Phase III randomized clinical trial of BV-4051, an Ayurvedic polyherbal formulation in moderate SARS-CoV-2 infections and its impact on inflammatory biomarkers
et al., Phytotherapy Research, doi:10.1002/ptr.7683, CTRI/2020/09/027817, Nov 2022
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 208 moderate COVID-19 patients in India, 103 treated with a combination of turmeric, ashwagandha, boswellia, and ginger, showing improved recovery with treatment. The dose of curcumin is unknown and bioavailability may be poor.
This is the 16th of 21 COVID-19 RCTs for curcumin, which collectively show efficacy with p=0.0000022.
This is the 22nd of 28 COVID-19 controlled studies for curcumin, which collectively show efficacy with p=0.0000000061.
|
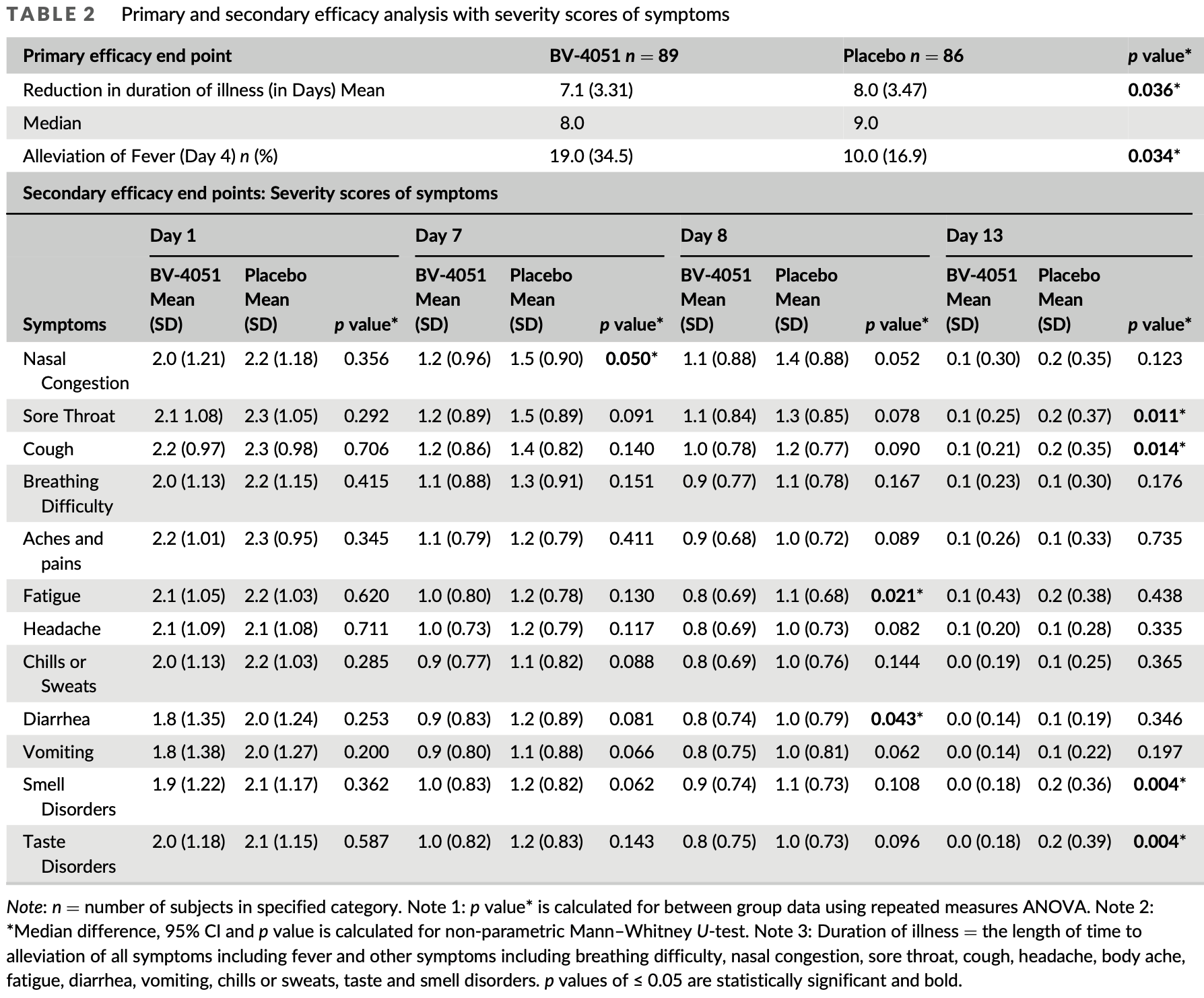
recovery time, 11.3% lower, relative time 0.89, p = 0.04, treatment 89, control 86.
