Ivermectin for COVID-19 in adults in the community (PRINCIPLE): an open, randomised, controlled, adaptive platform trial of short- and longer-term outcomes
et al., Journal of Infection, doi:10.1016/j.jinf.2024.106130, PRINCIPLE, ISRCTN86534580, Feb 2024
See also
Significantly improved recovery and significantly lower risk of
long COVID with ivermectin, despite very late treatment, low-risk patients,
and poor administration.
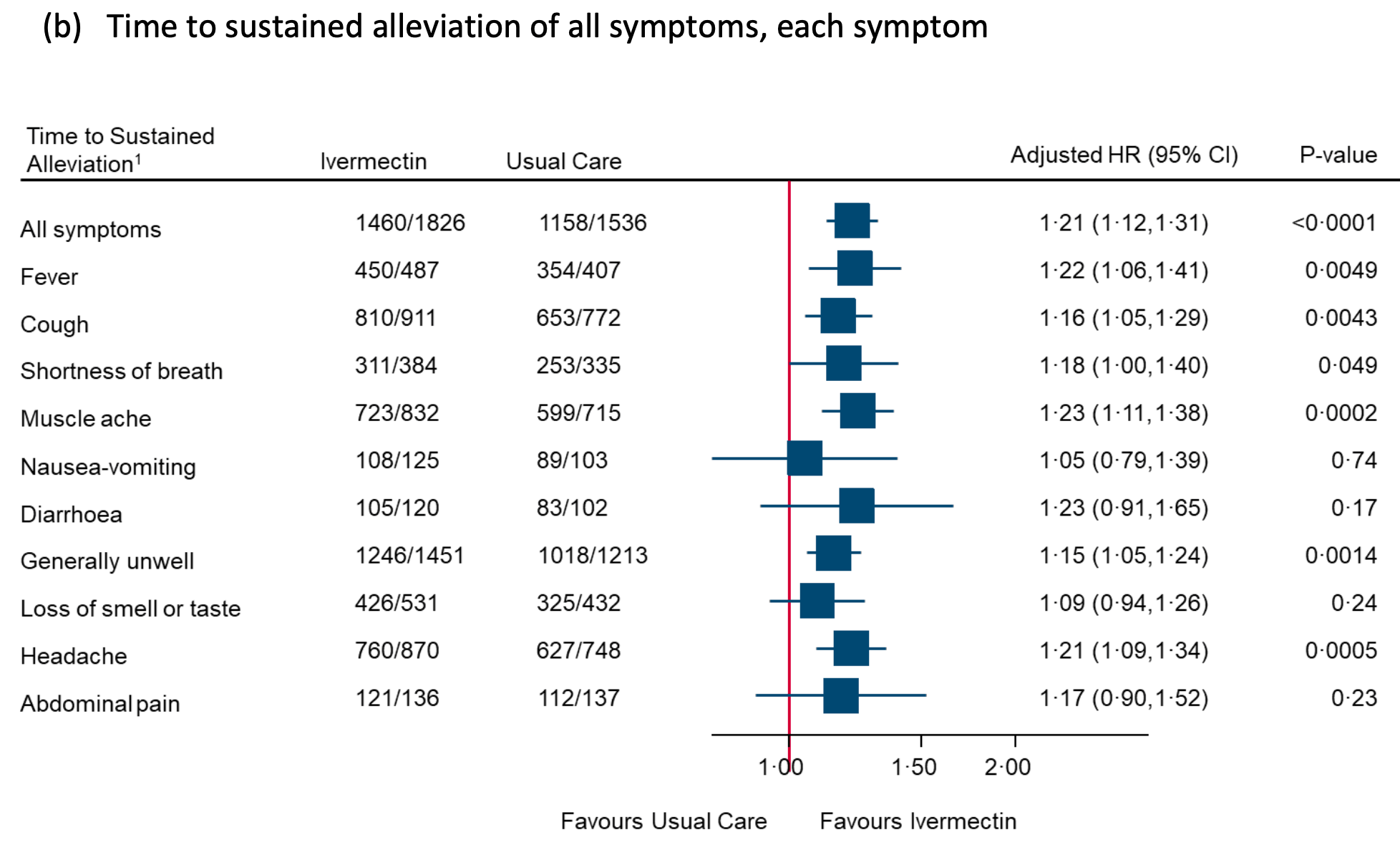
36% lower ongoing persistent COVID-19 specific symptoms,
p<0.0001 (details below). The primary recovery outcome shows superiority of
ivermectin (probability of superiority > 0.999), missing from the abstract
(details below). The p values for sustained recovery, early sustained
recovery, alleviation of all symptoms, and sustained alleviation are all <
0.0001.
The efficacy seen for ivermectin here is despite the trial
being the most clearly designed to fail trial, with major bias in design,
operation, analysis, and reporting. This trial is a great example of bias in
clinical trials which will be covered in detail in the future.
c19early.org
Designing trials to show or hide efficacy
Trials are often designed to show or hide efficacy. The contrast is clear in this case—these trials are for the same disease, from the same investigator, and overlap in time.
| Molnupiravir (PANORAMIC)1,2 | Ivermectin (PRINCIPLE)3 | |
|---|---|---|
| Investigator | Prof. Chris Butler | Prof. Chris Butler |
| Treatment delay | up to 5 days from onset | up to 14 days from onset |
| Population | 50+ or 18+ w/comorbidities — high-risk patients | 18+ (mid-trial change) — low-risk patients |
| Treatment | 5 days, 2x per day | 3 days, 1x per day, dose below real-world use |
| Administration | Per recommendation (with or without food) | Opposite of recommendation for COVID-19 - without food, greatly reducing concentration4,5 |
| Patients | 183 patients per day (25,783) | 11 patients per day (3,963 inc. concurrent control) |
| Publication delay | 4 months | 1.6 years (2.2 years from expected end) |
| Enrollment | Dec 2021 - Apr 2022 | May 2021 - Jul 2022 |
| Mutagenic | Yes6-20 | No |
| Trial funder stance | Approved | Denied—efficacy confirms liability for prior errors |
| Cost | $70721 | <$122 |
| Merck profit | >$8B sales to date23, estimated $18 to produce24 | No potential, many low-cost manufacturers |
| Design | Design better to show efficacy | Design better to hide efficacy |
Responses: authors have not responded to any of these issues.
36% lower long COVID hidden in appendix.
Page 358 in the appendix shows 36% lower ongoing persistent COVID-19 specific symptoms (p<0.0001) when combining the individual symptom results. The paper reports a 28% reduction (p=0.015), not mentioned in the abstract or conclusion. This appears to be a one of any symptom analysis, effectively increasing the weight of the more common “fatigue”, reducing the perceived effect (the difference does not appear to be due to adjustments - the adjustments in Table S6 to Table S39 make minimal difference). This is for very late and poorly administered treatment taken by only 89% of patients in a relatively low-risk population - benefits may be much greater with recommended usage and in high-risk patients.
Undisclosed null-biased Bayesian prior masks superiority.
Prof. Sander Greenland demonstrates (starting on slide 38)62 that the Bayesian model applied an extremely tight, null-centered prior that was never reported in the paper. This prior pulls the frequentist hazard ratio for time-to-recovery down and slashes the posterior probability that the benefit reaches the trial's own “clinically meaningful” threshold of HR≥1.2. Even with the shrinkage, the posterior probability of any benefit exceeds 0.9999, well above the protocol's 0.99 superiority bar, however authors label ivermectin “unlikely to provide clinically meaningful improvement.” Greenland argues that the unexplained prior acts like a hidden penalty, lacks empirical justification, and biases the analysis toward the null, thereby allowing the authors to downplay results their own decision rules would otherwise classify as superior. Author notes the strong priors are not justified by previous trials and suggests that they reflect social pressure to discredit ivermectin.
Ad-hoc "clinically meaningful" threshold contradicted by same authors later in the same year for an ARI RCT.
In the Immune Defence RCT on common acute respiratory illness (ARI)63, with two of the same senior investigators including the PI for PRINCIPLE, a similar reduction in illness-days from over-the-counter nasal sprays is highlighted as a clear therapeutic success without any extra “clinically meaningful” hurdle, is referred to as "clinically significant", and is promoted in the abstract and discussion64. By contrast, PRINCIPLE imposes a bespoke threshold and a very tight sceptical prior. This inverts the expected risk-benefit logic: improved recovery and minimizing viral replication is likely to be more significant for COVID-19, which showed higher risk of severity, systemic viral spread, and long-term sequelae.
False claims for long-term outcomes and recovery.
Authors claim that ivermectin is "unlikely to provide clinically meaningful improvement in recovery, hospital admissions, or longer-term outcomes", which is contradicted by their results. 36% (or the 28% from the author's calculation) lower long COVID is clearly clinical and very meaningful - it would represent an enormous global reduction in morbidity if adopted. The significantly faster recovery is also clearly clinically meaningful.
Significantly improved recovery is strongly associated with significantly lower mortality.
Authors report highly statistically significant improved recovery but claim no clinical relevance. Across all 216 treatments we cover, improved recovery is very significantly associated with lower mortality, p<0.000000001 (from all studies that report both).
Superiority of ivermectin hidden.
