A prospective, multi center, single blind, randomized controlled study evaluating “AyurCoro3” as an adjuvant in the treatment of mild to moderate COVID
et al., Journal of Ayurveda and Integrated Medical Sciences, doi:10.21760/jaims.6.4.6, Aug 2021
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 174 patients in India, 87 treated with AyurCoro-3 (turmeric, gomutra, potassium alum, khadisakhar, bos indicus milk, ghee), showing faster recovery with treatment. EC/NEW/INST/2019/245.
This is the 5th of 21 COVID-19 RCTs for curcumin, which collectively show efficacy with p=0.0000022.
This is the 8th of 28 COVID-19 controlled studies for curcumin, which collectively show efficacy with p=0.0000000061.
|
risk of death, 88.9% lower, RR 0.11, p = 0.12, treatment 0 of 87 (0.0%), control 4 of 87 (4.6%), NNT 22, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of mechanical ventilation, 75.0% lower, RR 0.25, p = 0.37, treatment 1 of 87 (1.1%), control 4 of 87 (4.6%), NNT 29.
|
|
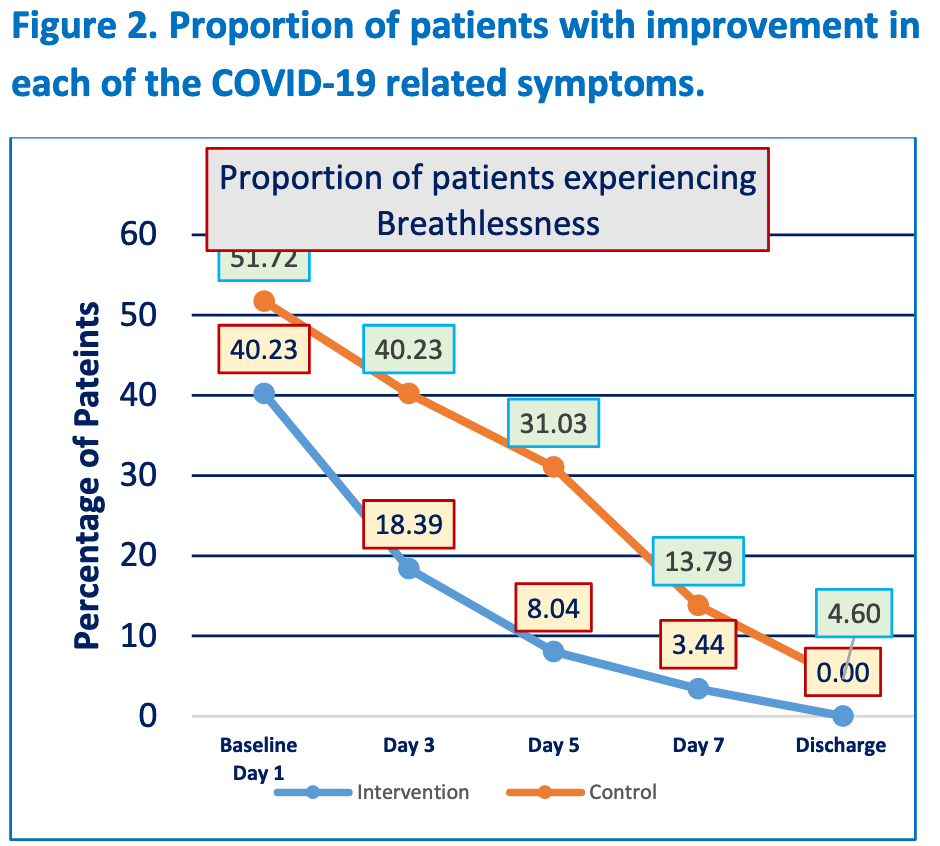
risk of no 2-point improvement, 46.5% lower, RR 0.54, p = 0.002, treatment 29 of 87 (33.3%), control 60 of 87 (69.0%), NNT 2.8, inverted to make RR<1 favor treatment, odds ratio converted to relative risk, day 7 mid-recovery.
|
|
hospitalization time, 10.0% lower, relative time 0.90, p = 0.40, treatment 87, control 87.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sankhe et al., 10 Aug 2021, Randomized Controlled Trial, India, peer-reviewed, 8 authors, study period October 2020 - March 2021, this trial uses multiple treatments in the treatment arm (combined with gomutra, potassium alum, khadisakhar, bos indicus milk, ghee) - results of individual treatments may vary.
A prospective, multi center, single blind, randomized controlled study evaluating "AyurCoro3" as an adjuvant in the treatment of mild to moderate COVID-19 patients
doi:10.21760/jaims.6.4.6
INTRODUCTION The Coronavirus disease (COVID-19) has affected the world worst with over 158 million patients infected
References
Bombardieri, Easthope, Convergence between orthodox and alternative medicine: A theoretical elaboration and empirical test, Health
Chauhan, Immunomodulatory properties of indigenous cow urine, MOJ Immunol
Chauhan, Semwal, Mishra, Semwal, Ayurvedic research and methodology: Present status and future strategies, Ayu
Consort, Transparent reporting of trials
Goyal, Threats and challenges of emerging viral diseases and scope of Ayurveda in its prevention, AYU
Jennings, Parks, Curcumin as an Antiviral Agent, Viruses
Kim, Lee, Hwang, Kwon, Jung et al., Alum Adjuvant Enhances Protection against Respiratory Syncytial Virus but Exacerbates Pulmonary Inflammation by Modulating Multiple Innate and Adaptive Immune Cells, PLoS One
Payyappallimana, Patwardhan, Mangalath, Kessler, Jayasundar et al., The COVID-19 Pandemic and the Relevance of Ayurveda's Whole Systems Approach to Health and Disease Management, J Altern Complement Med
Randhawa, Sharma, Chemotherapeutic potential of cow urine: A review, J Intercult Ethnopharmacol
Rotti, Raval, Anchan, Bellampalli, Bhale et al., Determinants of Prakriti, the human constitution types of Indian traditional medicine and its correlation with contemporary science, J Ayurveda Integr Med
Semwal, Mishra, Chauhan, Semwal, Adverse health effects of tobacco and role of Ayurveda in their reduction, J Med Sci
Tijare, Ambatkar, Tiwari, Clinical Improvement In COVID19 Patients With Timely Intervention Of Panchagavya Medicine: A Preliminary Finding, Int J of Pharmc Res [Internet