|
|
fever, 11.0% lower, RR 0.89, p = 0.03, treatment 70 of 89 (78.7%), control 76 of 86 (88.4%), NNT 10, day 4.
|
|
congestion, 20.0% lower, RR 0.80, p = 0.05, treatment 89, control 86, mid-recovery, day 7.
|
|
sore throat, 20.0% lower, RR 0.80, p = 0.09, treatment 89, control 86, mid-recovery, day 7.
|
|
cough, 14.3% lower, RR 0.86, p = 0.14, treatment 89, control 86, mid-recovery, day 7.
|
|
dyspnea, 15.4% lower, RR 0.85, p = 0.15, treatment 89, control 86, mid-recovery, day 7.
|
|
pain, 8.3% lower, RR 0.92, p = 0.41, treatment 89, control 86, mid-recovery, day 7.
|
|
fatigue, 16.7% lower, RR 0.83, p = 0.13, treatment 89, control 86, mid-recovery, day 7.
|
|
headache, 16.7% lower, RR 0.83, p = 0.12, treatment 89, control 86, mid-recovery, day 7.
|
|
chills, 18.2% lower, RR 0.82, p = 0.09, treatment 89, control 86, mid-recovery, day 7.
|
|
diarrhea, 25.0% lower, RR 0.75, p = 0.08, treatment 89, control 86, mid-recovery, day 7.
|
|
vomiting, 18.2% lower, RR 0.82, p = 0.07, treatment 89, control 86, mid-recovery, day 7.
|
|
smell, 16.7% lower, RR 0.83, p = 0.06, treatment 89, control 86, mid-recovery, day 7.
|
|
taste, 16.7% lower, RR 0.83, p = 0.14, treatment 89, control 86, mid-recovery, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Chitre et al., 23 Nov 2022, Double Blind Randomized Controlled Trial, placebo-controlled, India, peer-reviewed, 8 authors, study period September 2020 - April 2021, this trial uses multiple treatments in the treatment arm (combined with ashwagandha, boswellia, ginger) - results of individual treatments may vary, trial CTRI/2020/09/027817.
Contact: dchitre@bioved.com, snadkarni@bioved.com.
Phase III randomized clinical trial of BV ‐4051, an Ayurvedic polyherbal formulation in moderate SARS‐CoV ‐2 infections and its impact on inflammatory biomarkers
Phytotherapy Research, doi:10.1002/ptr.7683
SARS-CoV-2 virus and its variants continue to be a challenge inspite of widespread vaccination and preventive measures. We hypothesized an oral, safe polyherbal formulation with antiinflammatory properties may improve the clinical outcome of this disease. BV-4051, a formulation from four Ayurvedic plants namely Ashwagandha, Boswellia, Ginger and Turmeric was used for the treatment of hospitalized moderate COVID-19 patients along with standard of care (SOC). Patients were randomly assigned to receive BV-4051 or placebo tablets for 14 days, at four sites in India during late 2020 to early 2021. Among 208 randomized subjects, 175 completed the study. In BV-4051 group the mean reduction in duration of illness (p = 0.036), alleviation and severity scores of several symptoms like fever, cough, smell, and taste disorders were statistically significant (p ≤ 0.05). A sub-set analysis of subjects treated with or without Remdesivir as SOC showed mean reduction in duration of illness in BV-4051 (p = 0.030), and severity scores (p ≤ 0.05). Mean difference in Interleukin-6 was statistically significant (p = 0.042) on BV-4051 without Remdesivir. BV-4051 may reduce duration of illness, symptoms severity, Interleukin-6, and prevent the incidence of COVID-19 complications. It may have an adjunctive effect with other SOC. Larger extensive clinical testing may give a better understanding of its effect.
