Efficacy of Nanocurcumin as an Add-On Treatment for Patients Hospitalized with COVID-19: A Double-Blind, Randomized Clinical Trial
et al., International Journal of Clinical Practice, doi:10.1155/2023/5734675, IRCT20211126053183N1, Jul 2023
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
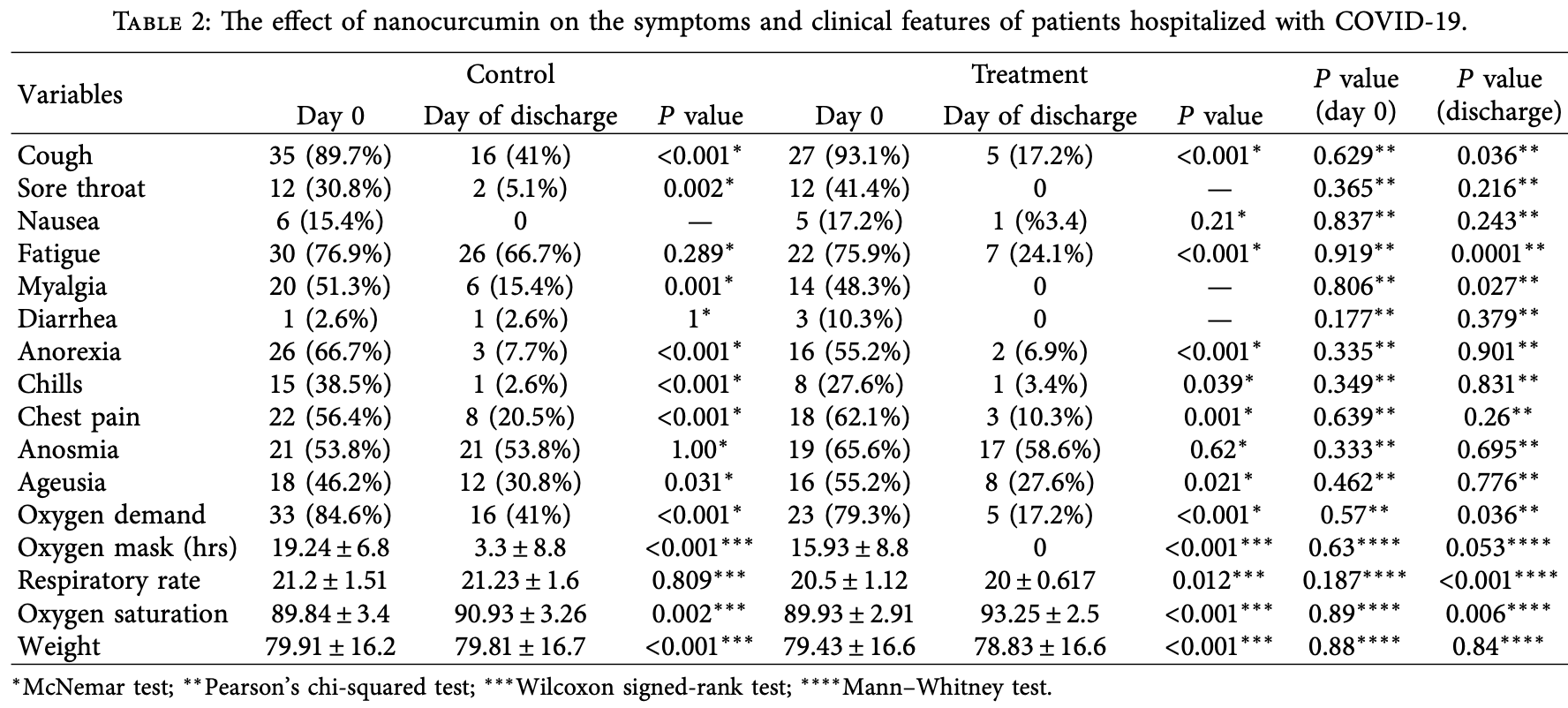
RCT 76 hospitalized patients, showing improved recovery with nanocurcumin. Authors note that pure curcumin is limited due to rapid metabolism, low bio-availability, weak aqueous solubility, and systemic deletion, and that the nanocurcumin formulation used improves curcumin’s solubility, stability, half-life, and bioavailability. The dropout rate was higher in the curcumin group, in part due to discontinuation for side effects. Authors do not provide detailed discharge criteria.
This is the 20th of 21 COVID-19 RCTs for curcumin, which collectively show efficacy with p=0.0000022.