The protocol states "If the Bayesian posterior probability of superiority (a log hazards ratio greater than 0 corresponding to quicker recovery) for a treatment versus Usual Care is sufficiently large (e.g. ≥ 0.99), the null hypothesis will be rejected and the intervention will be deemed superior..". The intervention is superior (probability > 0.9999), yet there was no press release and immediate call for use, and this is not even mentioned in the abstract or conclusion.
Superiority of budesonide not hidden.
The same trial's budesonide arm did not hide the superiority: "There was a benefit in time to first self-reported recovery ... with a probability of superiority greater than 0·999, meeting the prespecified superiority threshold of 0·99" (in the abstract).
Budesonide press release within 12 days.
The superiority for recovery for budesonide was announced within 12 days of trial completion56,68. Slow but perhaps acceptable. Ethical and moral obligations mandate release as soon as possible. However for ivermectin, the results were hidden for around 600 days, and then misrepresented.
Similar results, opposite conclusion.
Of the 6 arms reporting results (HCQ is still missing), three show superiority on the primary recovery outcome. Comparing ivermectin and budesonide, which both show probability of superiority >0.999 and similar results - several individual recovery results are better for ivermectin than budesonide - concurrent and COVID+: time to allevation of all symptoms, time to sustained alleviation of all symptoms, WHO5 wellbeing at day 28, all concurrent: time to allevation of all symptoms, time to sustained alleviation of all symptoms, and time to initial reduction of severity all show better results for ivermectin than budesonide. However the conclusions for each are the opposite - for budesonide authors concluded superiority, for ivermectin they conclude that ivermectin "is unlikely to provide clinically meaningful improvement in recovery". Note improvements are higher with ivermectin and the primary recovery outcome for several subgroups related to baseline severity - illness duration, baseline severity, and respiratory illness.
Pre-specified "meaningful effect" only for interim futility.
The protocol (even the post-hoc versions) only mentions clinically meaningful effects in terms of interim futility analyses: "If the Bayesian posterior probability of a clinically meaningful treatment effect is sufficiently small (e.g. < 0.01) for the first co-primary endpoint (time to recovery), the intervention arm may be dropped from the study for futility". In the body of the paper authors do note that the pre-specified HR of ≥1.2 was only for futility evaluation, however the abstract drops this, implying that there was a pre-specified HR of 1.2 for superiority.
Meaningful effect was 1.5 days for other arms.
Authors did not mention a "meaningful effect" for budesonide, but added a "meaningful effect" of 1.5 days for colchicine, azithromycin, and doxycycline - which was sufficient to ensure very low probabilities for those arms. For ivermectin they show an improvement of 2.06 days, i.e., clinically meaningful according to the authors for all prior arms (for most other people, smaller improvements are also clinically meaningful, and as above the improvements translate into lower mortality for high-risk patients). Additionally, the poor design of the trial means the actual improvement for recommended usage is likely much greater.
Pre-specified "meaningful effect" probability changed 0.01->0.25.
In June 2022, in view of interim (if not all at the time) results, authors changed the 0.01 probability to 0.25 (just above the 0.22 for the one outcome at the time). Authors note this change is only for ivermectin and favipiravir, and will return to 0.01 for future arms (appendix page 169). Authors claim a rationale for the change is in "Appendix A" but this appears to be missing.
Hospitalization/death probability of meaningful effect.
For hospitalization/death authors previously used a threshold of 2% for calculating the probability of meaningful effect. For ivermectin they changed it to 20%. Consider the families of 20% or 2% of COVID-19 deaths, perhaps 4 million or 400,000 people based on an estimated 20 million total. Do they believe those deaths were not clinically meaningful?
10x lower accuracy in reported results.
For budesonide authors reported key results with 10x greater accuracy, for example 10.9 vs. 13.3 days time to recovery, wherease for ivermectin all times are reported as integers, e.g., 14 vs. 16 days. This may be used to hide differences and to reduce efficacy (e.g., 3.6 vs. 5.4 becomes 4 vs. 5 and 3.6 vs. 4.4 becomes 4 vs. 4).
Adverse event data missing.
Other than a count of hospitalizations, no adverse event data was reported.
Mortality results missing for concurrent control arm.
Authors do not provide the main mortality results for the concurrent control arm.
Details of hospitalization/death missing, many could be prior to treatment.
Authors provide no details on the hospitalizations and deaths. Given the remote nature of the trial, the enrollment of very late stage patients, the very low event rate, and the very delayed time between patients signing up and actual enrollment indicated by some participants, many of the hospitalizations may have happened before medication was delivered and taken. This is supported by the subgroup analysis showing that patients >7 days from onset (likely >8-9 days to treatment initiation) contributed more to the ivermectin events. The patient information sheet for molnupiravir states that medication will be delivered by the next day2,69, while the patient information sheet for ivermectin has deleted "next day" only stating that medication will be delivered70.
False claim on administration.
Authors falsely claim that "the influence of food on absorption is not known"4. Guzzo et al.5 show that the plasma concentration of ivermectin is much higher when administered with food (geometric mean AUC 2.6 times higher). This is from 2002 and well-known among ivermectin researchers.
Results delayed 600 days.
Results were delayed around 600 days from the expected announcement time, with no reasonable excuse for hiding such positive results (or any results).
Authors coverage of prior research extremely biased and cherry-picked.
Authors perform extreme cherry-picking on their discussion of previous research, and even then highly misrepresent those studies. For example, authors discuss the TOGETHER trial, without mentioning the known impossible data, refusal to release data despite pledging to, external sharing of results during the trial, randomization/blinding failure, and many protocol violations; and without mentioning that the principal investigator said that "There is a clear signal that IVM works in COVID patients.." in private71.
Very late treatment.
Patients were enrolled up to 14 days after the onset of symptoms. Extensive research for COVID-19 and other viral diseases show that early antiviral treatment is critical.
Inclusion changed from 50+ to 18+ w/COVID dyspnea or comorbidity before start of ivermectin arm.
Inclusion was originally 50+ w/comorbidity or 65+, but was changed to 18+ w/COVID dyspnea or comorbidity or 65+ before the start of the ivermectin arm. The move to low-risk patients was specific to the ivermectin and favipiravir arms only72.
Mid-trial change to include lower risk patients.
Inclusion criteria were modified mid-trial to allow enrolling anyone 18+, i.e. very low risk patients. This change is first seen in protocol 9.0 on July 12, 202141
Exteme conflict of interest.
The chief investigator is also chief investigator for the PANORAMIC molnupiravir trial, with overlapping dates, and 7.2B+ financial conflict of interest between the two treatments.
Pause due to supply but medicine was stored at every study site.
The trial claimed to pause due to a supply problem but medicine was stored at every site73, It is unlikely that all sites would have run out, if there was no supply in some locations, recruitment could have continued at other locations.
Supply issue contradicated by manufacturer.
The trial was paused with a reported supply issue, however the manufacturer stated that there were no supply issues.
Design favors null result in contrast to molnupiravir trial by the same chief investigator.
Treatment delay, inclusion criteria, dosing, administration, and target size all show a design better for efficacy for molnupiravir, and worse for efficacy for ivermectin. Both trials have the same chief investigator and overlapping dates.
Other arm results delayed over 5 years.
The HCQ arm results were withheld for over 5 years52, consistent with extreme bias against reporting efficacy of low-cost treatments that were denied to the public by the funder.
Inclusion changed from 7 to 14 days.
Inclusion was originally within 7 days of symptoms, but was changed to 14 days, compared to the molnupiravir trial which was started with 5 days74.
"Gate-keeping" protection of serious outcome evaluation.
Authors declare a "gate-keeping" strategy to prevent evaluation of hospitalization/death if the recovery time difference is not significant4. Authors claim benefit for serious outcomes is unlikely without statistically significant benefit for recovery time, which is not logical, especially with low prevalence of progression - consider for example an intervention that prevented progression to mortality by 100%, but has no effect on resolution of a specific symptom, e.g., cough. The trial did not always have this gate-keeping strategy - protocol 4.0 had hospitalization/death as the primary outcome and protocol 5.0 added the new strategy (this was a post-hoc change for azithromycin and doxycycline related to the recruitment of low-risk patients and low event rates).
Long delay between registration and enrollment.
One participant reports filling out a form for the trial at the time of receiving a positive PCR result and not being called until much later on day 11 of COVID to complete enrollment75. A second participant reports waiting 9 days after online registration to receive an enrollment phone call76,77.
Subject to participant fraud.
There is no requirement for participants to have a face-to-face visit as part of trial participation. The self-reported design and the potential lack of professional medical examination results for many patients opens this kind of remote trial to participant fraud, which may be significant due to extreme politicization in the study country. Participant fraud has been reported for two other remote trials78,79, involving submission of fake surveys and repeated signups. Authors do not provide any information on attempts to limit participant fraud.
Inconsistent analysis - Bayesian vs. frequentist statistics.
The protocol specifies Bayesian analysis which is used for some outcomes. However, authors have used frequentist statistics for other outcomes, with no known reason. This results in avoiding reporting Bayesian probability of superiority showing superiority of treatment for those outcomes.
Age 65+ reduced from 47% to 16% for ivermectin.
From the non-concurrent to concurrent populations (Table 1), we can see a dramatic change in the population. 47% of patients were over 65 in the control group prior to the ivermectin arm. For ivermectin this was reduced to 16% (focusing on low-risk patients is one method to reduce the chance of showing a benefit).