CONFLICT OF INTEREST Deepa Chitre, MD has shares in Bioved Pharmaceuticals, Inc.; Satej Nadkarni, PhD and Debendranath Dey, PhD have stock options in Bioved Pharmaceuticals, Inc.
DATA AVAILABILITY STATEMENT All of the individual participant data collected during the trial, Study Protocol, Statistical Analytical Plan, Informed Consent Form and Clinical Study Report shall be available immediately after publication, for a period of 5 years. Researchers who provide an interest and methodologically sound proposal should contact dchitre@bioved.com with a copy to snadkarni@bioved.com. To gain access, researchers will need to sign a data access agreement. Placeholder Text
ORCID
SUPPORTING INFORMATION Additional supporting information can be found online in the Supporting Information section at the end of this article. How to cite this article: Chitre, D., Nadkarni, S., Jagtap, N., Tulle, R., Gitte, A., Rahate, P., Chaskar, S., & Dey, D. (2022) . Phase III randomized clinical trial of BV-4051, an Ayurvedic polyherbal formulation in moderate SARS-CoV-2 infections and its impact on inflammatory biomarkers. Phytotherapy Research, 1-10. https://doi.org/10.1002/ptr.7683
References
Abdalhamid, Donahue, Kamal-Ahmed, Strand, Mitchell et al., Identification of SARS-CoV-2 variants of concern in vaccine-breakthrough infections, Journal of Infection in Developing Countries, doi:10.3855/jidc.15458
Ablamunits, Lepsy, Blocking TNF signaling may save lives in COVID-19 infection, Molecular Biology Reports, doi:10.1007/s11033-022-07166-x
Accorsi, Britton, Fleming-Dutra, Smith, Shang et al., Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants, Journal of the American Medical Association, doi:10.1001/jama.2022.0470
Ahmad, Zahiruddin, Parveen, Basist, Parveen et al., Indian medicinal plants and formulations and their potential against COVID-19-preclinical and clinical research, Frontiers in Pharmacology, doi:10.3389/fphar.2020.578970
Batista, Foti, Anti-SARS-CoV-2 and anti-cytokine storm neutralizing antibody therapies against COVID-19: update, challenges, and perspectives, International Immunopharmacology, doi:10.1016/j.intimp.2021.108036
Bernal, Gomes Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, The New England Journal of Medicine, doi:10.1056/NEJMoa2116044
Birhane, Bressler, Chang, Clark, Dorough et al., COVID-19 Vaccine breakthrough infections reported to CDC -United States, Morbidity and Mortality Weekly Report, doi:10.15585/mmwr.mm7021e3
Boozari, Hosseinzadeh, Natural products for COVID-19 prevention and treatment regarding to previous coronavirus infections and novel studies, Phytotherapy Research, doi:10.1002/ptr.6873
Cairns, Dulko, Griffiths, Golan, Cohen et al., Efficacy of niclosamide vs placebo in SARS-CoV-2 respiratory viral clearance, viral shedding, and duration of symptoms among patients with mild to moderate COVID-19: A Phase 2 randomized clinical trial, JAMA Network Open, doi:10.1001/jamanetworkopen.2021.44942
Chang, Wang, Yeh, Shieh, Chiang, Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines, Journal of Ethnopharmacology, doi:10.1016/j.jep.2012.10.043
Chaskar, None
Chen, Lin, Zhao, Sun, Tian et al., A low-producing haplotype of interleukin-6 disrupting CTCF binding is protective against Severe COVID-19, Clinical Microbiology, doi:10.1128/mBio.01372-21
Chitre, Dey, Method for extraction of fractions containing pharmacologically active ingredients with less cytotoxicity from one or more plants, US Patent issued
Chopra, Lavin, Patwardhan, Chitre, Randomized double-blind placebo-controlled trial of efficacy and safety in rheumatoid arthritis, Journal of Rheumatology
Chopra, Lavin, Patwardhan, Chitre, Randomized double-blind placebo-controlled trial of efficacy and safety, in particular VAS and WOMAC, in osteoarthritis of Knee, Journal of Clinical Rheumatology