This is the 26th of 28 COVID-19 controlled studies for curcumin, which collectively show efficacy with p=0.0000000061.
|
risk of oxygen therapy, 58.0% lower, RR 0.42, p = 0.06, treatment 5 of 29 (17.2%), control 16 of 39 (41.0%), NNT 4.2.
|
|
relative improvement in SpO2, 67.2% better, RR 0.33, p = 0.04, treatment mean 3.32 (±3.84) n=29, control mean 1.09 (±4.71) n=39.
|
|
risk of no recovery, 49.6% lower, RR 0.50, p = 0.33, treatment 3 of 29 (10.3%), control 8 of 39 (20.5%), NNT 9.8, chest pain.
|
|
risk of no recovery, 34.5% higher, RR 1.34, p = 1.00, treatment 1 of 29 (3.4%), control 1 of 39 (2.6%), chills.
|
|
risk of no recovery, 58.0% lower, RR 0.42, p = 0.06, treatment 5 of 29 (17.2%), control 16 of 39 (41.0%), NNT 4.2, cough.
|
|
risk of no recovery, 77.7% lower, RR 0.22, p = 0.50, treatment 0 of 29 (0.0%), control 2 of 39 (5.1%), NNT 20, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), sore throat.
|
|
risk of no recovery, 63.8% lower, RR 0.36, p < 0.001, treatment 7 of 29 (24.1%), control 26 of 39 (66.7%), NNT 2.4, fatigue.
|
|
risk of no recovery, 91.3% lower, RR 0.09, p = 0.03, treatment 0 of 29 (0.0%), control 6 of 39 (15.4%), NNT 6.5, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), myalgia.
|
|
risk of no recovery, 8.9% higher, RR 1.09, p = 0.81, treatment 17 of 29 (58.6%), control 21 of 39 (53.8%), anosmia.
|
|
risk of no recovery, 10.3% lower, RR 0.90, p = 1.00, treatment 8 of 29 (27.6%), control 12 of 39 (30.8%), NNT 31, ageusia.
|
|
risk of no recovery, 10.3% lower, RR 0.90, p = 1.00, treatment 2 of 29 (6.9%), control 3 of 39 (7.7%), NNT 126, anorexia.
|
|
risk of no recovery, 63.6% lower, RR 0.36, p = 1.00, treatment 0 of 29 (0.0%), control 1 of 39 (2.6%), NNT 39, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), diarrhea.
|
|
risk of no recovery, 234.5% higher, RR 3.34, p = 0.43, treatment 1 of 29 (3.4%), control 0 of 39 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), nausea.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ahmadi et al., 28 Jul 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Iran, peer-reviewed, 5 authors, study period December 2021 - March 2022, trial IRCT20211126053183N1.
Contact: masoumi7415@gmail.com.
Efficacy of Nanocurcumin as an Add-On Treatment for Patients Hospitalized with COVID-19: A Double-Blind, Randomized Clinical Trial
International Journal of Clinical Practice, doi:10.1155/2023/5734675
Background. Curcumin is a polyphenol derivative of the Curcuma longa rhizome, with potential antioxidant, anticancer, antidepressant, antiviral, and anti-infammatory efects. Tis compound can be prepared as biodegradable polymer nanoparticles, called nanocurcumin, to improve its solubility, stability, half-life, and bioavailability. Aim. We explored nanocurcumin's efect on the clinical manifestations of patients hospitalized with mild-to-moderate COVID-19. Methods. Tis double-blind, randomized clinical trial involved 76 COVID-19 patients admitted to Ali-Asghar Hospital from December 2021 to March 2022. All patients received standard coronavirus treatment as per national guidelines. In addition, four times a day for two weeks, the curcumin group received 40 mg of nanocurcumin, while the control group received a placebo. Clinical manifestations were examined and recorded by the associate doctors working in the department. Statistical analysis was done using SPSS v. 21. Results. Tirty-nine people from the control group and 29 from the curcumin group completed the study. At baseline, the groups were comparable in age, gender, body mass index, hospitalization duration, and background diseases. Te mean age of patients in the control and treatment groups was 53.9 ± 11.9 and 54.6 ± 13.4, respectively. Compared with the placebo, nanocurcumin minimized coughs (P � 0.036), fatigue (P � 0.0001), myalgia (P � 0.027), oxygen demand (P � 0.036), oxygen usage (P � 0.05), and respiratory rate (P < 0.0001). By discharge, the curcumin group had a signifcantly greater increase in SPO 2 than the control group (P � 0.006). Conclusions. Tis preliminary study suggests that nanocurcumin has a potentiating anti-infammatory efect when combined with standard COVID-19 treatment, helping the recovery from the acute infammatory phase of the disease in hospitalized patients with mild-to-moderate disease severity. Tis trial is registered with Iranian Registry of Clinical Trials: IRCT20211126053183N1 (registered while recruiting on 13/12/2021).