Fatigue not included in secondary outcome symptoms, dominates long COVID results.
The protocol (page 17 of 55, section 2.3.2.2, appendix page 119) does not include fatigue as a symptom. It's unclear if this was added later to dilute long COVID results (the included long-term follow-up SAP is post-hoc, dated June 2023). The more general fatigue, which may not be related to COVID-19, is by far the most common long-COVID symptom reported and dilutes efficacy—excluding fatigue ongoing persistent COVID-19 specific symptoms are 43% lower with treatment.
Superiority and futility rules not mutually exclusive.
The superiority and futility rules are not even mutually exclusive—the treatment can be considered superior and futile at the same time (superiority: Pr(HR > 1) ≥ 0.99, "meaningful-effect” futility: Pr(HR ≥ 1.2) ≤ 0.01"80).
Delivery delay reduces perceived effect.
Recovery is defined as the time from randomization, however there are additional delays between randomization and delivery, and between delivery and the patient taking the medication. This has the effect of reducing the perceived effect of treatment. For example, the median time to alleviation of all symptoms was 4 and 5 days respectively, or 20% faster with treatment. If the delay until treatment is one day, this becomes 3 and 4 days, for 25% improvement, with progressively greater improvement with longer delays. The patient information sheet for molnupiravir states that medication will be delivered by the next day2,69, while the patient information sheet for ivermectin has deleted "next day" only stating that medication will be delivered70.
Recovery subgroup forest plot for all arms except ivermectin.
The main paper shows a forest plot with subgroups of the primary recovery outcome for all arms (colchicine, budesonide, azithromycin, doxycycline) except ivermectin. For ivermectin, authors only show the hospitalization/death forest plot in the main paper. The recovery results show superiority of ivermectin.
Even faster recovery with greater baseline severity.
Figure S2a shows that longer duration, at least one baseline major severity item, and respiratory illness all show greater improvement for recovery, consistent with the greater room for improvement in more severe cases. If authors truly believed that HR 1.2 is a required and valid threshold, they should not be ruling out use for higher-risk cases.
Lack of recovery inverted to reduce effect size.
Authors report the number of patients fully recovered at 3, 6, 12 months. It is typical to compare the risk of bad outcomes (failure to recover, hospitalization, death, etc.), however authors compare good outcomes. Authors method shows a significant improvement of 6% at 12 months, however the typical analysis shows a much larger 18% reduction in failure to recover. Consider a recovery rate of 90% in the control group, by the author's method it would be impossible for any intervention to create the HR ≥1.2 that they added.
Slow delivery.
The patient information sheet for molnupiravir states that medication will be delivered by the next day2,69, while the patient information sheet for ivermectin has deleted "next day" only stating that medication will be delivered70.
Administration on an empty stomach.
Authors instructed patients that "no food should be taken two hours before or after administration"4. Guzzo et al.5, from 2002 and well known to ivermectin investigators, shows that the plasma concentration of ivermectin is much higher when administered with food (geometric mean AUC 2.6 times higher).
Mismatch with original proposal.
The original proposal for the trial starts with: "COVID-19 disproportionately affects people over 50 years old with comorbidities and those over 65 years old. The infection causes considerable morbidity and mortality in this population group in particular."81, yet authors later modified the trial to include anyone 18+.
Eligibility criteria worse than concurrent favipiravir arm.
In addition to inferior eligibility compared with molnupiravir, eligibility was even inferior to the concurrent favipiravir arm, with ivermectin further favoring a null result. As of July 8, 2021 favipiravir started at 50+ while ivermectin started at 18+ w/dyspnea or comorbidity as per the trial newsletter82.
Recruitment questions varied.
Recruitment questions varied, for example the video instructions for ambulatory care show the system asking about only two symptons - cough and fever. Notably, cough may be less responsive to treatment and increased enrollment based on cough may reduce the chance of showing efficacy83.
Ivermectin from source chosen has shown lower efficacy.
Authors chose to source ivermectin from Edenbridge, which ranked 7 out of 11 brands in In Vitro tests for antiparasitic efficacy84, requiring 5 days compared to 2 days for the best performing brand, and 3 days for 4 other brands.
Ability to pickup medication quickly removed from information sheet.
Earlier versions of the patient information sheet (e.g., v3.185) allowed patients to pickup the medication from a local pharmacy instead of waiting for delivery. This was removed sometime before the ivermectin arm and the sheet now only lists delivery, excluding the possibility of very quick pickup of the medication after enrollment86.
Only three different doses, lower μg/kg dose for higher weights.
Only three different doses were used: 45-64kg (18mg), 65-84kg (24mg), and ≥84kg (30mg)4. Patients with higher weights will have progressively lower μg/kg dosing.
Duration == 7 missing.
The subgroup forest plot shows illness duration <7 and >7, without specifying what happens with == 7. The papers for other arms shows ≤7 and >7.
Efficacy not due to open label design.
Some people with strong prior claims of no efficacy have claimed that the efficacy here is due to the open label design. Moustgaard et al. showed there was no evidence to support this from analysis of 142 meta-analyses covering 1,153 trials. However, in this case we have multiple arms from the same exact trial that show this is not the case (which authors acknowledge in the paper, noting no evidence from the other arms). Moreover, any effect would be reversed because at the time and in the study country, the government and almost all media claimed that ivermectin was ineffective (and contrary information was censored).
The PANORAMIC trial for molnupiravir and the PRINCIPLE trial
for ivermectin provide a good example of extreme bias in trial design. For
molnupiravir investigators randomized 25,000 patients a median of 2 days from
onset25. For ivermectin, they allow inclusion up to 14 days
after onset — a delay incompatible with the recommended use of antiviral
treatments, and incompatible with current real-world protocols. This delay
alone would normally be more than enough to guarantee a null effect for an
early treatment. However, authors also bias the population, treatment dose and
duration, treatment administration, and sample size to favor a null result
with ivermectin.
PANORAMIC and PRINCIPLE have the same chief investigator and
primary contact1,3,
and the molnupiravir and ivermectin arms overlap in time.
It is unclear why results were not released December 2021, why
a reported supply issue was contradicted by the manufacturer, why the trial
continued, and why results were delayed 19 months26.
Ivermectin was added to the PRINCIPLE trial on May 12, 202127
(June 2021 according to28),
and favipiravir on April 26, 202127.
4,731 patients were enrolled as of April 8, 202129,
by which time the azithromycin, doxycycline, and budesonide arms had
completed. The colchicine arm had been running for one month and was later
terminated with 156 patients. With an estimated enrollment of 1,000 per arm for
ivermectin, favipiravir, and concurrent control, the trial would end when total
enrollment reached around 8,000.
8,010 patients were enrolled and ivermectin was removed from
the list of treatments under investigation on the website on or before Dec 2,
202130,31,
suggesting that enrollment was complete and results would be available shortly
thereafter.
By Dec 9 ivermectin was added back to the list32
with a note that the arm was paused due to supply issues. MedPage Today
reported on the pause on Dec 1433. Notably, Merck's
statement at the time shows a significantly softer stance compared to their
previous comments33,34.
The reported supply issue is unusual - trials normally secure
medication in advance, the reported trial manufacturer stated there were no
supply issues35,
investigators did not respond to journalist queries, there was reportedly no
response to Freedom of Information requests36,
alternate sources of ivermectin in the specified dosage were readily
available, and there was no need for an identical match in appearance. The trial
manufacturer was Edenbridge37,
participants received standard standard foil strips38,39.
The trial later restarted the ivermectin arm. As of January 27,
2022, the trial was paused without explanation. As of February 11, 2022, the
trial was open intermittently (twice daily between Sunday and Thursday), a
change which further decreases the chance of participants receiving relatively
early treatment. Delaying and restarting the trial at a later time may also
reduce observed efficacy due to less severe variants in combination with the
trial design.
