/j.foodchem.2021.131594",
"article-title": "Inhibition of the SARS-CoV-2 3CL(pro) main protease by plant polyphenols.",
"author": "Bahun",
"doi-asserted-by": "publisher",
"journal-title": "Food Chem.",
"key": "B31",
"volume": "373",
"year": "2022"
},
{
"DOI": "10.3390/v13101914",
"article-title": "Turmeric root and its bioactive ingredient curcumin effectively neutralize SARS-CoV-2 in vitro.",
"author": "Bormann",
"doi-asserted-by": "publisher",
"journal-title": "Viruses.",
"key": "B32",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7463",
"article-title": "In vitro antiviral activity against SARS-CoV-2 of common herbal medicinal extracts and their bioactive compounds.",
"author": "Leka",
"doi-asserted-by": "publisher",
"first-page": "3013",
"journal-title": "Phytother Res.",
"key": "B33",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.3390/molecules26226900",
"article-title": "Curcumin inhibits in vitro SARS-CoV-2 infection in vero E6 cells through multiple antiviral mechanisms.",
"author": "Marin-Palma",
"doi-asserted-by": "publisher",
"journal-title": "Molecules.",
"key": "B34",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1016/j.drudis.2019.11.001",
"article-title": "Enhancing the potential preclinical and clinical benefits of quercetin through novel drug delivery systems.",
"author": "Khursheed",
"doi-asserted-by": "publisher",
"first-page": "209",
"journal-title": "Drug Discovery Today.",
"key": "B35",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1155/2020/8825387",
"article-title": "Quercetin: its main pharmacological activity and potential application in clinical medicine.",
"author": "Yang",
"doi-asserted-by": "publisher",
"journal-title": "Oxid Med Cell Longev.",
"key": "B36",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1016/j.bmc.2006.09.014",
"article-title": "Binding interaction of quercetin-3-beta-galactoside and its synthetic derivatives with SARS-CoV 3CL(pro): structure-activity relationship studies reveal salient pharmacophore features.",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "8295",
"journal-title": "Bioorg Med Chem.",
"key": "B37",
"volume": "14",
"year": "2006"
},
{
"DOI": "10.1007/s10529-011-0845-8",
"article-title": "Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris.",
"author": "Nguyen",
"doi-asserted-by": "publisher",
"first-page": "831",
"journal-title": "Biotechnol Lett.",
"key": "B38",
"volume": "34",
"year": "2012"
},
{
"DOI": "10.1002/ptr.7309",
"article-title": "Quercetin and its derivates as antiviral potentials: a comprehensive review.",
"author": "Di Petrillo",
"doi-asserted-by": "publisher",
"first-page": "266",
"journal-title": "Phytother Res.",
"key": "B39",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.1016/j.ijbiomac.2020.07.235",
"article-title": "Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening.",
"author": "Abian",
"doi-asserted-by": "publisher",
"first-page": "1693",
"journal-title": "Int J Biol Macromol.",
"key": "B40",
"volume": "164",
"year": "2020"
},
{
"DOI": "10.3390/biomedicines9040375",
"article-title": "Rutin is a low micromolar inhibitor of SARS-CoV-2 main protease 3CLpro: implications for drug design of quercetin analogs.",
"author": "Rizzuti",
"doi-asserted-by": "publisher",
"journal-title": "Biomedicines.",
"key": "B41",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03995-1",
"article-title": "Virus-induced senescence is a driver and therapeutic target in COVID-19.",
"author": "Lee",
"doi-asserted-by": "publisher",
"first-page": "283",
"journal-title": "Nature.",
"key": "B42",
"volume": "599",
"year": "2021"
},
{
"DOI": "10.2307/2986225",
"article-title": "Graphical comparison of nonparametric curves.",
"author": "Bowman",
"doi-asserted-by": "publisher",
"first-page": "83",
"journal-title": "Appl Statist.",
"key": "B43",
"volume": "45",
"year": "1996"
},
{
"DOI": "10.2307/2532993",
"article-title": "Non-parametric analysis of covariance.",
"author": "Young",
"doi-asserted-by": "publisher",
"first-page": "920",
"journal-title": "Biometrics.",