Ethical Approval Tis study was conducted in line with the ethical principles of Shiraz University of Medical Sciences (Ethics Committee approval code: IR.SUMS.SCHEANUT.REC.1400.031) and the Helsinki Declaration of 1964 and its later amendments.
Consent Informed consent was obtained from all subjects.
Disclosure Te present article was extracted from the dissertation written by Sedigheh Ahmadi.
Conflicts of Interest Te authors declare that they have no conficts of interest.
Authors' Contributions SA was responsible for study design, implementation, data interpretation, original draft preparation, and manuscript revision. ZM was responsible for study design, data collection, and manuscript revision. MZ was responsible for study design, data analysis, and manuscript revision. SG was responsible for study implementation, data interpretation, and original draft preparation. SJM was responsible for study concept, design, funding acquisition, implementation, supervision, and manuscript revision. All authors have read and approved the fnal version of the manuscript.
References
Adibian, Hodaei, Nikpayam, Sohrab, Hekmatdoost et al., Te efects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profle in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial, Phytotherapy Research
Allegra, Innao, Russo, Gerace, Alonci et al., Anticancer activity of curcumin and its analogues: preclinical and clinical studies, Cancer Investigation
Avasarala, Zhang, Liu, Wang, London et al., Curcumin modulates the infammatory response and inhibits subsequent fbrosis in a mouse model of viralinduced acute respiratory distress syndrome, PLoS One
Bhaskar, Sinha, Banach, Mittoo, Weissert et al., Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper, Frontiers in Immunology
Boroumand, Samarghandian, Hashemy, Immunomodulatory, anti-infammatory, and antioxidant efects of curcumin, Journal of Herbmed Pharmacology
Canoglu, Çalıs ¸kan, COVID-19 and thrombosis: prophylaxis and management, Tuberkuloz Ve Toraks
Cohen, Veena, Srivatsan, Wang, Suppression of interleukin 6 and 8 production in head and neck cancer cells with curcumin via inhibition of Iκβ kinase
Conti, Ronconi, Carafa, Gallenga, Ross et al., Induction of pro-infammatory cytokines (IL-1 and IL-6) and lung infammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-infammatory strategies, Journal of Biological Regulators & Homeostatic Agents
Dai, Gu, Su, Wang, Zhao et al., Inhibition of curcumin on infuenza A virus infection and infuenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways, International Immunopharmacology
Dei Cas, Ghidoni, Dietary curcumin: correlation between bioavailability and health potential, Nutrients
Dolati, Ahmadi, Rikhtegar, Babaloo, Ayromlou et al., Changes in T17 cells function after nanocurcumin use to treat multiple sclerosis, International Immunopharmacology
Garg, Ahuja, Sankar, Kumar, Moss, Curcumin for maintenance of remission in ulcerative colitis, Cochrane Database of Systematic Reviews
Gera, Sharma, Ghosh, Lee, Min et al., Nanoformulations of curcumin: an emerging paradigm for improved remedial application, Oncotarget
Goel, Kunnumakkara, Aggarwal, Curcumin as "Curecumin": from kitchen to clinic, Biochemical Pharmacology
Haftcheshmeh, Khosrojerdi, Aliabadi, Lotf, Mohammadi et al., Immunomodulatory efects of curcumin in rheumatoid arthritis: evidence from molecular mechanisms to clinical outcomes, Biochemistry & Pharmacology
Han, Xu, Guo, Huang, Curcumin ameliorates severe infuenza pneumonia via attenuating lung injury and regulating macrophage cytokines production, Clinical and Experimental Pharmacology and Physiology
Hassaniazad, Eftekhar, Inchehsablagh, Kamali, Tousi et al., A triple-blind, placebo-controlled, randomized clinical trial to evaluate the efect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients, Phytotherapy Research
He, Yue, Zheng, Zhang, Chen et al., Curcumin, infammation, and chronic diseases: how are they linked?, Molecules
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Te Lancet
Hurley, Akinfresoye, Nwulia, Kamiya, Kulkarni et al., Antidepressant-like efects of curcumin in WKY rat model of depression is associated with an increase in hippocampal BDNF, Behavioural Brain Research
Jennings, Parks, Curcumin as an antiviral agent, Viruses
Jobin, Bradham, Russo, Juma, Narula et al., Curcumin blocks cytokine-mediated NF-κB activation and proinfammatory gene expression by inhibiting inhibitory factor I-κB kinase activity, Te Journal of Immunology