We are pre-specifying subgroup analysis for enrollment up to
Dec 2, 2021, for treatment within 2 days of onset, and for treatment of high
risk patients (as originally defined by the trial).
| PRINCIPLE trial timeline | |
| Date | Change |
|---|---|
| March 22, 2020 | Inclusion ≤7 days, age 50+ w/comorbidity or 65+. |
| June 16, 2020 | Inclusion changed to ≤14 days. |
| February 14, 2021 | Inclusion changed to 18+ w/COVID dyspnea or comorbidity or 65+40. |
| May 12, 2021 | Ivermectin listed as current intervention in protocol27. |
| June 2021 | Ivermectin added according to web site28. |
| Jul 12, 2021 | Inclusion changed to 18+41. |
| December 2021 | Anticipated completion of ivermectin arm. |
| December 3, 2021 | Ivermectin arm ends, removed from web site between Dec 2 and Dec 331, |
| December 2021 | No press release or rapid top-tier publication, indicating positive results. |
| December 9, 2021 | Ivermectin added back to web site with claim of pause due to supply issues. |
| December 14, 2021 | Trial does not respond to MedPage Today regarding supply problems. A statement from Merck is dramatically different to their previous position and is consistent with them knowing that a trial they cannot ignore has positive results and them being unsure if they can suppress the results44. |
| December 25, 2021 | The trial supplier, Edenbridge, denies any supply issue. Prof. Chris Butler declines to comment45. The trial used standard widely available tablets38,39. |
| January 14, 2022 | Prof. Paul Little, TSC chair, is removed from the trial in protocol version 1346. The TSC is responsible for reporting ethical issues. |
| January 27, 2022 | Trial paused without explanation47. |
| February 11, 2022 | Trial only open intermittently (twice daily between Sunday and Thursday), adding further enrollment delays48. |
| July 8, 2022 | Extended ivermectin arm ends49. |
| July 2022 | No press release or rapid top-tier publication, indicating positive results. |
| June 2, 2023 | Sometime between May 2 and June 2, authors add a note on the web site indicating that, against protocol, they are delaying and will release in a rigorous and transparent way after extended 1 year followup ends in July50,51. Note that the analysis code for professional trials is written and tested in advance. |
| November 6, 2023 | Links to the protocol, amendments, and other supporting documents were removed from the web site. |
| December 2023 | Still no results or update. A link was added to a version 14 of the protocol dated August 8, 2022 (after all arms had completed). The link does not work, pointing to an internal University of Oxford site. The latest version available is 13.0, dated January 14, 202246 (during the ivermectin arm, after the expected end in December 2021). |
c19early.org
PRINCIPLE trial selective reporting delays
Results for effective but politically inconvenient treatments were withheld for very long periods.
| Treatment patients | Duration | Results delay | |
|---|---|---|---|
| HCQ52 | 413 | 2 months | Over 5 years52 |
| Azithromycin53 | 540 | 6 months | 56 days54 |
| Budesonide55 | 1,073 | 4 months | 12 days56 |
| Doxycycline57 | 780 | 5 months | 42 days54 |
| Colchicine58 | 156 | 3 months | 120 days59 |
| Ivermectin60 | 2,157 | 14 months | 600 days (810 days from ~1,000 per arm enrollment) |
| Favipiravir61 | ~2,250 | 15 months | 780 days (1,000 days from ~1,000 per arm enrollment) |
This is the 48th of 53 COVID-19 RCTs for ivermectin, which collectively show efficacy with p=0.000000087.
This is the 101st of 106 COVID-19 controlled studies for ivermectin, which collectively show efficacy with p<0.0000000001.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments88.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death/hospitalization, 1.0% higher, HR 1.01, p = 0.97, treatment 34 of 2,157 (1.6%), control 27 of 1,806 (1.5%), concurrent and eligible, primary outcome.
|
|
risk of death, 62.3% lower, RR 0.38, p = 0.18, treatment 3 of 2,157 (0.1%), control 12 of 3,256 (0.4%), NNT 436, non-concurrent, authors do not provide details for concurrent deaths.
|
|
risk of mechanical ventilation, 151.4% higher, RR 2.51, p = 0.63, treatment 3 of 2,149 (0.1%), control 1 of 1,801 (0.1%).
|
|
risk of ICU admission, 319.0% higher, RR 4.19, p = 0.23, treatment 5 of 2,149 (0.2%), control 1 of 1,801 (0.1%).
|
|
time to sustained recovery, 16.0% lower, HR 0.84, p < 0.001, treatment 2,157, control 1,806, inverted to make HR<1 favor treatment, concurrent and eligible.
|
|
early sustained recovery, 23.1% lower, HR 0.77, p < 0.001, treatment 2,154, control 1,805, inverted to make HR<1 favor treatment, concurrent and eligible.
|
|
sustained alleviation, 17.4% lower, HR 0.83, p < 0.001, treatment 1,826, control 1,535, inverted to make HR<1 favor treatment, concurrent and eligible.
|
|
alleviation of all symptoms, 16.0% lower, HR 0.84, p < 0.001, treatment 2,154, control 1,805, inverted to make HR<1 favor treatment, concurrent and eligible.
|
|
first reported recovery, 13.0% lower, HR 0.87, p < 0.001, treatment 2,157, control 1,806, inverted to make HR<1 favor treatment, concurrent and eligible, primary outcome.
|
|
no recovery at 3/6/12 months, 28.0% lower, HR 0.72, p = 0.02, treatment 94 of 1,941 (4.8%), control 109 of 1,624 (6.7%), NNT 54.
|
|
risk of no recovery, 17.6% lower, RR 0.82, p = 0.001, treatment 417 of 1,848 (22.6%), control 420 of 1,533 (27.4%), NNT 21, day 365.
|
|
risk of long COVID, 36.3% lower, RR 0.64, p < 0.001, treatment 1,886, control 1,567, all symptoms combined.
|
|
risk of long COVID, 63.2% higher, RR 1.63, p = 1.00, treatment 2 of 1,507 (0.1%), control 1 of 1,230 (0.1%), ongoing/persistent, fever.
|
|
risk of long COVID, 72.7% lower, RR 0.27, p = 0.33, treatment 1 of 1,819 (0.1%), control 3 of 1,489 (0.2%), NNT 683, ongoing/persistent, cough.
|
|
risk of long COVID, 50.1% lower, RR 0.50, p = 0.04, treatment 15 of 1,886 (0.8%), control 25 of 1,567 (1.6%), NNT 125, ongoing/persistent, dyspnea.
|
|
risk of long COVID, 35.3% higher, RR 1.35, p = 0.74, treatment 5 of 1,808 (0.3%), control 3 of 1,468 (0.2%), ongoing/persistent, chest pain.
|
|
risk of long COVID, 25.2% lower, RR 0.75, p = 0.36, treatment 21 of 1,831 (1.1%), control 23 of 1,501 (1.5%), NNT 259, ongoing/persistent, smell.
|
|
risk of long COVID, 58.7% lower, RR 0.41, p = 0.42, treatment 2 of 1,821 (0.1%), control 4 of 1,503 (0.3%), NNT 640, ongoing/persistent, diarrhoea.
|
|
risk of long COVID, 70.2% lower, RR 0.30, p < 0.001, treatment 10 of 1,739 (0.6%), control 27 of 1,400 (1.9%), NNT 74, ongoing/persistent, headache.
|
|
risk of long COVID, 29.8% lower, RR 0.70, p = 0.25, treatment 23 of 1,739 (1.3%), control 27 of 1,433 (1.9%), NNT 178, ongoing/persistent, muscle ache.
|
|
risk of long COVID, 47.1% lower, RR 0.53, p = 0.03, treatment 19 of 1,724 (1.1%), control 30 of 1,441 (2.1%), NNT 102, ongoing/persistent, generally unwell.
|
|
risk of long COVID, 20.1% lower, RR 0.80, p = 0.19, treatment 66 of 1,876 (3.5%), control 69 of 1,567 (4.4%), NNT 113, ongoing/persistent, fatigue.
|
|
risk of long COVID, 42.9% lower, RR 0.57, p < 0.001, treatment 1,886, control 1,567, all symptoms combined.
|
|
risk of long COVID, 63.2% higher, RR 1.63, p = 1.00, treatment 2 of 1,507 (0.1%), control 1 of 1,230 (0.1%), ongoing/persistent, fever.
|
|
risk of long COVID, 72.7% lower, RR 0.27, p = 0.33, treatment 1 of 1,819 (0.1%), control 3 of 1,489 (0.2%), NNT 683, ongoing/persistent, cough.
|
|
risk of long COVID, 50.1% lower, RR 0.50, p = 0.04, treatment 15 of 1,886 (0.8%), control 25 of 1,567 (1.6%), NNT 125, ongoing/persistent, dyspnea.
|
|
risk of long COVID, 35.3% higher, RR 1.35, p = 0.74, treatment 5 of 1,808 (0.3%), control 3 of 1,468 (0.2%), ongoing/persistent, chest pain.
|
|
risk of long COVID, 25.2% lower, RR 0.75, p = 0.36, treatment 21 of 1,831 (1.1%), control 23 of 1,501 (1.5%), NNT 259, ongoing/persistent, smell.
|
|
risk of long COVID, 58.7% lower, RR 0.41, p = 0.42, treatment 2 of 1,821 (0.1%), control 4 of 1,503 (0.3%), NNT 640, ongoing/persistent, diarrhoea.
|
|
risk of long COVID, 70.2% lower, RR 0.30, p < 0.001, treatment 10 of 1,739 (0.6%), control 27 of 1,400 (1.9%), NNT 74, ongoing/persistent, headache.
|
|
risk of long COVID, 29.8% lower, RR 0.70, p = 0.25, treatment 23 of 1,739 (1.3%), control 27 of 1,433 (1.9%), NNT 178, ongoing/persistent, muscle ache.
|
|
risk of long COVID, 47.1% lower, RR 0.53, p = 0.03, treatment 19 of 1,724 (1.1%), control 30 of 1,441 (2.1%), NNT 102, ongoing/persistent, excluding non-pre-specified fatigue, generally unwell.
|
|
risk of long COVID, 28.5% lower, RR 0.72, p < 0.001, treatment 1,513, control 1,238, adjusted per study, all symptoms combined.
|
|
risk of long COVID, 39.9% lower, RR 0.60, p = 0.01, treatment 46 of 1,435 (3.2%), control 51 of 1,136 (4.5%), relatedness (yes + unsure) 9.1% (treatment) 11.2% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, cough.