Chopra, Saluja, Kianifard, Chitre, Venugopalan, Long term effectiveness of RA-1 as a monotherapy and in combination with disease modifying anti-rheumatic drugs in the treatment of rheumatoid arthritis, Journal of Ayurveda and Integrative Medicine, doi:10.4103/0975-9476.72620
Dey, Chaskar, Athavale, Chitre, Acute and chronic toxicity, cytochrome p450 enzyme inhibition, and HERG channel blockade studies with a polyherbal, ayurvedic formulation for inflammation, doi:10.1155/2015/971982
Dey, Chaskar, Athavale, Chitre, Inhibition of LPSinduced TNF-α and NO production in mouse macrophage and inflammatory response in rat animal models by a novel ayurvedic formulation, BV-9238, Phytotherapy Research, doi:10.1002/ptr.5151
Dey, None
Emadi, Chua, Talwani, Bentzen, Baddley, Safety and efficacy of imatinib for hospitalized adults with COVID-19: A structured summary of a study protocol for a randomized controlled trial, Trials, doi:10.1186/s13063-020-04819-9
Fan, Gu, Alemi, Chinese herbal medicine for COVID-19 current evidence with systematic review and meta-analysis, Journal of Integrative Medicine, doi:10.1016/j.joim.2020.07.008
Feldman, Maini, Woody, Holgate, Winter et al., Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed, Lancet, doi:10.1016/S0140-6736(20)30858-8
Gao, Xiuying, Jiao, Wang, Reinfection and breakthrough Infection of SARS-CoV-2: An emerging challenge that is threatening our world, Infectious Diseases and Immunity, doi:10.1097/ID9.0000000000000027
Gomaa, Mohamed, Abd-Ellatief, Gomaa, Boswellic acids/Boswellia serrata extract as a potential COVID-19 therapeutic agent in the elderly, Inflammopharmacology, doi:10.1007/s10787-021-00841-8
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, The New England Journal of Medicine, doi:10.1056/nejmoa2116846
Han, Ma, Li, Liu, Zhao et al., Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors, Emerging Microbes & Infections, doi:10.1080/22221751.2020.1770129
Herold, Jurinovic, Arnreich, Lipworth, Hellmuth et al., Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19, The Journal of Allergy and Clinical Immunology, doi:10.1016/j.jaci.2020.05.008
Ichsyani, Ridhanya, Risanti, Desti, Ceria et al., Antiviral effects of Curcuma longa L. against dengue virus in vitro and in vivo, IOP Conference Series: Earth and Environmental Science, doi:10.1088/1755-1315/101/1/012005
Izadi, Brenner, Mahil, Dand, Yiu et al., Association between tumor necrosis factor inhibitors and the risk of hospitalization or death among patients with immune-mediated inflammatory disease and COVID-19, JAMA Network Open, doi:10.1001/jamanetworkopen.2021.29639
Kumar, Rai, Khan, Amit, Haque et al., Role of herbal medicines in the management of patients with Covid-19: A systematic review and meta-analysis of randomized controlled trials, Journal of Traditional and Complementary Medicine, doi:10.1016/j.jtcme.2022.01.002
Magro, SARS-CoV-2 and COVID-19: Is interleukin-6 (IL-6) the 'culprit lesion' of ARDS onset? What is there besides Tocilizumab?, Cytokine X, doi:10.1016/j.cytox.2020.100029
Mandel, Harari, Gurevich, Achiron, Cytokine prediction of mortality in COVID19 patients, Cytokine, doi:10.1016/j.cyto.2020.155190
Patil, Hupparage, Malgi, Deshpande, Patil et al., Dual inhibition of COVID-19 spike glycoprotein and main protease 3CLpro by Withanone from Withania somnifera, Chinese Herbal Medicines, doi:10.1016/j.chmed.2021.06.002
Rahate, None
Rasool, Khan, Ali, Anjum, Ahmed et al., Anti-Avian influenza virus H9N2 activity of aqueous extracts of Zingiber officinalis (Ginger) and Allium sativum (Garlic) in chick embryos, Pakistan Journal of Pharmaceutical Sciences
Saggam, Limgaokar, Borse, Dixit, Tillu et al., Withania somnifera (L.) Dunal: Opportunity for Clinical repurposing in COVID-19 Management, Frontiers in Pharmacology, doi:10.3389/fphar.2021.623795