Kannan, Kolandaivel, Antiviral potential of natural compounds against infuenza virus hemagglutinin, Computational Biology and Chemistry
Karimi, Mahmoodpoor, Kooshki, Niazkar, Shoorei et al., Efects of nanocurcumin on infammatory factors and clinical outcomes in critically ill patients with sepsis: a pilot randomized clinical trial, European Journal of Integrative Medicine
Khan, Khan, Nano-gold displayed antiinfammatory property via NF-kB pathways by suppressing COX-2 activity, Nanomedicine, and Biotechnology
Kocaadam, ¸anlier, Curcumin, an active component of turmeric (Curcuma longa), and its efects on health, Critical Reviews in Food Science and Nutrition
Mollazadeh, Cicero, Blesso, Pirro, Majeed et al., Immune modulation by curcumin: the role of interleukin-10, Critical Reviews in Food Science and Nutrition
Rahmanzade, Rahmanzadeh, Hashemian, Tabarsi, Iran's approach to COVID-19: evolving treatment protocols and ongoing clinical trials, Frontiers in Public Health
Rodriguez-Morales, Cardona-Ospina, Gutiérrez-Ocampo, Villamizar-Peña, Holguin-Rivera et al., Clinical, laboratory and imaging features of COVID-19: a systematic review and metaanalysis, Travel Medicine and Infectious Disease
Saadati, Sadeghi, Mansour, Yari, Poustchi et al., Curcumin and infammation in non-alcoholic fatty liver disease: a randomized, placebo controlled clinical trial, BMC Gastroenterology
Saber-Moghaddam, Salari, Hejazi, Amini, Taherzadeh et al., Oral nano-curcumin formulation efcacy in management of mild to moderate hospitalized coronavirus disease-19 patients: an open label nonrandomized clinical trial, Phytotherapy Research
Safarzadeh Kozani, Safarzadeh, Kozani, Mirarefn, Sheikhi et al., COVID-19 Vaccines in Iran
Sordillo, Helson, Curcumin suppression of cytokine release and cytokine storm. A potential therapy for patients with Ebola and other severe viral infections, Vivo
Tahmasebi, El-Esawi, Mahmoud, Timoshin, Valizadeh et al., Immunomodulatory efects of Nanocurcumin on T17 cell responses in mild and severe COVID-19 patients, Journal of Cellular Physiology
Tahmasebi, Saeed, Temirgalieva, Yumashev, El-Esawi et al., Nanocurcumin improves Treg cell responses in patients with mild and severe SARS-CoV2, Life Sciences
Wen, Kuo, Jan, Liang, Wang et al., Specifc plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus, Journal of Medicinal Chemistry
Xu, Liu, Curcumin alleviates macrophage activation and lung infammation induced by infuenza virus infection through inhibiting the NF-κB signaling pathway, Infuenza and other respiratory viruses
Yang, Li, Li, Wang, Huang, Synergistic antiviral efect of curcumin functionalized graphene oxide against respiratory syncytial virus infection, Nanoscale
Yazdanpanah, Hamblin, Rezaei, Te immune system and COVID-19: friend or foe?, Life Sciences
Zahedipour, Hosseini, Sathyapalan, Majeed, Jamialahmadi et al., Potential efects of curcumin in the treatment of COVID-19 infection, Phytotherapy Research
Zhao, Liu, Yi, Wang, Qiao et al., Curcumin inhibiting T17 cell diferentiation by regulating the metabotropic glutamate receptor-4 expression on dendritic cells, International Immunopharmacology
DOI record:
{
"DOI": "10.1155/2023/5734675",
"ISSN": [
"1742-1241",
"1368-5031"
],
"URL": "http://dx.doi.org/10.1155/2023/5734675",
"abstract": "<jats:p>Background. Curcumin is a polyphenol derivative of the Curcuma longa rhizome, with potential antioxidant, anticancer, antidepressant, antiviral, and anti-inflammatory effects. This compound can be prepared as biodegradable polymer nanoparticles, called nanocurcumin, to improve its solubility, stability, half-life, and bioavailability. Aim. We explored nanocurcumin’s effect on the clinical manifestations of patients hospitalized with mild-to-moderate COVID-19. Methods. This double-blind, randomized clinical trial involved 76 COVID-19 patients admitted to Ali-Asghar Hospital from December 2021 to March 2022. All patients received standard coronavirus treatment as per national guidelines. In addition, four times a day for two weeks, the curcumin group received 40 mg of nanocurcumin, while the control group received a placebo. Clinical manifestations were examined and recorded by the associate doctors working in the department. Statistical analysis was done using SPSS v. 21. Results. Thirty-nine people from the control group and 29 from the curcumin group completed the study. At baseline, the groups were comparable in