|
|
risk of long COVID, 41.6% lower, RR 0.58, p < 0.001, treatment 96 of 1,513 (6.3%), control 106 of 1,238 (8.6%), relatedness (yes + unsure) 14.4% (treatment) 18.5% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, shortness of breath.
|
|
risk of long COVID, 22.2% lower, RR 0.78, p = 0.27, treatment 39 of 1,426 (2.7%), control 36 of 1,117 (3.2%), relatedness (yes + unsure) 7.6% (treatment) 8.4% (control)
, NNT 205, adjusted per study and for relatedness, moderate/major symptoms at 12 months, chest pain.
|
|
risk of long COVID, 38.0% lower, RR 0.62, p = 0.02, treatment 46 of 1,427 (3.2%), control 44 of 1,140 (3.9%), relatedness (yes + unsure) 7.7% (treatment) 10.3% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, palpitations.
|
|
risk of long COVID, 11.5% higher, RR 1.12, p = 0.52, treatment 77 of 1,403 (5.5%), control 57 of 1,131 (5.0%), relatedness (yes + unsure) 13.2% (treatment) 12.9% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, smell.
|
|
risk of long COVID, 36.4% lower, RR 0.64, p = 0.02, treatment 51 of 1,375 (3.7%), control 51 of 1,116 (4.6%), relatedness (yes + unsure) 1.1% (treatment) 1.4% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, taste.
|
|
risk of long COVID, 22.1% lower, RR 0.78, p = 0.37, treatment 26 of 1,290 (2.0%), control 25 of 1,057 (2.4%), relatedness (yes + unsure) 4.8% (treatment) 5.3% (control)
, NNT 286, adjusted per study and for relatedness, moderate/major symptoms at 12 months, ear ache.
|
|
risk of long COVID, 9.6% higher, RR 1.10, p = 0.73, treatment 37 of 1,325 (2.8%), control 25 of 1,089 (2.3%), relatedness (yes + unsure) 6.7% (treatment) 7.4% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, sore throat.
|
|
risk of long COVID, 34.8% lower, RR 0.65, p = 0.12, treatment 24 of 1,308 (1.8%), control 27 of 1,077 (2.5%), relatedness (yes + unsure) 6.0% (treatment) 6.9% (control)
, NNT 149, adjusted per study and for relatedness, moderate/major symptoms at 12 months, hoarse voice.
|
|
risk of long COVID, 16.6% higher, RR 1.17, p = 0.42, treatment 60 of 1,341 (4.5%), control 46 of 1,082 (4.3%), relatedness (yes + unsure) 8.3% (treatment) 7.4% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, tinnitus.
|
|
risk of long COVID, 34.7% lower, RR 0.65, p = 0.29, treatment 13 of 1,382 (0.9%), control 12 of 1,139 (1.1%), relatedness (yes + unsure) 2.2% (treatment) 3.0% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, vomiting.
|
|
risk of long COVID, 46.5% higher, RR 1.46, p = 0.12, treatment 44 of 1,439 (3.1%), control 25 of 1,175 (2.1%), relatedness (yes + unsure) 5.9% (treatment) 5.8% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, abdominal pain.
|
|
risk of long COVID, 1.6% lower, RR 0.98, p = 0.95, treatment 33 of 1,416 (2.3%), control 24 of 1,167 (2.1%), relatedness (yes + unsure) 4.4% (treatment) 5.1% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, diarrhoea.
|
|
risk of long COVID, 43.8% higher, RR 1.44, p = 0.23, treatment 30 of 1,417 (2.1%), control 17 of 1,160 (1.5%), relatedness (yes + unsure) 4.5% (treatment) 4.6% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, reduced appetite.
|
|
risk of long COVID, 89.8% higher, RR 1.90, p = 0.27, treatment 11 of 1,375 (0.8%), control 4 of 1,129 (0.4%), relatedness (yes + unsure) 1.8% (treatment) 2.2% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, weight loss.
|
|
risk of long COVID, 41.3% lower, RR 0.59, p < 0.001, treatment 89 of 1,287 (6.9%), control 94 of 973 (9.7%), relatedness (yes + unsure) 13.2% (treatment) 16.2% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, anxiety.
|
|
risk of long COVID, 38.6% lower, RR 0.61, p < 0.001, treatment 95 of 1,298 (7.3%), control 92 of 992 (9.3%), relatedness (yes + unsure) 13.7% (treatment) 17.4% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, depression.
|
|
risk of long COVID, 49.0% lower, RR 0.51, p < 0.001, treatment 129 of 1,376 (9.4%), control 143 of 1,105 (12.9%), relatedness (yes + unsure) 19.6% (treatment) 27.3% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, brain fog.
|
|
risk of long COVID, 32.9% lower, RR 0.67, p = 0.08, treatment 37 of 1,187 (3.1%), control 33 of 874 (3.8%), relatedness (yes + unsure) 7.1% (treatment) 9.2% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, confusion.
|
|
risk of long COVID, 41.9% lower, RR 0.58, p < 0.001, treatment 78 of 1,278 (6.1%), control 81 of 964 (8.4%), relatedness (yes + unsure) 12.5% (treatment) 15.7% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, headache.
|
|
risk of long COVID, 45.4% lower, RR 0.55, p = 0.001, treatment 53 of 1,254 (4.2%), control 54 of 955 (5.7%), relatedness (yes + unsure) 11.5% (treatment) 15.8% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, dizziness.
|
|
risk of long COVID, 73.5% lower, RR 0.27, p = 0.10, treatment 3 of 1,105 (0.3%), control 3 of 802 (0.4%), relatedness (yes + unsure) 0.4% (treatment) 1.1% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, fainting.
|
|
risk of long COVID, 38.7% lower, RR 0.61, p = 0.02, treatment 42 of 1,200 (3.5%), control 39 of 894 (4.4%), relatedness (yes + unsure) 7.9% (treatment) 10.7% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, numbness.
|
|
risk of long COVID, 42.1% lower, RR 0.58, p < 0.001, treatment 123 of 1,288 (9.5%), control 121 of 982 (12.3%), relatedness (yes + unsure) 13.9% (treatment) 18.5% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, sleeping problems.
|
|
risk of long COVID, 11.8% lower, RR 0.88, p = 0.37, treatment 99 of 1,180 (8.4%), control 88 of 967 (9.1%), relatedness (yes + unsure) 15.1% (treatment) 16.1% (control)
, NNT 141, adjusted per study and for relatedness, moderate/major symptoms at 12 months, body pains.
|
|
risk of long COVID, 32.2% lower, RR 0.68, p < 0.001, treatment 136 of 1,220 (11.1%), control 146 of 1,034 (14.1%), relatedness (yes + unsure) 16.1% (treatment) 19.0% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, joint pains.
|
|
risk of long COVID, 30.2% lower, RR 0.70, p < 0.001, treatment 205 of 1,398 (14.7%), control 209 of 1,181 (17.7%), relatedness (yes + unsure) 24.1% (treatment) 28.3% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, fatigue.
|
|
risk of long COVID, 31.9% lower, RR 0.68, p = 0.006, treatment 91 of 1,208 (7.5%), control 89 of 997 (8.9%), relatedness (yes + unsure) 14.5% (treatment) 18.1% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, weakness.
|
|
risk of long COVID, 35.9% lower, RR 0.64, p < 0.001, treatment 99 of 1,211 (8.2%), control 107 of 1,002 (10.7%), relatedness (yes + unsure) 14.3% (treatment) 17.4% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, generally unwell.
|
|
risk of long COVID, 9.2% lower, RR 0.91, p = 0.81, treatment 16 of 1,066 (1.5%), control 11 of 855 (1.3%), relatedness (yes + unsure) 3.0% (treatment) 3.8% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, fever.
|
|
risk of long COVID, 42.4% lower, RR 0.58, p = 0.21, treatment 10 of 1,065 (0.9%), control 11 of 853 (1.3%), relatedness (yes + unsure) 3.0% (treatment) 3.7% (control)
, adjusted per study and for relatedness, moderate/major symptoms at 12 months, rashes.
|
|
risk of long COVID, 10.0% lower, RR 0.90, p = 0.62, treatment 47 of 1,090 (4.3%), control 41 of 873 (4.7%), NNT 260, adjusted per study, moderate/major symptoms at 12 months, other.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
2.
Gbinigie et al., Platform adaptive trial of novel antivirals for early treatment of COVID-19 In the community (PANORAMIC): protocol for a randomised, controlled, open-label, adaptive platform trial of community novel antiviral treatment of COVID-19 in people at increased risk of more severe disease, BMJ Open, doi:10.1136/bmjopen-2022-069176.
5.
Guzzo et al., Safety, Tolerability, and Pharmacokinetics of Escalating High Doses of Ivermectin in Healthy Adult Subjects, J. Clinical Pharmacology, doi:10.1177/009127002237994.
6.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
7.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
8.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
11.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
12.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
13.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
14.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
15.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
16.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
17.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
18.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
19.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
20.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
21.
fiercepharma.com, www.fiercepharma.com/pharma/icer-says-pfizers-paxlovid-mercks-molnupiravir-both-cost-effective-drugs-benefit-far-equal.
25.
Butler et al., Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, The Lancet, doi:10.1016/S0140-6736(22)02597-1.
33.