Salama, Han, Yau, Reiss, Kramer et al., Tocilizumab in patients hospitalized with Covid-19 pneumonia, The New England Journal of Medicine, doi:10.1056/NEJMoa2030340
Sarkar, Chakrabarti, Dutta, Covid-19 infection in India: A comparative analysis of the second wave with the first wave, Pathogens, doi:10.3390/pathogens10091222
Sheikh, Pal, Javed, Shekhar, COVID-19 vaccination in developing nations: Challenges and opportunities for innovation, Infectious Disease Reports, doi:10.3390/idr13020041
Sornpet, Potha, Tragoolpua, Pringproa, Antiviral activity of five Asian medicinal plant crude extracts against highly pathogenic H5N1 avian influenza virus, Asian Pacific Journal of Tropical Medicine, doi:10.1016/j.aptm.2017.08.010
Srivastava, Rengaraju, Srivastava, Narayanan, Gupta et al., Efficacy of two siddha polyherbal decoctions, Nilavembu Kudineer and Kaba Sura Kudineer, along with standard allopathy treatment in the management of mild to moderate symptomatic COVID-19 patients-a double-blind, placebo-controlled, clinical trial, Trials, doi:10.1186/s13063-021-05478-0
Tiwari, Gupta, Pathak, A double-blind, randomized, placebo-controlled trail on the effect of Ashwagandha (Withania somnifera dunal) root extract in improving cardiorespiratory endurance and recovery in healthy athletic adults, Journal of Ethnopharmacology, doi:10.1016/j.jep.2021.113929
Treanor, Hayden, Vrooman, Barbarash, Bettis et al., Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial, Journal of the American Medical Association, doi:10.1001/jama.283.8.1016
Trunfio, Verga, Ghisetti, Burdino, Emanuele et al., Clinical phenotype and contagiousness of early breakthrough SARS-CoV-2 infections after BNT162b2 COVID-19 mRNA vaccine: A Parallel Cohort Study in Healthcare Workers, Vaccine, doi:10.3390/vaccines9121377
Udani, India grapples with second wave of Covid-19, Lancet, doi:10.1016/S2666-5247(21)00123-3
Whitley, Molnupiravir -A Step toward orally bioavailable therapies for Covid-19, The New England Journal of Medicine, doi:10.1056/NEJMe2117814
Xiong, Wang, Su, Cho, Xing, Chinese herbal medicine for coronavirus disease 2019: A systematic review and meta-analysis, Pharmacological Research, doi:10.1016/j.phrs.2020.105056
Zhao, Li, Zhong, Mechanism of action of small-molecule agents in ongoing clinical trials for SARS-CoV-2: A review, Frontiers in Pharmacology, doi:10.3389/fphar.2022.840639
DOI record:
{
"DOI": "10.1002/ptr.7683",
"ISSN": [
"0951-418X",
"1099-1573"
],
"URL": "http://dx.doi.org/10.1002/ptr.7683",
"alternative-id": [
"10.1002/ptr.7683"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-04-13"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-10-25"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-11-23"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9392-4709",
"affiliation": [
{
"name": "Bioved Pharmaceuticals, Inc. San Jose California USA"
}
],
"authenticated-orcid": false,
"family": "Chitre",
"given": "Deepa",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1545-1408",
"affiliation": [
{
"name": "Bioved Pharmaceuticals, Inc. San Jose California USA"
}
],
"authenticated-orcid": false,
"family": "Nadkarni",
"given": "Satej",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5271-0632",
"affiliation": [
{
"name": "Vishwaraj Hospital Loni Kalbhor, Pune India"
}
],
"authenticated-orcid": false,
"family": "Jagtap",
"given": "Namdev",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0845-9970",
"affiliation": [
{
"name": "Vedant Hospital Thane, Mumbai India"
}
],
"authenticated-orcid": false,
"family": "Tulle",
"given": "Rahul",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0908-4768",
"affiliation": [
{
"name": "Siddhivinayak Hospital Thane, Mumbai India"
}
],
"authenticated-orcid": false,
"family": "Gitte",
"given": "Amol",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5075-8245",
"affiliation": [
{
"name": "Seven Star Hospital Nagpur India"