age, gender, body mass index, hospitalization duration, and background diseases. The mean age of patients in the control and treatment groups was 53.9 ± 11.9 and 54.6 ± 13.4, respectively. Compared with the placebo, nanocurcumin minimized coughs (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M1\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.036</mn>\n </math>\n </jats:inline-formula>), fatigue (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M2\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.0001</mn>\n </math>\n </jats:inline-formula>), myalgia (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M3\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.027</mn>\n </math>\n </jats:inline-formula>), oxygen demand (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M4\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.036</mn>\n </math>\n </jats:inline-formula>), oxygen usage (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M5\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.05</mn>\n </math>\n </jats:inline-formula>), and respiratory rate (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M6\">\n <mi>P</mi>\n <mo><</mo>\n <mn>0.0001</mn>\n </math>\n </jats:inline-formula>). By discharge, the curcumin group had a significantly greater increase in SPO2 than the control group (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M7\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.006</mn>\n </math>\n </jats:inline-formula>). Conclusions. This preliminary study suggests that nanocurcumin has a potentiating anti-inflammatory effect when combined with standard COVID-19 treatment, helping the recovery from the acute inflammatory phase of the disease in hospitalized patients with mild-to-moderate disease severity. This trial is registered with Iranian Registry of Clinical Trials: IRCT20211126053183N1 (registered while recruiting on 13/12/2021).</jats:p>",
"alternative-id": [
"5734675",
"5734675"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2969-3125",
"affiliation": [
{
"name": "Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran"
}
],
"authenticated-orcid": true,
"family": "Ahmadi",
"given": "Sedigheh",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-5855-2153",
"affiliation": [
{
"name": "Department of Internal Medicine, Shiraz University of Medical Sciences, Shiraz, Iran"
}
],
"authenticated-orcid": true,
"family": "Mehrabi",
"given": "Zeinab",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7157-9265",
"affiliation": [
{
"name": "Nutrition Research Center, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran"
}
],
"authenticated-orcid": true,
"family": "Zare",
"given": "Morteza",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9976-5887",
"affiliation": [
{
"name": "Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran"
}
],
"authenticated-orcid": true,
"family": "Ghadir",
"given": "Sara",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6712-6802",
"affiliation": [
{
"name": "Nutrition Research Center, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran"
},
{
"name": "Gastroenterohepatology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran"
},
{
"name": "Center for Cohort Study of SUMS Employees’ Health, Shiraz University of Medical Sciences, Shiraz, Iran"
}
],
"authenticated-orcid": true,
"family": "Masoumi",
"given": "Seyed Jalil",
"sequence": "additional"
}
],
"container-title": "International Journal of Clinical Practice",
"container-title-short": "International Journal of Clinical Practice",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
7,
29
]
],
"date-time": "2023-07-29T02:20:12Z",
"timestamp": 1690597212000
},
"deposited": {
"date-parts": [
[
2023,
7,
29
]
],
"date-time": "2023-07-29T02:20:40Z",
"timestamp": 1690597240000
},
"editor": [
{
"affiliation": [],
"family": "de Souza",
"given": "Leandro Napier",
"sequence": "additional"
}
],
"funder": [
{
"DOI": "10.13039/501100004320",
"award": [
"23550"
],
"doi-asserted-by": "publisher",
"name": "Shiraz University of Medical Sciences"
}
],
"indexed": {
"date-parts": [
[
2023,
7,
29
]
],
"date-time": "2023-07-29T04:24:28Z",
"timestamp": 1690604668584
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
7,
28
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
7,
28
]
],
"date-time": "2023-07-28T00:00:00Z",
"timestamp": 1690502400000
}
}
],
"link": [
{