"key": "B44",
"volume": "51",
"year": "1995"
},
{
"journal-title": "R: a language and environment for statistical computing.",
"key": "B45",
"year": "2015"
},
{
"DOI": "10.1038/s41598-022-14664-2",
"article-title": "Quercetin and luteolin are single-digit micromolar inhibitors of the SARS-CoV-2 RNA-dependent RNA polymerase.",
"author": "Munafò",
"doi-asserted-by": "publisher",
"journal-title": "Sci Rep.",
"key": "B46",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/j.csbj.2020.11.010",
"article-title": "Chinese herbal compounds against SARS-CoV-2: puerarin and quercetin impair the binding of viral S-protein to ACE2 receptor.",
"author": "Pan",
"doi-asserted-by": "publisher",
"first-page": "3518",
"journal-title": "Comput Struct Biotechnol J.",
"key": "B47",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1002/fsn3.2226",
"article-title": "Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: a randomized triple-blind placebo-controlled clinical trial.",
"author": "Ahmadi",
"doi-asserted-by": "publisher",
"first-page": "4068",
"journal-title": "Food Sci Nutr.",
"key": "B48",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.7759/cureus.17829",
"article-title": "Turmeric as a possible treatment for COVID-19-induced anosmia and ageusia.",
"author": "Chabot",
"doi-asserted-by": "publisher",
"journal-title": "Cureus.",
"key": "B49",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7374",
"article-title": "Effect of nanocurcumin supplementation on the severity of symptoms and length of hospital stay in patients with COVID-19: a randomized double-blind placebo-controlled trial.",
"author": "Honarkar Shafie",
"doi-asserted-by": "publisher",
"first-page": "1013",
"journal-title": "Phytother Res.",
"key": "B50",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.1155/2021/8447545",
"article-title": "A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of a nutritional supplement (ImmuActive(TM)) for COVID-19 patients.",
"author": "Majeed",
"doi-asserted-by": "publisher",
"journal-title": "Evid Based Complement Alternat Med.",
"key": "B51",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.669362",
"article-title": "Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: a randomized clinical trial.",
"author": "Pawar",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol.",
"key": "B52",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7004",
"article-title": "Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: an open label nonrandomized clinical trial.",
"author": "Saber-Moghaddam",
"doi-asserted-by": "publisher",
"first-page": "2616",
"journal-title": "Phytother Res.",
"key": "B53",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7375",
"article-title": "Antiinflammatory potential of nano-curcumin as an alternative therapeutic agent for the treatment of mild-to-moderate hospitalized COVID-19 patients in a placebo-controlled clinical trial.",
"author": "Asadirad",
"doi-asserted-by": "publisher",
"first-page": "1023",
"journal-title": "Phytother Res.",
"key": "B54",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.1002/ptr.7294",
"article-title": "A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients.",
"author": "Hassaniazad",
"doi-asserted-by": "publisher",
"first-page": "6417",
"journal-title": "Phytother Res.",
"key": "B55",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.eujim.2020.101122",
"article-title": "Effects of nanocurcumin on inflammatory factors and clinical outcomes in critically ill patients with sepsis: a pilot randomized clinical trial.",
"author": "Karimi",
"doi-asserted-by": "publisher",
"journal-title": "Eur J Int Med.",
"key": "B56",
"volume": "36",
"year": "2020"
},
{
"DOI": "10.1002/jcp.30233",
"article-title": "Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID-19 patients.",
"author": "Tahmasebi",
"doi-asserted-by": "publisher",
"first-page": "5325",
"journal-title": "J Cell Physiol.",
"key": "B57",
"volume": "236",
"year": "2021"
},
{
"DOI": "10.1016/j.lfs.2021.119437",