}
],
"authenticated-orcid": false,
"family": "Rahate",
"given": "Prashant",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6488-2079",
"affiliation": [
{
"name": "Bioved Pharmaceuticals Pvt. Ltd. Pune India"
}
],
"authenticated-orcid": false,
"family": "Chaskar",
"given": "Sunetra",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9651-2080",
"affiliation": [
{
"name": "Bioved Pharmaceuticals, Inc. San Jose California USA"
}
],
"authenticated-orcid": false,
"family": "Dey",
"given": "Debendranath",
"sequence": "additional"
}
],
"container-title": "Phytotherapy Research",
"container-title-short": "Phytotherapy Research",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
11,
24
]
],
"date-time": "2022-11-24T13:18:20Z",
"timestamp": 1669295900000
},
"deposited": {
"date-parts": [
[
2022,
11,
24
]
],
"date-time": "2022-11-24T13:18:32Z",
"timestamp": 1669295912000
},
"indexed": {
"date-parts": [
[
2022,
11,
25
]
],
"date-time": "2022-11-25T05:51:48Z",
"timestamp": 1669355508498
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
11,
23
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
23
]
],
"date-time": "2022-11-23T00:00:00Z",
"timestamp": 1669161600000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
23
]
],
"date-time": "2022-11-23T00:00:00Z",
"timestamp": 1669161600000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7683",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/ptr.7683",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7683",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2022,
11,
23
]
]
},
"published-online": {
"date-parts": [
[
2022,
11,
23
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.3855/jidc.15458",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"DOI": "10.1007/s11033-022-07166-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"DOI": "10.1001/jama.2022.0470",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_4_1"
},
{
"DOI": "10.3389/fphar.2020.578970",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_1"
},
{
"DOI": "10.1016/j.intimp.2021.108036",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.15585/mmwr.mm7021e3",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1002/ptr.6873",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1001/jamanetworkopen.2021.44942",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.1016/j.jep.2012.10.043",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.1128/mBio.01372-21",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"key": "e_1_2_10_13_1",
"unstructured": "ChitreD.andDeyD.(2014).Method for extraction of fractions containing pharmacologically active ingredients with less cytotoxicity from one or more plants. US Patent issued 8 808 769.https://uspto.gov"
},
{
"article-title": "Randomized double‐blind placebo‐ controlled trial of efficacy and safety in rheumatoid arthritis",
"author": "Chopra A.",
"first-page": "1365",
"journal-title": "Journal of Rheumatology",
"key": "e_1_2_10_14_1",
"volume": "27",
"year": "2000"
},
{
"article-title": "Randomized double‐blind placebo‐controlled trial of efficacy and safety, in particular VAS and WOMAC, in osteoarthritis of Knee",
"author": "Chopra A.",
"first-page": "236",
"journal-title": "Journal of Clinical Rheumatology",
"key": "e_1_2_10_15_1",
"volume": "10",
"year": "2004"
},
{
"DOI": "10.4103/0975-9476.72620",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.1002/ptr.5151",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.1155/2015/971982",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.1186/s13063-020-04819-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.1016/j.joim.2020.07.008",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_1"
},
{
"DOI": "10.1016/S0140-6736(20)30858-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_1"
},
{
"DOI": "10.1097/ID9.0000000000000027",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