"URL": "http://downloads.hindawi.com/journals/ijclp/2023/5734675.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/ijclp/2023/5734675.xml",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/ijclp/2023/5734675.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "98",
"original-title": [],
"page": "1-7",
"prefix": "10.1155",
"published": {
"date-parts": [
[
2023,
7,
28
]
]
},
"published-print": {
"date-parts": [
[
2023,
7,
28
]
]
},
"publisher": "Hindawi Limited",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "C. Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"issue": "10223",
"journal-title": "The Lancet",
"key": "1",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.lfs.2020.117900",
"article-title": "The immune system and COVID-19: friend or foe?",
"author": "F. Yazdanpanah",
"doi-asserted-by": "crossref",
"journal-title": "Life Sciences",
"key": "2",
"volume": "256",
"year": "2020"
},
{
"article-title": "Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies",
"author": "P. Conti",
"first-page": "327",
"issue": "2",
"journal-title": "Journal of Biological Regulators & Homeostatic Agents",
"key": "3",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.5578/tt.20219818",
"article-title": "COVID-19 and thrombosis: prophylaxis and management",
"author": "K. Canoğlu",
"doi-asserted-by": "crossref",
"first-page": "269",
"issue": "2",
"journal-title": "Tuberkuloz Ve Toraks",
"key": "4",
"volume": "69",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2020.01648",
"article-title": "Cytokine storm in COVID-19—immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper",
"author": "S. Bhaskar",
"doi-asserted-by": "crossref",
"first-page": "1648",
"journal-title": "Frontiers in Immunology",
"key": "5",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1080/10408398.2015.1077195",
"article-title": "Curcumin, an active component of turmeric (Curcuma longa), and its effects on health",
"author": "B. Kocaadam",
"doi-asserted-by": "crossref",
"first-page": "2889",
"issue": "13",
"journal-title": "Critical Reviews in Food Science and Nutrition",
"key": "6",
"volume": "57",
"year": "2017"
},
{
"DOI": "10.1016/j.bcp.2007.08.016",
"article-title": "Curcumin as “Curecumin”: from kitchen to clinic",
"author": "A. Goel",
"doi-asserted-by": "crossref",
"first-page": "787",
"issue": "4",
"journal-title": "Biochemical Pharmacology",
"key": "7",
"volume": "75",
"year": "2008"
},
{
"DOI": "10.15171/jhp.2018.33",
"article-title": "Immunomodulatory, anti-inflammatory, and antioxidant effects of curcumin",
"author": "N. Boroumand",
"doi-asserted-by": "crossref",
"first-page": "211",
"issue": "4",
"journal-title": "Journal of Herbmed Pharmacology",
"key": "8",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1080/07357907.2016.1247166",
"article-title": "Anticancer activity of curcumin and its analogues: preclinical and clinical studies",
"author": "A. Allegra",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "Cancer Investigation",
"key": "9",
"volume": "35",
"year": "2017"
},
{
"DOI": "10.1016/j.bbr.2012.10.049",
"article-title": "Antidepressant-like effects of curcumin in WKY rat model of depression is associated with an increase in hippocampal BDNF",
"author": "L. L. Hurley",
"doi-asserted-by": "crossref",
"first-page": "27",
"journal-title": "Behavioural Brain Research",
"key": "10",
"volume": "239",
"year": "2013"
},
{
"article-title": "Curcumin for maintenance of remission in ulcerative colitis",
"author": "S. K. Garg",
"issue": "10",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "11",
"year": "2012"
},
{
"DOI": "10.3390/molecules20059183",
"article-title": "Curcumin, inflammation, and chronic diseases: how are they linked?",
"author": "Y. He",
"doi-asserted-by": "crossref",
"first-page": "9183",
"issue": "5",
"journal-title": "Molecules",
"key": "12",
"volume": "20",
"year": "2015"
},
{
"article-title": "Curcumin suppression of cytokine release and cytokine storm. A potential therapy for patients with Ebola and other severe viral infections",
"author": "P. P. Sordillo",
"first-page": "1",
"issue": "1",
"journal-title": "Vivo",
"key": "13",
"volume": "29",
"year": "2015"
},
{
"DOI": "10.1016/j.intimp.2017.11.009",
"article-title": "Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways",
"author": "J. Dai",
"doi-asserted-by": "crossref",
"first-page": "177",
"journal-title": "International Immunopharmacology",
"key": "14",
"volume": "54",
"year": "2018"
},
{
"DOI": "10.1016/j.compbiolchem.2017.11.001",
"article-title": "Antiviral potential of natural compounds against influenza virus hemagglutinin",