Henderson, J., Ivermectin Arm of PRINCIPLE Trial Put on Hold — Trial website cites supply issues, MedPage Today, www.medpagetoday.com/special-reports/exclusives/96194.
35.
web.archive.org (E), web.archive.org/web/20220104000601/https://www.theepochtimes.com/mkt_app/no-supply-issues-with-ivermectin-pharmaceutical-supplying-principle-oxford-trial_4177066.html.
39.
dailymed.nlm.nih.gov, dailymed.nlm.nih.gov/dailymed/getFile.cfm?setid=847a1dd7-d65b-4a0e-a67d-d90392059dac&type=pdf.
45.
web.archive.org (H), web.archive.org/web/20220104000601/https://www.theepochtimes.com/mkt_app/no-supply-issues-with-ivermectin-pharmaceutical-supplying-principle-oxford-trial_4177066.html.
52.
Hobbs et al., The PRINCIPLE randomised controlled open label platform trial of hydroxychloroquine for treating COVID19 in community based patients at high risk, Scientific Reports, doi:10.1038/s41598-025-09275-6.
53.
Butler (B) et al., Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, The Lancet, doi:10.1016/S0140-6736(21)00461-X.
54.
nihr.ac.uk, www.nihr.ac.uk/news/principle-trial-finds-no-benefit-from-antibiotics-azithromycin-and-doxycycline-for-covid-19-patients/26680.
55.
Yu et al., Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, The Lancet, doi:10.1016/S0140-6736(21)01744-X.
56.
principletrial.org (B), www.principletrial.org/news/asthma-drug-budesonide-shortens-recovery-time-in-non-hospitalised-patients-with-covid-19.
58.
Dorward et al., Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial, British Journal of General Practice, doi:10.3399/BJGP.2022.0083.
60.
Hayward et al., Ivermectin for COVID-19 in adults in the community (PRINCIPLE): an open, randomised, controlled, adaptive platform trial of short- and longer-term outcomes, Journal of Infection, doi:10.1016/j.jinf.2024.106130.
61.
Hobbs (B) et al., Favipiravir for COVID-19 in adults in the community in PRINCIPLE, an open-label, randomised, controlled, adaptive platform trial of short- and longer-term outcomes, Journal of Infection, doi:10.1016/j.jinf.2024.106248.
65.
england.nhs.uk, www.england.nhs.uk/statistics/wp-content/uploads/sites/2/2023/07/COVID-THERAPEUTICS-WEEKLY-PUBLICATION-week-ending-020723-V2.0-OCT-22-Onwards.xlsx.
66.
Behzad et al., Real world Effectiveness of Sotrovimab in Preventing COVID-19–related Hospitalisation or Death in Patients Infected with Omicron BA.2, Journal of Infection and Public Health, doi:10.1016/j.jiph.2023.11.029.
67.
De Vito et al., What Is the Efficacy of Sotrovimab in Reducing Disease Progression and Death in People with COVID-19 during the Omicron Era? Answers from a Real-Life Study, Viruses, doi:10.3390/v15081757.
68.
ox.ac.uk, www.ox.ac.uk/news/2021-04-12-asthma-drug-budesonide-shortens-recovery-time-non-hospitalised-patients-covid-19.
71.
Reis et al., Effect of Early Treatment with Ivermectin among Patients with Covid-19, New England Journal of Medicine, doi:10.1056/NEJMoa2115869.
72.
webcache.googleusercontent.com, webcache.googleusercontent.com/search?q=cache:xaz8W3zZ0TgJ:https://rfs2.healthbureau.gov.hk/images/jsn_is_thumbs/images/past_event/Health_Research_Symposium_2021/Materials/HRS2021_K1_powepoint.pdf&hl=en&gl=ie.
79.
Lindsell et al., ACTIV-6: Operationalizing a decentralized, outpatient randomized platform trial to evaluate efficacy of repurposed medicines for COVID-19, Journal of Clinical and Translational Science, doi:10.1017/cts.2023.644.
81.
hra.nhs.uk, www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/principle-covid-19-uph/.
84.
Williams, T., Not All Ivermectin Is Created Equal: Comparing The Quality of 11 Different Ivermectin Sources, Do Your Own Research, doyourownresearch.substack.com/p/not-all-ivermectin-is-created-equal.
Hayward et al., 29 Feb 2024, Randomized Controlled Trial, placebo-controlled, United Kingdom, peer-reviewed, 25 authors, study period 23 June, 2021 - 1 July, 2022, dosage 350μg/kg days 1-3, trial ISRCTN86534580 (PRINCIPLE).
Contact: principle@phc.ox.ac.uk.
Ivermectin for COVID-19 in adults in the community (PRINCIPLE): an open, randomised, controlled, adaptive platform trial of short- and longer-term outcomes
Journal of Infection, doi:10.1016/j.jinf.2024.106130
Background The evidence for whether ivermectin impacts recovery, hospital admissions, and longer-term outcomes in COVID-19 is contested. The WHO recommends its use only in the context of clinical trials.
Methods In this multicentre, open-label, multi-arm, adaptive platform randomised controlled trial, we included participants aged ≥18 years in the community, with a positive SARS-CoV-2 test, and symptoms lasting ≤14 days. Participants were randomised to usual care, usual care plus ivermectin tablets (target 300-400 g/kg per dose, once daily for 3 days), or usual care plus other interventions. Co-primary endpoints were time to first self-reported recovery, and COVID-19 related hospitalisation/death within 28 days, analysed using Bayesian models. Recovery at 6 months was the primary, longer term outcome. Trial registration: ISRCTN86534580.
Findings The primary analysis included 8811 SARS-CoV-2 positive participants (median symptom duration 5 days), randomised to ivermectin (n=2157), usual care (n=3256), and other treatments (n=3398) from June 23, 2021 to July 1, 2022. Time to self-reported recovery was shorter in the ivermectin group compared with usual care (hazard ratio 1•15 [95% Bayesian credible interval, 1•07 to 1•23], median decrease 2.06 days [1•00 to 3•06]), probability of meaningful effect (pre-specified hazard ratio 1.2) 0•192). COVID-19-related J o u r n a l P r e -p r o o f 4 hospitalisations/deaths (odds ratio 1•02 [0•63 to 1•62]; estimated percentage difference 0% [-1% to 0•6%]), serious adverse events (three and five respectively), and the proportion feeling fully recovered were similar in both groups at 6 months (74•3% and 71•2% respectively (RR = 1•05, [1•02 to 1•08]) and also at 3 and 12 months.,.
Interpretation Ivermectin for COVID-19 is unlikely to provide clinically meaningful improvement in recovery, hospital admissions, or longer-term outcomes. Further trials of ivermectin for SARS-Cov-2 infection in vaccinated community populations appear unwarranted.
Conflict of 0% (-0•9% to 0•6%) 3 J o u r n a l P r e -p r o o f ivermectin. Pr(Superiority) is the probability of superiority and treatment superiority is declared if Pr(superiority) ≥ 0•975 versus usual care. Pr(Meaningful) is the probability that the odds ratio for Ivermectin versus usual care is 0.80 or smaller. 4 All secondary outcome analyses were conducted on the concurrent and eligible randomisation SARS-CoV-2 positive population, but restricted to those who are in the ivermectin and usual care group only. Secondary outcomes were analysed using frequentist statistics. 5 Defined as recovered within 14 days and reports feeling recovered for the next 14 days (or recovered at 14 and 28 days if only call data available) 6 Relative risks adjusted for age, comorbidity at baseline, duration of illness, and vaccination status at baseline. 7 Estimated hazard ratio derived from a Cox proportional hazard model adjusted for age, comorbidity at baseline, duration of illness, and vaccination status at baseline, with 95% confidence interval. 8 Mixed effect model adjusting age, comorbidity, duration of illness, vaccination status at baseline, and time. Participant was fitted as a random effect. WHO well-being score was also adjusted for the score at baseline 9 Unadjusted relative risks due to low event rate. --Ivermectin versus concurrent and eligible usual care. 1 Relative risks (RR), derived from frequentist approach mixed effect logistic regression model, adjusted..