},
{
"DOI": "10.1007/s10787-021-00841-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_23_1"
},
{
"DOI": "10.1056/nejmoa2116846",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_24_1"
},
{
"DOI": "10.1080/22221751.2020.1770129",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_25_1"
},
{
"DOI": "10.1016/j.jaci.2020.05.008",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_26_1"
},
{
"DOI": "10.1088/1755-1315/101/1/012005",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_27_1"
},
{
"DOI": "10.1001/jamanetworkopen.2021.29639",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_28_1"
},
{
"DOI": "10.1016/j.jtcme.2022.01.002",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_29_1"
},
{
"DOI": "10.1016/j.cytox.2020.100029",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_30_1"
},
{
"DOI": "10.1016/j.cyto.2020.155190",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_31_1"
},
{
"DOI": "10.1016/j.chmed.2021.06.002",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_32_1"
},
{
"article-title": "Anti‐Avian influenza virus H9N2 activity of aqueous extracts of Zingiber officinalis (Ginger) and Allium sativum (Garlic) in chick embryos",
"author": "Rasool A.",
"first-page": "1341",
"issue": "4",
"journal-title": "Pakistan Journal of Pharmaceutical Sciences",
"key": "e_1_2_10_33_1",
"volume": "30",
"year": "2017"
},
{
"DOI": "10.3389/fphar.2021.623795",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_34_1"
},
{
"DOI": "10.1056/NEJMoa2030340",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_35_1"
},
{
"DOI": "10.3390/pathogens10091222",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_36_1"
},
{
"DOI": "10.3390/idr13020041",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_37_1"
},
{
"DOI": "10.1016/j.aptm.2017.08.010",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_38_1"
},
{
"DOI": "10.1186/s13063-021-05478-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_39_1"
},
{
"key": "e_1_2_10_40_1",
"unstructured": "Tamiflu® Prescribing Data(2012).TAMIFLU® (oseltamivir phosphate) capsules for oral use TAMIFLU® (oseltamivir phosphate) capsules for oral suspension. Full prescribing information. Page 18.https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021087s062lbl.pdf"
},
{
"DOI": "10.1016/j.jep.2021.113929",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_41_1"
},
{
"DOI": "10.1001/jama.283.8.1016",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_42_1"
},
{
"DOI": "10.3390/vaccines9121377",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_43_1"
},
{
"DOI": "10.1016/S2666-5247(21)00123-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_44_1"
},
{
"key": "e_1_2_10_45_1",
"unstructured": "United Nations News. (2022).UN analysis shows link between lack of vaccine equity and widening poverty gap.https://news.un.org/en/story/2022/03/1114762"
},
{
"DOI": "10.1056/NEJMe2117814",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_46_1"
},
{
"key": "e_1_2_10_47_1",
"unstructured": "World Bank Report(2022).https://www.worldbank.org/en/publication/wdr2022/brief/chapter-1-introduction-the-economic-impacts-of-the-covid-19-crisis"
},
{
"DOI": "10.1016/j.phrs.2020.105056",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_48_1"
},
{
"DOI": "10.3389/fphar.2022.840639",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_49_1"
}
],
"reference-count": 48,
"references-count": 48,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/ptr.7683"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology"
],
"subtitle": [],
"title": "Phase\n <scp>III</scp>\n randomized clinical trial of\n <scp>BV</scp>\n ‐4051, an Ayurvedic polyherbal formulation in moderate\n <scp>SARS‐CoV</scp>\n ‐2 infections and its impact on inflammatory biomarkers",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}