"author": "S. Kannan",
"doi-asserted-by": "crossref",
"first-page": "207",
"journal-title": "Computational Biology and Chemistry",
"key": "15",
"volume": "71",
"year": "2017"
},
{
"DOI": "10.1039/C7NR06520E",
"article-title": "Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection",
"author": "X. X. Yang",
"doi-asserted-by": "crossref",
"first-page": "16086",
"issue": "41",
"journal-title": "Nanoscale",
"key": "16",
"volume": "9",
"year": "2017"
},
{
"DOI": "10.1021/jm070295s",
"article-title": "Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus",
"author": "C.-C. Wen",
"doi-asserted-by": "crossref",
"first-page": "4087",
"issue": "17",
"journal-title": "Journal of Medicinal Chemistry",
"key": "17",
"volume": "50",
"year": "2007"
},
{
"DOI": "10.3390/v12111242",
"article-title": "Curcumin as an antiviral agent",
"author": "M. R. Jennings",
"doi-asserted-by": "crossref",
"first-page": "1242",
"issue": "11",
"journal-title": "Viruses",
"key": "18",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1002/ptr.6738",
"article-title": "Potential effects of curcumin in the treatment of COVID‐19 infection",
"author": "F. Zahedipour",
"doi-asserted-by": "crossref",
"first-page": "2911",
"issue": "11",
"journal-title": "Phytotherapy Research",
"key": "19",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.18632/oncotarget.19164",
"article-title": "Nanoformulations of curcumin: an emerging paradigm for improved remedial application",
"author": "M. Gera",
"doi-asserted-by": "crossref",
"issue": "39",
"journal-title": "Oncotarget",
"key": "20",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1016/j.eujim.2020.101122",
"article-title": "Effects of nanocurcumin on inflammatory factors and clinical outcomes in critically ill patients with sepsis: a pilot randomized clinical trial",
"author": "A. Karimi",
"doi-asserted-by": "crossref",
"journal-title": "European Journal of Integrative Medicine",
"key": "21",
"volume": "36",
"year": "2020"
},
{
"DOI": "10.3389/fpubh.2020.551889",
"article-title": "Iran’s approach to COVID-19: evolving treatment protocols and ongoing clinical trials",
"author": "R. Rahmanzade",
"doi-asserted-by": "crossref",
"journal-title": "Frontiers in Public Health",
"key": "22",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.tmaid.2020.101623",
"article-title": "Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis",
"author": "A. J. Rodriguez-Morales",
"doi-asserted-by": "crossref",
"journal-title": "Travel Medicine and Infectious Disease",
"key": "23",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1002/ptr.7004",
"article-title": "Oral nano‐curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease‐19 patients: an open label nonrandomized clinical trial",
"author": "N. Saber‐Moghaddam",
"doi-asserted-by": "crossref",
"first-page": "2616",
"issue": "5",
"journal-title": "Phytotherapy Research",
"key": "24",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7294",
"article-title": "A triple‐blind, placebo‐controlled, randomized clinical trial to evaluate the effect of curcumin‐containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID‐19 patients",
"author": "M. Hassaniazad",
"doi-asserted-by": "crossref",
"first-page": "6417",
"issue": "11",
"journal-title": "Phytotherapy Research",
"key": "25",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.intimp.2017.02.017",
"article-title": "Curcumin inhibiting Th17 cell differentiation by regulating the metabotropic glutamate receptor-4 expression on dendritic cells",
"author": "G. Zhao",
"doi-asserted-by": "crossref",
"first-page": "80",
"journal-title": "International Immunopharmacology",
"key": "26",
"volume": "46",
"year": "2017"
},
{
"DOI": "10.1186/s12876-019-1055-4",
"article-title": "Curcumin and inflammation in non-alcoholic fatty liver disease: a randomized, placebo controlled clinical trial",
"author": "S. Saadati",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "BMC Gastroenterology",
"key": "27",
"volume": "19",
"year": "2019"
},
{
"DOI": "10.1002/ptr.6328",
"article-title": "The effects of curcumin supplementation on high‐sensitivity C‐reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled trial",
"author": "M. Adibian",
"doi-asserted-by": "crossref",
"first-page": "1374",
"issue": "5",
"journal-title": "Phytotherapy Research",
"key": "28",
"volume": "33",
"year": "2019"
},
{
"DOI": "10.1016/j.intimp.2018.05.018",