References
Anand, Bradley, Mcauley, Clarke, Fool's gold? Why blinded trials are not always best, BMJ
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of Covid-19 -Final Report, N Engl J Med
Bitterman, Martins, Cices, Nadendla, Comparison of Trials Using Ivermectin for COVID-19 Between Regions With High and Low Prevalence of Strongyloidiasis: A Meta-analysis, JAMA network open
Bramante, Buse, Liebovitz, Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadrupleblind, parallel-group, phase 3 trial, Lancet Infect Dis
Bramante, Huling, Tignanelli, Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19, N Engl J Med
Bramante, Huling, Tignanelli, Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19, The New England Journal of Medicine
Butler, Dorward, Yu, Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, The Lancet
Butler, Yu, Dorward, Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet Respir Med
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral research
Dorward, Yu, Hayward, Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial, Br J Gen Pract
Eastman, Rusinova, Herold, Nonspecific membrane bilayer perturbations by ivermectin underlie SARS-CoV-2 in vitro activity, bioRxiv
England, None
Gupta, Biswal, Panda, Ray, Rana, Binding mechanism and structural insights into the identified protein target of COVID-19 and importin-α with in-vitro effective drug ivermectin, Journal of Biomolecular Structure and Dynamics
Horby, Lim, Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med
Investigators, Gordon, Mouncey, Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19, N Engl J Med
Izcovich, Peiris, Ragusa, Bias as a source of inconsistency in ivermectin trials for COVID-19: A systematic review. Ivermectin's suggested benefits are mainly based on potentially biased results, J Clin Epidemiol
Leber, Lammel, Siebenhofer, Redlberger-Fritz, Panovska-Griffiths et al., Comparing the diagnostic accuracy of point-of-care lateral flow antigen testing for SARS-CoV-2 with RT-PCR in primary care (REAP-2), EClinicalMedicine
Lind, Lovegrove, Geller, Uyeki, Datta et al., Increase in Outpatient Ivermectin Dispensing in the US During the COVID-19 Pandemic: A Cross-Sectional Analysis, J Gen Intern Med
Macpherson, Pragmatic clinical trials, Complementary therapies in medicine
Marcolino, Meira, Guimaraes, Systematic review and meta-analysis of ivermectin for treatment of COVID-19: evidence beyond the hype, BMC Infect Dis
Moustgaard, Clayton, Jones, Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study, BMJ
Naggie, Boulware, Lindsell, Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients With COVID-19: A Randomized Clinical Trial, JAMA
Naggie, Boulware, Lindsell, Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA
Nguyen, Hees, Hofner, Adaptive platform trials: the impact of common controls on type one error and power, J Biopharm Stat
Om, Spillane, Byrne, Neill, Harrington et al., Interventions in an Ambulatory Setting to Prevent Progression to Severe Disease in Patients With COVID-19: A Systematic Review, Ann Pharmacother
Patel, Dorward, Yu, Hobbs, Butler, Inclusion and diversity in the PRINCIPLE trial, The Lancet
Popp, Reis, Schießer, Ivermectin for preventing and treating COVID-19, Cochrane Database Syst Rev
Reis, Silva, Silva, Effect of Early Treatment with Ivermectin among Patients with Covid-19, N Engl J Med
Roman, Burela, Pasupuleti, Piscoya, Vidal et al., Ivermectin for the treatment of coronavirus disease 2019: a systematic review and meta-analysis of randomized controlled trials, Clinical Infectious Diseases
Saville, Berry, Berry, Viele, Berry, The Bayesian Time Machine: Accounting for temporal drift in multi-arm platform trials, Clinical Trials
Shafiee, Athar, Gargari, Jafarabady, Siahvoshi et al., Ivermectin under scrutiny: a systematic review and meta-analysis of efficacy and possible sources of controversies in COVID-19 patients, Virol J
Skipper, Pastick, Engen, Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19 : A Randomized Trial, Ann Intern Med
Topp, Østergaard, Søndergaard, Bech, The WHO-5 Well-Being Index: A Systematic Review of the Literature, Psychotherapy and Psychosomatics
Verity, Okell, Dorigatti, Estimates of the severity of coronavirus disease 2019: a model-based analysis, The Lancet Infectious Diseases
Woodcock, Lavange, Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both, N Engl J Med
Yang, Shen, Hou, Is Ivermectin Effective in Treating COVID-19?, Front Pharmacol
Yu, Bafadhel, Dorward, Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, The Lancet
Zhang, Song, Ci, Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflammation Research
DOI record:
{
"DOI": "10.1016/j.jinf.2024.106130",
"ISSN": [
"0163-4453"
],
"URL": "http://dx.doi.org/10.1016/j.jinf.2024.106130",
"alternative-id": [
"S0163445324000641"
],
"article-number": "106130",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Ivermectin for COVID-19 in adults in the community (PRINCIPLE): an open, randomised, controlled, adaptive platform trial of short- and longer-term outcomes"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jinf.2024.106130"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Author(s). Published by Elsevier Ltd on behalf of The British Infection Association."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-0852-627X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hayward",
"given": "Gail",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-0331-7364",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yu",
"given": "Ly-Mee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Little",
"given": "Paul",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2963-4491",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gbinigie",
"given": "Oghenekome",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4842-9247",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shanyinde",
"given": "Milensu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harris",
"given": "Victoria",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6072-1430",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dorward",
"given": "Jienchi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saville",
"given": "Benjamin R",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8701-3258",
"affiliation": [],
"authenticated-orcid": false,
"family": "Berry",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evans",
"given": "Philip H",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thomas",
"given": "Nicholas PB",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Mahendra G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richards",
"given": "Duncan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hecke",
"given": "Oliver Van",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Detry",
"given": "Michelle A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saunders",
"given": "Christina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fitzgerald",
"given": "Mark",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8872-3646",
"affiliation": [],
"authenticated-orcid": false,
"family": "Robinson",
"given": "Jared",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Latimer-Bell",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allen",
"given": "Julie",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7643-572X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ogburn",
"given": "Emma",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7498-1276",
"affiliation": [],
"authenticated-orcid": false,
"family": "Grabey",
"given": "Jenna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Lusignan",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hobbs",
"given": "FD Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Butler",
"given": "Christopher C",
"sequence": "additional"
}
],
"container-title": "Journal of Infection",
"container-title-short": "Journal of Infection",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"journalofinfection.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
2,
29
]
],
"date-time": "2024-02-29T17:09:19Z",
"timestamp": 1709226559000
},
"deposited": {
"date-parts": [
[
2024,
2,
29
]
],
"date-time": "2024-02-29T17:09:41Z",
"timestamp": 1709226581000
},
"funder": [
{
"DOI": "10.13039/501100000272",
"doi-asserted-by": "publisher",
"name": "NIHR"
}
],
"indexed": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T00:36:26Z",
"timestamp": 1709253386593
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
2
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
1
]
],
"date-time": "2024-02-01T00:00:00Z",
"timestamp": 1706745600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 21,
"start": {
"date-parts": [
[
2024,
2,
22
]
],
"date-time": "2024-02-22T00:00:00Z",
"timestamp": 1708560000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445324000641?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445324000641?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "106130",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
2
]
]
},
"published-print": {
"date-parts": [
[
2024,
2
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral research",
"key": "10.1016/j.jinf.2024.106130_bib1",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1007/s00011-008-8007-8",
"article-title": "Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "524",
"issue": "11",
"journal-title": "Inflammation Research",
"key": "10.1016/j.jinf.2024.106130_bib2",
"volume": "57",
"year": "2008"
},
{
"article-title": "Nonspecific membrane bilayer perturbations by ivermectin underlie SARS-CoV-2 in vitro activity",
"author": "Eastman",
"journal-title": "bioRxiv",
"key": "10.1016/j.jinf.2024.106130_bib3",
"year": "2023"
},
{
"DOI": "10.1080/07391102.2020.1839564",
"article-title": "Binding mechanism and structural insights into the identified protein target of COVID-19 and importin-α with in-vitro effective drug ivermectin",
"author": "Sen Gupta",
"doi-asserted-by": "crossref",
"first-page": "2217",
"issue": "5",
"journal-title": "Journal of Biomolecular Structure and Dynamics",
"key": "10.1016/j.jinf.2024.106130_bib4",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.1016/j.jclinepi.2021.12.018",
"article-title": "Bias as a source of inconsistency in ivermectin trials for COVID-19: A systematic review. Ivermectin's suggested benefits are mainly based on potentially biased results",
"author": "Izcovich",
"doi-asserted-by": "crossref",
"first-page": "43",
"journal-title": "J Clin Epidemiol",
"key": "10.1016/j.jinf.2024.106130_bib5",
"volume": "144",
"year": "2022"
},
{
"DOI": "10.1007/s11606-021-06948-6",
"article-title": "Increase in Outpatient Ivermectin Dispensing in the US During the COVID-19 Pandemic: A Cross-Sectional Analysis",
"author": "Lind",
"doi-asserted-by": "crossref",
"first-page": "2909",
"issue": "9",
"journal-title": "J Gen Intern Med",
"key": "10.1016/j.jinf.2024.106130_bib6",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1038/d41586-020-02958-2",
"article-title": "Latin America’s embrace of an unproven COVID treatment is hindering drug trials Unchecked ivermectin use in the region is making it difficult to test the anti-parasite drug’s effectiveness against the coronavirus",