"article-title": "Nanocurcumin improves Treg cell responses in patients with mild and severe SARS-CoV2.",
"author": "Tahmasebi",
"doi-asserted-by": "publisher",
"journal-title": "Life Sci.",
"key": "B58",
"volume": "276",
"year": "2021"
},
{
"DOI": "10.1016/j.intimp.2020.107088",
"article-title": "Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients.",
"author": "Valizadeh",
"doi-asserted-by": "publisher",
"journal-title": "Int Immunopharmacol.",
"key": "B59",
"volume": "89",
"year": "2020"
},
{
"DOI": "10.2147/IJGM.S318720",
"article-title": "Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: a prospective, randomized, controlled, and open-label study.",
"author": "Di Pierro",
"doi-asserted-by": "publisher",
"first-page": "2359",
"journal-title": "Int J Gen Med.",
"key": "B60",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.2147/IJGM.S318949",
"article-title": "Potential clinical benefits of quercetin in the early stage of COVID-19: results of a second, pilot, randomized, controlled and open-label clinical trial.",
"author": "Di Pierro",
"doi-asserted-by": "publisher",
"first-page": "2807",
"journal-title": "Int J Gen Med.",
"key": "B61",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1101/2020.12.22.20245993",
"article-title": "Evaluation of the effect of zinc, quercetin, bromelain and vitamin C on COVID-19 patients.",
"author": "Kamel",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "B62",
"year": "2020"
},
{
"DOI": "10.3906/biy-2104-16",
"article-title": "Treatment of COVID-19 patients with quercetin: a prospective, single center, randomized, controlled trial.",
"author": "Onal",
"doi-asserted-by": "publisher",
"first-page": "518",
"journal-title": "Turk J Biol.",
"key": "B63",
"volume": "45",
"year": "2021"
},
{
"DOI": "10.3390/life12010066",
"article-title": "Promising effects of 3-month period of quercetin phytosome((R)) supplementation in the prevention of symptomatic COVID-19 disease in healthcare workers: a pilot study.",
"author": "Rondanelli",
"doi-asserted-by": "publisher",
"journal-title": "Life.",
"key": "B64",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/j.ejphar.2021.174615",
"article-title": "The therapeutic efficacy of quercetin in combination with antiviral drugs in hospitalized COVID-19 patients: a randomized controlled trial.",
"author": "Shohan",
"doi-asserted-by": "publisher",
"journal-title": "Eur J Pharmacol.",
"key": "B65",
"volume": "914",
"year": "2022"
},
{
"DOI": "10.14739/2310-1210.2021.5.231714",
"article-title": "Efficacy of quercetin in patients with pneumonia associated with coronavirus disease (COVID-19).",
"author": "Zupanets",
"doi-asserted-by": "publisher",
"first-page": "636",
"journal-title": "Zaporizhia Med J.",
"key": "B66",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2022.898062",
"article-title": "Oral co-supplementation of curcumin, quercetin, and vitamin D3 as an adjuvant therapy for mild to moderate symptoms of COVID-19-results from a pilot open-label, randomized controlled trial.",
"author": "Khan",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol.",
"key": "B67",
"volume": "13",
"year": "2022"
},
{
"article-title": "Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions.",
"author": "Cheng",
"first-page": "2895",
"journal-title": "Anticancer Res.",
"key": "B68",
"volume": "21",
"year": "2001"
},
{
"DOI": "10.1016/j.fct.2007.05.015",
"article-title": "A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties.",
"author": "Harwood",
"doi-asserted-by": "publisher",
"first-page": "2179",
"journal-title": "Food Chem Toxicol.",
"key": "B69",
"volume": "45",
"year": "2007"
}
],
"reference-count": 69,
"references-count": 69,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fnut.2022.1023997/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Nutrition and Dietetics",
"Endocrinology, Diabetes and Metabolism",
"Food Science"
],
"subtitle": [],
"title": "The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19—Results from a pragmatic randomized clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "9"
}
dinujjan