"article-title": "Changes in Th17 cells function after nanocurcumin use to treat multiple sclerosis",
"author": "S. Dolati",
"doi-asserted-by": "crossref",
"first-page": "74",
"journal-title": "International Immunopharmacology",
"key": "29",
"volume": "61",
"year": "2018"
},
{
"article-title": "Immunomodulatory effects of curcumin in rheumatoid arthritis: evidence from molecular mechanisms to clinical outcomes",
"author": "S. Mohammadian Haftcheshmeh",
"first-page": "1",
"journal-title": "Reviews of Physiology, Biochemistry & Pharmacology",
"key": "30",
"year": "2020"
},
{
"DOI": "10.1080/10408398.2017.1358139",
"article-title": "Immune modulation by curcumin: the role of interleukin-10",
"author": "H. Mollazadeh",
"doi-asserted-by": "crossref",
"first-page": "89",
"issue": "1",
"journal-title": "Critical Reviews in Food Science and Nutrition",
"key": "31",
"volume": "59",
"year": "2019"
},
{
"DOI": "10.1002/jcp.30233",
"article-title": "Immunomodulatory effects of Nanocurcumin on Th17 cell responses in mild and severe COVID‐19 patients",
"author": "S. Tahmasebi",
"doi-asserted-by": "crossref",
"first-page": "5325",
"issue": "7",
"journal-title": "Journal of Cellular Physiology",
"key": "32",
"volume": "236",
"year": "2021"
},
{
"DOI": "10.1016/j.lfs.2021.119437",
"article-title": "Nanocurcumin improves Treg cell responses in patients with mild and severe SARS-CoV2",
"author": "S. Tahmasebi",
"doi-asserted-by": "crossref",
"journal-title": "Life Sciences",
"key": "33",
"volume": "276",
"year": "2021"
},
{
"DOI": "10.4049/jimmunol.163.6.3474",
"article-title": "Curcumin blocks cytokine-mediated NF-κB activation and proinflammatory gene expression by inhibiting inhibitory factor I-κB kinase activity",
"author": "C. Jobin",
"doi-asserted-by": "crossref",
"first-page": "3474",
"issue": "6",
"journal-title": "The Journal of Immunology",
"key": "34",
"volume": "163",
"year": "1999"
},
{
"DOI": "10.1001/archotol.135.2.190",
"article-title": "Suppression of interleukin 6 and 8 production in head and neck cancer cells with curcumin via inhibition of Iκβ kinase",
"author": "A. N. Cohen",
"doi-asserted-by": "crossref",
"first-page": "190",
"issue": "2",
"journal-title": "Archives of Otolaryngology-Head and Neck Surgery",
"key": "35",
"volume": "135",
"year": "2009"
},
{
"DOI": "10.1111/1440-1681.12848",
"article-title": "Curcumin ameliorates severe influenza pneumonia via attenuating lung injury and regulating macrophage cytokines production",
"author": "S. Han",
"doi-asserted-by": "crossref",
"first-page": "84",
"issue": "1",
"journal-title": "Clinical and Experimental Pharmacology and Physiology",
"key": "36",
"volume": "45",
"year": "2018"
},
{
"DOI": "10.1111/irv.12459",
"article-title": "Curcumin alleviates macrophage activation and lung inflammation induced by influenza virus infection through inhibiting the NF‐κB signaling pathway",
"author": "Y. Xu",
"doi-asserted-by": "crossref",
"first-page": "457",
"issue": "5",
"journal-title": "Influenza and other respiratory viruses",
"key": "37",
"volume": "11",
"year": "2017"
},
{
"DOI": "10.1080/21691401.2018.1446968",
"article-title": "Nano-gold displayed anti-inflammatory property via NF-kB pathways by suppressing COX-2 activity",
"author": "M. A. Khan",
"doi-asserted-by": "crossref",
"first-page": "1149",
"issue": "1",
"journal-title": "Artificial Cells, Nanomedicine, and Biotechnology",
"key": "38",
"volume": "46",
"year": "2018"
},
{
"DOI": "10.1371/journal.pone.0057285",
"article-title": "Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome",
"author": "S. Avasarala",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "PLoS One",
"key": "39",
"volume": "8",
"year": "2013"
},
{
"DOI": "10.3390/nu11092147",
"article-title": "Dietary curcumin: correlation between bioavailability and health potential",
"author": "M. Dei Cas",
"doi-asserted-by": "crossref",
"first-page": "2147",
"issue": "9",
"journal-title": "Nutrients",
"key": "40",
"volume": "11",
"year": "2019"
},
{
"author": "P. Safarzadeh Kozani",
"issue": "2",
"journal-title": "COVID-19 Vaccines in Iran",
"key": "41",
"volume": "2",
"year": "2022"
}
],
"reference-count": 41,
"references-count": 41,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.hindawi.com/journals/ijclp/2023/5734675/"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Efficacy of Nanocurcumin as an Add-On Treatment for Patients Hospitalized with COVID-19: A Double-Blind, Randomized Clinical Trial",
"type": "journal-article",
"volume": "2023"
}