"doi-asserted-by": "crossref",
"first-page": "481",
"journal-title": "Nature",
"key": "10.1016/j.jinf.2024.106130_bib7",
"volume": "586",
"year": "2020"
},
{
"DOI": "10.1177/10600280211028242",
"article-title": "Interventions in an Ambulatory Setting to Prevent Progression to Severe Disease in Patients With COVID-19: A Systematic Review",
"author": "E",
"doi-asserted-by": "crossref",
"first-page": "309",
"issue": "3",
"journal-title": "Ann Pharmacother",
"key": "10.1016/j.jinf.2024.106130_bib8",
"volume": "56",
"year": "2022"
},
{
"DOI": "10.1186/s12985-022-01829-8",
"article-title": "Ivermectin under scrutiny: a systematic review and meta-analysis of efficacy and possible sources of controversies in COVID-19 patients",
"author": "Shafiee",
"doi-asserted-by": "crossref",
"first-page": "102",
"issue": "1",
"journal-title": "Virol J",
"key": "10.1016/j.jinf.2024.106130_bib9",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciab591",
"article-title": "Ivermectin for the treatment of coronavirus disease 2019: a systematic review and meta-analysis of randomized controlled trials",
"author": "Roman",
"doi-asserted-by": "crossref",
"first-page": "1022",
"issue": "6",
"journal-title": "Clinical Infectious Diseases",
"key": "10.1016/j.jinf.2024.106130_bib10",
"volume": "74",
"year": "2022"
},
{
"article-title": "Ivermectin for preventing and treating COVID-19",
"author": "Popp",
"issue": "6",
"journal-title": "Cochrane Database Syst Rev",
"key": "10.1016/j.jinf.2024.106130_bib11",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1186/s12879-022-07589-8",
"article-title": "Systematic review and meta-analysis of ivermectin for treatment of COVID-19: evidence beyond the hype",
"author": "Marcolino",
"doi-asserted-by": "crossref",
"first-page": "639",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "10.1016/j.jinf.2024.106130_bib12",
"volume": "22",
"year": "2022"
},
{
"article-title": "Is Ivermectin Effective in Treating COVID-19?",
"author": "Yang",
"journal-title": "Front Pharmacol",
"key": "10.1016/j.jinf.2024.106130_bib13",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2022.3079",
"article-title": "Comparison of Trials Using Ivermectin for COVID-19 Between Regions With High and Low Prevalence of Strongyloidiasis: A Meta-analysis",
"author": "Bitterman",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "JAMA network open",
"key": "10.1016/j.jinf.2024.106130_bib14",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2115869",
"article-title": "Effect of Early Treatment with Ivermectin among Patients with Covid-19",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "1721",
"issue": "18",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2024.106130_bib15",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jama.2022.18590",
"article-title": "Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial",
"author": "Naggie",
"doi-asserted-by": "crossref",
"first-page": "1595",
"issue": "16",
"journal-title": "JAMA",
"key": "10.1016/j.jinf.2024.106130_bib16",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2201662",
"article-title": "Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19",
"author": "Bramante",
"doi-asserted-by": "crossref",
"first-page": "599",
"issue": "7",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2024.106130_bib17",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1001/jama.2023.1650",
"article-title": "Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients With COVID-19: A Randomized Clinical Trial",
"author": "Naggie",
"doi-asserted-by": "crossref",
"first-page": "888",
"issue": "11",
"journal-title": "JAMA",
"key": "10.1016/j.jinf.2024.106130_bib18",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00299-2",
"article-title": "Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial",
"author": "Bramante",
"doi-asserted-by": "crossref",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.jinf.2024.106130_bib19",
"year": "2023"
},
{
"DOI": "10.1056/NEJMra1510062",
"article-title": "Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both",
"author": "Woodcock",
"doi-asserted-by": "crossref",
"first-page": "62",
"issue": "1",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2024.106130_bib20",
"volume": "377",
"year": "2017"
},
{
"DOI": "10.1016/S0140-6736(21)00461-X",
"article-title": "Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "1063",
"issue": "10279",
"journal-title": "The Lancet",
"key": "10.1016/j.jinf.2024.106130_bib21",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)01744-X",
"article-title": "Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial",
"author": "Yu",
"doi-asserted-by": "crossref",
"journal-title": "The Lancet",
"key": "10.1016/j.jinf.2024.106130_bib22",
"year": "2021"
},
{
"DOI": "10.3399/BJGP.2022.0083",
"article-title": "Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial",
"author": "Dorward",
"doi-asserted-by": "crossref",
"first-page": "e446",
"issue": "720",
"journal-title": "Br J Gen Pract",
"key": "10.1016/j.jinf.2024.106130_bib23",
"volume": "72",
"year": "2022"
},
{
"DOI": "10.1136/bmj.l6228",
"article-title": "Fool's gold? Why blinded trials are not always best.",
"author": "Anand",
"doi-asserted-by": "crossref",
"first-page": "l6228",
"journal-title": "BMJ",
"key": "10.1016/j.jinf.2024.106130_bib24",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1136/bmj.l6802",
"article-title": "Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study",
"author": "Moustgaard",
"doi-asserted-by": "crossref",
"first-page": "l6802",
"journal-title": "BMJ",
"key": "10.1016/j.jinf.2024.106130_bib25",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)00945-4",
"article-title": "Inclusion and diversity in the PRINCIPLE trial",
"author": "Patel",
"doi-asserted-by": "crossref",
"first-page": "2251",
"issue": "10291",
"journal-title": "The Lancet",
"key": "10.1016/j.jinf.2024.106130_bib26",
"volume": "397",
"year": "2021"
},
{
"author": "National Institute for Health and Care Excellence (NICE)",
"key": "10.1016/j.jinf.2024.106130_bib27",
"series-title": "COVID-19 rapid guideline: managing symptoms (including at the end of life) in the community",
"year": "2020"
},
{
"key": "10.1016/j.jinf.2024.106130_bib28",
"unstructured": "National Institute for Health and Care Excellence. COVID-19 rapid guideline: Managing COVID-19. 2022. 〈https://app.magicapp.org/#/guideline/L4Qb5n/section/nBMk69〉 (accessed 16th August 2022 2022)."
},
{
"key": "10.1016/j.jinf.2024.106130_bib29",
"unstructured": "England N. 2023. 〈https://www.england.nhs.uk/statistics/statistical-work-areas/covid-therapeutics-antivirals-and-neutralising-monoclonal-antibodies/〉."
},
{
"key": "10.1016/j.jinf.2024.106130_bib30",
"unstructured": "The United Kingdom Government. Coronavirus (COVID-19) in the UK February 12, 2021 2020. 〈https://coronavirus.data.gov.uk/〉 (accessed February 12, 2021."
},
{
"DOI": "10.1016/S1473-3099(20)30243-7",
"article-title": "Estimates of the severity of coronavirus disease 2019: a model-based analysis",
"author": "Verity",
"doi-asserted-by": "crossref",
"first-page": "669",
"issue": "6",
"journal-title": "The Lancet Infectious Diseases",
"key": "10.1016/j.jinf.2024.106130_bib31",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.7326/M20-4207",
"article-title": "Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19: A Randomized Trial",
"author": "Skipper",
"doi-asserted-by": "crossref",
"first-page": "623",
"issue": "8",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.jinf.2024.106130_bib32",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the Treatment of Covid-19 - Final Report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2024.106130_bib33",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1159/000376585",
"article-title": "The WHO-5 Well-Being Index: A Systematic Review of the Literature",
"author": "Topp",
"doi-asserted-by": "crossref",
"first-page": "167",
"issue": "3",
"journal-title": "Psychotherapy and Psychosomatics",
"key": "10.1016/j.jinf.2024.106130_bib34",
"volume": "84",
"year": "2015"
},
{
"DOI": "10.1177/17407745221112013",
"article-title": "The Bayesian Time Machine: Accounting for temporal drift in multi-arm platform trials",
"author": "Saville",
"doi-asserted-by": "crossref",
"first-page": "490",
"issue": "5",
"journal-title": "Clinical Trials",
"key": "10.1016/j.jinf.2024.106130_bib35",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1080/10543406.2023.2275765",
"article-title": "Adaptive platform trials: the impact of common controls on type one error and power",
"author": "Nguyen",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J Biopharm Stat",
"key": "10.1016/j.jinf.2024.106130_bib36",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in Hospitalized Patients with Covid-19",
"author": "Recovery Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2024.106130_bib37",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2100433",
"article-title": "Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19",
"author": "REMAP-CAP Investigators",
"doi-asserted-by": "crossref",
"first-page": "1491",
"issue": "16",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2024.106130_bib38",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/j.ctim.2004.07.043",
"article-title": "Pragmatic clinical trials",
"author": "MacPherson",
"doi-asserted-by": "crossref",
"first-page": "136",
"issue": "2-3",
"journal-title": "Complementary therapies in medicine",
"key": "10.1016/j.jinf.2024.106130_bib39",
"volume": "12",
"year": "2004"
},
{
"DOI": "10.1016/S2213-2600(21)00310-6",
"article-title": "Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "1010",
"issue": "9",
"journal-title": "Lancet Respir Med",
"key": "10.1016/j.jinf.2024.106130_bib40",
"volume": "9",
"year": "2021"
},
{
"article-title": "Ivermectin for Treatment of Mild-to-Moderate COVID-19 in the Outpatient Setting: A Decentralized, Placebo-controlled, Randomized, Platform Clinical Trial",
"author": "Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)-6 Study Group",
"journal-title": "medRxiv",
"key": "10.1016/j.jinf.2024.106130_bib41",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2201662",
"article-title": "Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19",
"author": "Bramante",
"doi-asserted-by": "crossref",
"first-page": "599",
"journal-title": "The New England Journal of Medicine",
"key": "10.1016/j.jinf.2024.106130_bib42",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2021.101011",
"article-title": "Comparing the diagnostic accuracy of point-of-care lateral flow antigen testing for SARS-CoV-2 with RT-PCR in primary care (REAP-2)",
"author": "Leber",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/j.jinf.2024.106130_bib43",
"volume": "38",
"year": "2021"
}
],
"reference-count": 43,
"references-count": 43,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0163445324000641"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Ivermectin for COVID-19 in adults in the community (PRINCIPLE): an open, randomised, controlled, adaptive platform trial of short- and longer-term outcomes",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}
hayward2